



It became evident that glial activation was not merely just correlated with pain, but that it was critically involved in inducing pathological pain.
Suddenly glial cells are turning up in spades in Chronic Fatigue Syndrome and Fibromyalgia. The Japanese neuroinflammation study found widespread glial cell activation in the brains of people with ME/CFS. Younger’s leptin finding suggests the glial cells may be going bonkers in ME/CFS, does his successful low dose naltrexone study in Fibromyalgia. VanElzakker’s Vagus Nerve Infection Hypothesis proposes chronic glial cell activation near the vagus nerve is causing ME/CFS.
“Fatigue appears to arise when some stimulus induces increased proinflammatory cytokines in the brain and/or activated microglia and increased expression of chemokines” Harrington
On the fatigue side, a 2012 paper, “Neurobiological Studies of Fatigue”, asserted that all animal models of fatigue had one thing in common – activation of the microglia and/or cytokine/chemokine storms in the brain.” This 48 page paper noted that treatments for fatigue are ‘scarce’ with the most effective exercise being ‘difficult to administer’ in the severely fatigued (lol).
Fortunately glial cells are getting a lot of research and treatment possibilities are popping up. It’s time to take a look at a fascinating new entry in ME/CFS and FM research.
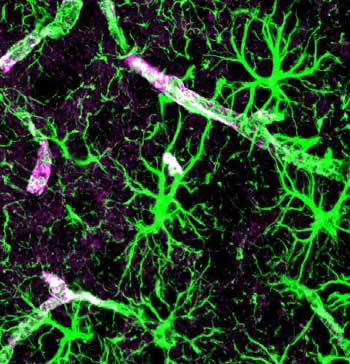
Glial Cell Activation
How turned-on or activated the glial cells are may determine how much pain or fatigue or other symptoms you have. Watkins
Pathological pain and the neuroimmune interface. Grace PM, Hutchinson MR, Maier SF, Watkins LR. Nat Rev Immunol. 2014 Apr;14(4):217-31. doi: 10.1038/nri3621. Epub 2014 Feb 28.
Toll-like receptors in chronic pain. Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Exp Neurol. 2012 Apr;234(2):316-29. doi: 10.1016/j.expneurol.2011.09.038. Epub 2011 Oct 6. Review.
Until the early 1990s pain was thought to primarily be ‘nerve-based’; i.e., it was caused by activation of the sensory nerves and could be ameliorated by drugs, mostly opioids, that affected nerve transmission in the brain. With more and more findings that non-neuronal cells called glial cells play a major role in producing chronic pain, that view no longer holds.
These non-neuronal components of the brain are abundant, outnumbering the neurons ten to one, and make up greater than 70% of all the brains cells. Two types of glial cells exist: the microglia that provide the scaffolding for the neurons in the brain, and the astrocytes which participate in several functions including formation of the blood-brain barrier and blood flow regulation in the brain.
With the glial cells it’s all about activation. When the glia cells are inactive they’re fine but when they’re pumped they tend to turn into hairy monsters.
Several realizations in the 1990s, two of which indirectly involve ME/CFS and FM, opened researchers’ eyes to the role glial cells play in chronic pain production. One involved the recognition that substance P – a pain producing factor which is increased in fibromyalgia – triggers glial cell activation. The other was the understanding that glial cell activation plays a key role in inducing ‘sickness behavior’ – the fatigue, fevers, pain, etc. associated with infection.
A decade later glial cell activation in the spinal cord had been implicated in a wide array of animal pain models including trauma, inflammation, chemotherapy, multiple sclerosis, migraine, back pain, and others. Glial cells are now also known to play critical roles in immune surveillance in the brain and in cleansing the brain of unwanted substances.
Research indicates that activated glial cells release substances that trigger the neurons to release excitatory substances (proinflammatory cytokines IL-1, TNF, and IL-6; chemokines; arachidonic acids; ATP; prostaglandins; nitric oxide) that enhance pain, downregulate GABA, upregulate glutamate expression, and more.
Rapid Pace of Learning
The pace of learning in this field has been fast and furious with review papers just over the last few years showing remarkable differences.
