



Will the Real Immune Issue Please Step Forward?

McGowan asks if all of these are happening at once or are different studies finding different issues in different study populations. The question of what’s really going on is big one in a field that generally gets very small studies which use varying methodological approaches in a disorder with a vague definition.
(Webinar on Epigenetics and ME/CFS – Dr. McGowan will talk about his study in a webinar tomorrow, Thursday, August 21, 2014, 2-3:00pm Eastern (1pm Central/Noon Mountain/11am Pacific) time. Space is limited – RSVP to Reserve Your Slot – Click HERE)
The “Second Genetics”
Epigenetics refers to the changes in sites in our DNA that are susceptible to change over time which can modify the expression of our genes. Our genetic heritage, it turns out, only sets the tableau for how our genes express themselves. Just because you’re borne with a genetic makeup that suggests, say, your immune system will immediately jump on and take out any pathogen that dares disturb you, that doesn’t mean it’s going to stay that way.
Things happen as you age that will change how your genes respond to events. Epigenetics isn’t so much an ‘if’ as it is a ‘how much’. These processes occur in our body all the time. The older we get the less we are our mother’s and father’s sons and daughters and the more our genetic makeup takes on an identity of its own. Twin studies indicating identical twins are epigenetically indistinguishable at first but diverge widely over time indicate how malleable our patterns of gene expression are.
How this change in gene expression over time occurs and the implications it has for health and disease is what epigenetics is all about. In a short time it’s become an enormous field.
There’s no question epigenetic modifications play a role in disease – the only question is how much of a role. A couple of years ago the National Institutes of Health was interested enough in the possible effects of epigenetic changes on aging, heart disease, and mental illness to devote 190 million dollars to its study. Some researchers think epigenetics will end up having a greater impact on disease than genetics.
Epigenetic modifications of immune genes have been implicated in autoimmune and other inflammatory diseases as well as neurodegenerative diseases. Researchers are now arguing which factor – genes, the environment, or epigenetics – plays the biggest role in autoimmunity. A recent review paper described probable epigenetic effects on neuroinflammation.
An identical twin study containing one twin with lupus and one twin without lupus, for instance, found altered methylation rates in the sick twin. Increased rates of hypomethylated immune genes suggested epigenetic changes had increased immune activation in the afflicted twin.
Epigenetic Processes
Over fifteen types of epigenetic processes have been identified. Damage to our DNA from toxic chemicals such as benzene and styrene can also, through increased oxidative stress, cause epigenetic changes. This, of course, suggests that the increased rates of oxidative stress present in ME/CFS may cause epigenetic changes.
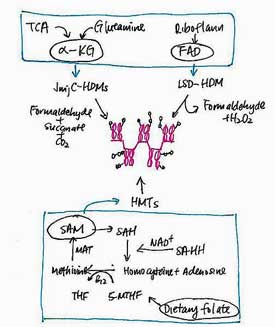
DNA Methylation
DNA methylation which involves the addition of methyl groups at CpG sites that convert cytosine to 5-methylcytosine may be the most common way epigenetic changes occur. CpG sites can either be hypomethylated (exhibit reduced methylation) or hypermethylated (exhibit increased methylation). Hypomethylation results in increased gene expression, while hypermethlation results in reduced gene expression.
The miRNA Slant
Micro RNA (miRNA) will not be talked about in McGowan’s study, but they are entangled in the epigenetic question. These small bits of RNA down-regulate many biological functions by decaying mRNA. As many as 60% of proteins in our body are believed to be regulated by miRNAs. One miRNA (miR-146a) that has been proposed as a ‘master regulator of immunity’ impacts both the innate immune and T-cell responses.
A study suggests that one miRNA may play a significant role in the natural killer cell problems in ME/CFS. Because miRNAs themselves appear to be strongly regulated by epigenetic processes, they’re something to keep an eye on in disorders characterized by high rates of epigenetic alterations.
The Study
Small Patient Set: Big Data Set
This study looked at the epigenetics of females with post-infectious onset, post-exertional malaise, and who met certain other criteria on symptom scores and who had not been taking immune-altering medications.