Glial cells have been implicated in two of the most puzzling problems facing the pain research field: opioid intolerance and opioid-induced hyperalgesia. Opioid intolerance occurs when opioids provide reduced pain relief after a period of use, and opioid hyperalgesia occurs when opioids cause increased sensitivity to pain over time.
As recently as 2011 a new term — ‘central immune signaling’ — was coined to describe how the brain’s immune system affects nervous system activity. The immune findings in glial cells in turn opened up a new window on the immune system in the brain.
Enter the Toll-like Receptors (TLRs) – A Major Focus in Immune Activated Nervous System Disorders Emerges
“The abundance of TLRs in pain responsive regions makes them a critical potential component of pain signaling”
The discovery of the Toll-like immune pathway (in flies) led to the 1995 Nobel Prize in Physiology or Medicine for Edward B. Lewis, Christiane Nüsslein-Volhard, and Eric F. Wieschaus. TLRs are now understood to be the critical link between the innate immune system and central nervous system.
The role they play in producing pathological pain was verified when researchers were able to reverse the most difficult pain to treat in the human body — neuropathic pain — in rodent models simply by using two TLR4 inhibitors, naloxone and naltrexone. These receptors dot the surface of the glial cells.
Infection Possible But Not Needed
These receptors respond to indicators of cellular stress and damage termed ‘endogenous danger signals’ or ‘alarmins’ (fibronectin, β-defensins, high mobility group box-1 HMGB1, ATP, heat shock proteins) produced during inflammation and injury. Increased levels of these alarmins have been implicated in a variety of central nervous system disorders including Alzheimer’s disease, stroke, and chronic pain.
The TLR4 response to environmental factors indicates that damage, not infection, is the key element. Of course, infection could cause damage, but infection is not necessary to produce the so-called ‘alarmins’ that TLR4s respond to.
Some evidence suggests alarmins may be present in FM and ME/CFS. Five studies have now found evidence of small-fiber neuropathy in fibromyalgia — a potential trigger for TLR4s. Several studies have found increased heat shock proteins in ME/CFS — another potential trigger for TLR4s. The Lights’ studies suggest small amounts of ATP and other indicators of cellular stress associated with muscle damage may be upregulating gene expression in ME/CFS, and ATP has been shown to activate glial cells.
ATP also binds to some of the same purinergic receptors (P2X4/P2X7) that studies of gene expression following exercise suggest are increased in ME/CFS. This means these triggers could be turning on the production of pro-inflammatory cytokines in ME/CFS.
The Opioid Connection
Interestingly, opioid drugs trigger glial cell activation via the TLR4 receptors. This means the drugs most commonly used to reduce pain can, in some people, trigger an immune response that increases pain, leading to either opioid tolerance or opioid-induced hyperalgesia (increased sensitivity to pain).
Jekyll and Hyde Cells
Recent evidence suggests the microglia have a Jekyll and Hyde-like nature; they can pump out neuron damaging pro-inflammatory factors or they can pump out anti-inflammatory factors that are neuroprotective. Turning the microglia back into their neuroprotective form could be the key to resolving chronic pain and other states.
High Cytokine Levels in the Body NOT Required Either
Increased cytokine levels in the body do clearly cause fatigue, pain, and other symptoms in some people with Fibromyalgia and ME/CFS, but it may also be that the glia have become so sensitized to immune system signals that only small alterations are required to set them off.
VanElzakker’s Vagus Nerve Infection Hypothesis (VNIF) and Broderick’s and Younger’s findings suggest that slight elevations of immune factors in the periphery may be all that’s needed to spark a cytokine storm in the brain. The big cytokine increases for some patients may occur in the brain.
‘Hairy Monsters’ Stuck in Hyperexcitable Mode
This is most likely to occur when glial cells get stuck in ‘hyperexcitable mode’. It’s now known that injury and environmental stressors can cause the microglial cells to become ‘stuck’ in a kind of panic-ridden defensive state. Once in this state, even mild stimuli can cause them to begin releasing proinflammatory cytokines that tweak the neurons to produce symptoms of ‘sickness behavior’.
Once neurons become activated they, in turn, can produce factors (substance P, glutatmate, nitric oxide and ATP) that keep the microglia whipped into a frenzy.
The Problem of Female Pain
The increased risk of chronic pain women face is at least partly due to sex hormones. Pain sensitivity in girls and boys prior to puberty is equal, and the sensitivity increases in females after puberty, and then it nears parity again after menopause. Some studies suggest increased estrogen levels are responsible. Estrogen in vivo has been found to increase pro-inflammatory cytokine gene expression by the microglia.
Conclusion
Glial cell dysregulation presents a fascinating possibility for people with Chronic Fatigue Syndrome and Fibromyalgia. Given the possible connections and the abundant research being done on them it’s a bit surprising they haven’t been connected to these disorders before. Given the role they play in producing chronic pain it would be remarkable if they were not implicated in Fibromyalgia. Treatment possibilities seem pretty abundant but clinical trials are only beginning to swing into gear.
Depending on how the neuroinflammation studies turn out we may be hearing much more about glial cells in the future.
- Coming up shortly – Glial Cell Inhibitors










Hi Cort,
Very interesting article. I’m curious to know your thoughts on infected microglial cells in the spine. I have CFS/ME and was recently diagnosed with a spinal syrinx. There are no tumors or cysts to explain this current symptom. From all my research I’m thinking it’s being caused by viral agents attacking my spine. Have you come across this before?
Many animal studies indicate that microglial cells in the spine are definitely players in chronic pain. I don’t have any knowledge on infection but Van Elzakker proposes that a mild infection in or near the vagus nerve is triggering the microglial cells there to produce proinflammatory cytokines that are causing the vagus nerve to initiate ‘sickness behavior’ via the brain.
Cort,
Really, really well writen, especially considering the complexity of the neural-immune interface.
How do the glia cells get stuck in ‘hyperexcitable mode’?
This blog motivated me to hit the “donate” button.
Thanks and thanks for introducing me to the subject and providing references. How do they gut stuck in hyperexcitable mode? That’s good question. I think it’s pretty clear that repeated triggers can do it and the TLR4 receptors are clearly involved but I don’t know if anyone knows why.
Thanks for the donation. I’m glad somebody is using that button.
Looking further it may be the number of TLR4 receptors on a cell that determine how ‘excitable’ it is. Why they could be high or low I don’t know.
“Alarmins” – I do like it when scientists come up with a name that we can all understand.
From what I’ve read they’re just starting to investigate the range of signals that act as Alarmins and you have to suspect that there may be any number of danger signals in ME/CFS and FMS going – “the engines cannae take it captain!”
I found (and lost unfortunately) one paper that showed that neuroinflammation persists long after an acute EBV infection has cleared which does seem to tie in to the Dubbo findings.
PS – For your next blog on this subject – the antiviral drug ganciclovir appears to have more than antiviral effects :
Antiviral drug ganciclovir is a potent inhibitor of microglial proliferation and neuroinflammation
“We discovered unexpectedly that the antiviral drug ganciclovir (GCV) inhibits the proliferation of microglia in experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis (MS), as well as in kainic acid–induced excitotoxicity.”
http://jem.rupress.org/content/211/2/189.abstract
” The engines cannae take it any longer Captain” “Full speed ahead Scotty!”
Uh oh….
I had no idea on ganciclovir; how interesting that an antiviral affects the glial cells! Wow…
I don’t really care how they get stuck in that mode I just want to see treatments that ‘unstuck’ them…my understanding is that is realistic so there is hope here.
Hi Cort,
It’s really great to see someone think outside of the box and actually try to get to the root of the problem instead of just trying to get rid of the patient with a prescription that will keep him away for awhile.
“VanElzakker’s Vagus Nerve Infection Hypothesis proposes chronic glial cell activation near the vagus nerve is causing ME/CFS.”
Have you taken a look into why the vagus nerve might be acting up? Seeing as it’s so common the cause might also be something quite common, such as streptococcus A. The bacterial infection damages the vagus nerve causing chronic glial cell activation. The vagus nerve acts up and causes IBS, nerve pain and sensations and keeps your body awake, not letting you sleep. Perhaps even leaving you with a mystery cough. You get flus, muscle inflammations and torn ligaments, but still the CRP values stay put. On paper you’re very healthy.