Even a relatively small epigenetic study (12 patients and 12 controls) generates an enormous amount of data. This study of the DNA ‘methylome’ of peripheral blood lymphocytes started out with an array covering 480,000 DNA sites subject to methylation (CpG sites). After microarray normalization 327,409 probes remained. Applying ‘probe selection criteria’ dropped the number of different methylation sites found to 1,192 sites. That corresponded to 826 genes. But then there was the ‘region analysis’, ‘gene pathway enrichment’, and much more. It’s complex stuff – far too complex for me.
Let’s get to the results.
Results
McGowan found significant differences in DNA methylation in the immune cells of healthy controls and the ME/CFS patients in almost 1200 CpG sites in over 800 genes. Genes involved in metabolic regulation, kinase activities, cellular processes and the immune response were highlighted. Significant numbers of both hypermethylated and hypomethylated CpG sites were found.
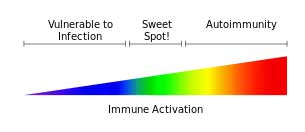
High rates of epigenetically altered immune regulating genes suggested ME/CFS patient’s immune systems are skewed to a Th2 immune response. Altered methylation rates in the genes involved in regulating the adaptive immune response (T and B cells) could help explain the inflammation present in ME/CFS.
The authors highlighted a few genes that had been epigenetically altered. One hypermethylated gene (BCL10) in the ME/CFS patients activates a transcription factor called NF-kB that’s responsible for triggering a wide inflammatory response. Maes has proposed that the NF-kB inflammatory pathway is enhanced in both ME/CFS and depression. Another epigenetically altered gene in ME/CFS is associated with EBV infection of B-cells.
It was interesting that no genes expressly associated with natural killer cells were highlighted although many genes associated with T-cells were.
The rubber meets the road in epigenetics when epigenetic modifications are shown to actually result in changes in gene expression. The study did not assess gene expression, but the authors noted that the epigenetic changes they found fit with prior ME/CFS gene expression results.
Different Epigenetic Patterns in Fibromyalgia
They also noted that a similar analysis of whole blood (not PBMCs) in Fibromyalgia suggested a very different pattern of epigenetic modifications existed in ME/CFS and FM. Methylation appears to be primarily affecting structural nervous system development and neuronal genes in FM, and immune and metabolic genes in ME/CFS (with the proviso that immune cells were examined in ME/CFS).
Epigenetics has the potential to alter gene expression in so many ways that it simply has to be addressed. This study suggested that epigenetically modified immune dysregulation could play a role in ME/CFS.
How to go from 800 plus methylated immune genes to identifying a gene or set of genes that can provide a basis for treatment is another question.
A Look at Epigenetic Effects on Rheumatic Disorders
“It is clear that aberrant epigenetic regulatory mechanisms play important roles in the development and pathogenesis of rheumatic disease.” – Gray, 2014
(Much of the information in this section is taken from a review paper – Perspectives on epigenetic-based immune intervention for rheumatic diseases Steven G Gray*, Arthritis Research & Therapy 2013, 15:207)
Epigenetic studies in other disorders are beginning to do that. Lupus studies, for example, have identified epigenetic modifications that alter the expression of CD4 T-cells and contribute to the development of autoreactivity and increased antibody production in that disease.
The fact that epigenetic modifications of the histone strands in our DNA create prime targets for autoantibodies suggests that epigenetic modifications may have special relevance to autoimmune disorders. Researchers have identified damaging epigenetic processes in Sjogren’s Syndrome that were reversed using Rituximab.
One epigenetic targeting drug (vorinostat) that is able to damp down pro-inflammatory cytokines and regulate T-cells may be effective in a range of autoimmune disorders. It is currently being used to treat lymphoma.
Given the broad pattern of hypomethylation found in ME/CFS and other disorders, it’s possible that drugs able to target factors that increase methylation rates across the board could be helpful. Lupus researchers appear to have uncovered a hypomethylated CpG site that regulates DNA methyltransferase – a prime factor in the methylation process.
McGowan noted that a DNA methylating drug that alters the methylation of immune pathways (including HLA Class I pathways) affected in ME/CFS has been produced. That drug, now apparently being tested in lung cancer cell lines, could be applicable to ME/CFS at some point.
Dietary Control of Methylation
A large number of naturally-occurring compounds that can regulate epigenetics suggest that dietary modification or supplementation may be helpful. Cucurmin, for instance, is able to regulate epigenetic processes believed active in some rheumatic and autoimmune disorders.