It might also account for the fact that even children suffer from FMS. Strep A does not discriminate in age, but being in a stressful stage of life would leave your immune system vulnerable to a strep attack.
I think I’ll have chat with my doctor about naltrexone.
VanElzakker believes an infection – just as you propose – perhaps with herpesviruses or others in the vagus nerve starts the process.
In the POTS community, there are some children being found to have PANDAs and it is thought to start from a strep infection. Along with this goes dysautonomia symptoms. One boys mom, whom I stay in touch with, just found out he has antibodies in his brain and heart connected to rheumatic fever which can be caused by streep. He just underwent his first IVIG infusion. His mom is addressing his diet and using alternative supplements to try to get his immune system to kick in. His cardio doc is the one figuring out his issues and that it is autoimmune related.
I think she is following your blogs here Cort, so maybe she can give more details.
Issie
Adults are beginning to have a variety of drugs available even if to little relief. But what can we do with children?
Do you have an opinion on quercetin, glial cells and children?
Quercetin and ldn have been successfully used in clinical trials involving children with excellent safety outcomes – I would not hesitate to give either one to my own children if they suffered from ME/CFS or FM.
Many of us are using quercetin to help with mast cell disorders.
Unfortunately, LDN caused me terrible depression. It did help my pain and sleep. But, the depression was not tolerable.
Issie
Hi Cort, how do you place Rituximab in this puzzle?
I don’t know…Check out an upcoming blog on EBV and autoimmunity, though – it’s fascinating stuff!
Gijs
Let’s see further results on rituximab first. When are the Norway results due?
Just a hunch here, maybe autoimmune stuff is happening in CFS that leads to microglia issues.
Maybe, just maybe, treat the autoimmune condition (via rituximab, for example) and you are also addressing the microglia?
Excellent article, Cort
I’m having some luck on Minocycline. (microglial inhibitor)
Only taken it for two weeks but definitely less pain, fatigue and PEM.
I know better than to get my hopes up but i couldn’t help mentioning it in light of this article.
:). You never know…good luck on it 🙂
Yayyyy, doxy helps me! Also, helps autoimmune function.
http://www.biomedsearch.com/nih/Doxycycline-in-autoimmune-central-nervous/17990580.html
http://www.prohealth.com/library/showarticle.cfm?libid=8949
Issie
I recently saw a physiotherapist for visceral manipulation and craniosacral therapy. Interesting enough, when she did mild manipulation on the vagus nerve in my ear it was extremely painful. She said the vagus nerve felt inflamed and very tight.
Now that I’ve read this article, that is really beginning to make sense. She did give me some manipulation exercises I can do to try to loosen the nerve and hopefully reduce inflammation.
It should be interesting to see how this plays out.
Interesting! That combines for the ‘tightness’ I associate with ME/CFS and inflammation…Please let us know how it goes. Good luck.
I currently take a low dose Naltrexone, 4.5 mg. I haven’t noticed a big difference as far as the pain goes but it had definitely helped my immune system out. I have FM and was constantly sick. Now, I get breaks where I’m not always ill but still suffer with pain, fibro fog, etc. I was treated for what my doctor said looked like shingles on the top of my pallet for 14 days with Valtrex and noticed I felt much better while taking that antiviral medication. Since then, I have always thought something viral had to do with causing the FM. The vagus nerve has come up quite often with me. I have a lot of issues with my left ear, and was even talked into having surgery on it before my diagnoses of FM. I always have a tightness around it and in the C5 area.
Have you been following the Pridgen Antiviral FM trial? If not check out this video – we’ll be reporting on this as it unfolds.
Cort,
So glad you sent this to me. I’ve never followed this before, but it’s very interesting. Do you know about how long ago this was recorded? Looks like he’s hoping to market a drug in about 3 years from the recording of this interview.
It was just recorded….I think we should know about the drug combo soon…Here’s another recent blog on his efforts.
http://www.cortjohnson.org/blog/2014/03/24/pridgen-reports-fibromyalgia-antiviral-trial-results-positive/
This makes so much sense to me and always has. EBV has always played a part in my health issues too. Hoping it’s our answer. Thanks for sharing this.