Two placebo-controlled studies, one with a product called Meriva (Thorne Research, Inc.,Dover, ID, USA), containing a formulation of curcumin complexed with phosphatidylcholine, was found to have significant benefit on rheumatoid arthritis patients, including decreased expression of pro-inflammatory cytokines IL-6 and IL-8. A cucurmin analog appears to be able to induce the expression of the major miRNA immune modulator (miR- 146a).
Delphinidum appears to be able to reduce activity in the NK-kB inflammatory pathway that the authors highlighted in their results. Resveratrol is a natural compound that appears to be able to modulate inflammation by regulating pro-inflammatory cytokines.
Sulforaphane’s(SFN) ability to reduce immune responsiveness is currently being assessed in clinical trials using ‘broccoli smoothies’. SFN’s ability to reduce the Th2 response, if proven, could come in handy in a disease like ME/CFS that appears to be characterized by an increased Th2 response. Injecting SNF into mice with arthritis reduced synovial problems and bone destruction as well as pro-inflammatory cytokine production.
The Future
McGowan outlined several steps that are needed to validate the effects epigenetics is having in ME/CFS. Longitudinal studies following ME/CFS patients over time which associate epigenetic alterations with specific symptoms is important. Researchers need to be able to show that epigenetic changes show up in changes in symptom expression.
Noting that immune abnormalities can be unmasked by challenges such as exercise, McGowan et. al. proposed that both epigenetic modifications and gene expression be analyzed before and after exercise. Researchers also need to be able to show that the epigenetic modifications are actually altering the expression of the genes they’re associated with. A combined epigenetics/gene expression study is a necessity at some point.
Getting the next studies done will clearly require substantial financial input, and that probably means NIH funding. Gathering the data needed to get the big bucks from the NIH is, of course, what these pilot studies are all about.
Webinar on Epigenetics and ME/CFS
Dr. McGowan will talk about his study in a webinar tomorrow, Thursday, August 21, 2014, 2-3:00pm Eastern (1pm Central/Noon Mountain/11am Pacific) Space is limited – RSVP to Reserve Your Slot – Click HERE)







I do believe this is a part of our key to possibly change what may have been turned on.
I know for me with all my autoimmune disorders, turmeric is absolutely necessary.
Looking forward to the webinar.
Issie
I really like the SMCI’s approach with these pilot studies. They get outside investigators into the field, they provide pilot data for larger NIH-funded studies, they don’t cost an arm and a leg to fund, etc. It seems like a really good model.
Excellent, excellent article Cort! Now if only we could have a CPET coupled with gene expression analysis, it would be amazing.
I wish I could follow the webinar, but I’m bound for my first of the 2 days CPET, today …
Good luck VBee 🙂
Love to hear how it goes.
Is this for disability?
Dr. Amy Yasko has been using protocols based on epigenetics for years. Her initial emphasis was on treating children with autism, but her research and insights apply to ME/CFS as well. Getting my genetics tested through her and having her panel of tests along with her recommendations and interpretation of my results has been well worth the money and time invested. In the space of one year, The dietary and supplement protocol she laid out for me based on my test results has brought me from 5years of being so sick I was planning my funeral and wishing nearly every day I would just die (severe ME Canadian definition,POTS, and more) to a place where now I am glad to be alive to watch my children move into young adulthood. I moved from about 5% functional level to 50-60% so far. That,my friend,is huge to me. My progress is not in a straight line upwards, but more like the trend line of a good stock. I actually started participating in life again. My husband has his wife back, my children have their mother back.
The great news is that you don’t have to travel to see her to get on the protocol. All the tests can be done from home and mailed to the lab. She writes her recommendations and encourages you to work with your Dr. to implement them.
I would encourage you to google Dr. Amy Yasko and read about her protocol and watch the videos of her seminars to see what you think of her and her reasoning behind her protocol. Don’t just assume it’s just about autism, the epigenetics of methylation apply to everyone.
It has helped me too. But you can figure it out yourself. If you do a 23&me test and enter your raw data into Promethesis, do the research, you can figure it out. There is a certain order you go for certain flaws. It must be done in that order and very, very small amounts to work. One of Dr Amy’s colleagues gives free webinars weekly that give a way to ask questions. Diet change is also important.
Issie
Thanks Cort for providing just the right amount of scientific knowledge. I think this study is very important and I hope NIH takes note.