

The preliminary results from the K Pax Synergy trial are in. Run at four centers across the country, this placebo-controlled, double-blinded 120 person trial combined a central nervous system stimulant (methyphenidate/Ritalin) with a nutrient formula. The idea was that as the stimulant boosted central nervous system activity the nutrient formula would enhance mitochondrial and immune functioning and protect against side effects. Dr. Kaiser, the originator of the Synergy formula, believes mitochondrial dysfunction plays a key role in ME/CFS.

This “phase-two” treatment trial was designed to determine if the combination formula was safe and efficacious, and to inform the team how to best produce a possible, much larger, phase-three trial.
Results
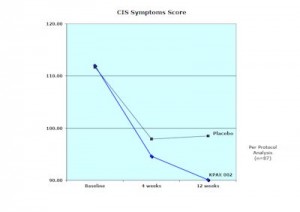
The patients in the placebo arm of the Synergy trial averaged a 13% decline in their Checklist Individual Strength (CIS) scores. The patients taking the Synergy formula averaged 22% decline in their CIS scores. The difference between the two did not have statistical significance. In the FDA’s eyes the study would have been a failure.
But what does a 22% decline in a CIS score mean for a patient? The average beginning CIS score was about 110. A 22% decline in that score would put a patient at about 88. Seventy-six is considered the cutoff point for remaining in the workforce. Go above 76 and you’re probably going to drop out of the workforce. Go below 76 and you’re probably going to stay in the workforce.
The average response didn’t put the “average” patient back into the workforce but it put them within about 10 points of possibly entering it. That indicates that a significant increase in functionality occurred.
Nor does the overall result say anything about the really positive responders. I asked Dr. Kaiser if anyone had a response similar to that of the disabled woman who was able to return to the workforce. He said about 10-15 people had responses like that. That’s about 20% of those getting the drug.
Relativity Strikes: High Placebo Response Complicates Findings
The large amount of patients in the placebo arm with a very positive placebo response complicated the results. Dr. Kaiser said ten to fifteen of the patients had high enough placebo responses to, by the measures of the study, be considered cured.
- 01-007 -27 point decline
- 01-018 -39 point decline
- 01-056 -60 point decline
- 01-070 -62 point decline
- 02-035 -47 point decline
- 02-038 -30 point decline
- 03-019 -68 point decline
- 03-028 -48 point decline
- 03-029 -83 point decline
- 04-003 -84 point decline
Because the most important results of the trial for the FDA involve the difference between the placebo and the treatment arm responses, the high placebo responses found were problematic.
I asked Dr. Kaiser if he thought high expectations played a role? That was one possibility. A statistical anomaly was another; it may be the Synergy group just happened to bump into some patients with very high placebo response rates.

One result suggested the trials was successful; the other – the one FDA relies on – suggested it wasn’t
The two methylphenidate studies highlight what complicated beasts clinical trials can be. They indicate that a drug that provides less benefit to the patients but has a lower placebo response rate could be considered a success by the FDA. A drug that provides more benefits to the patients, on the other hand, but also has a higher placebo response rate could be considered a failure.
In the 2006 methylphenidate trial the ME/CFS patients responded less well (11% improvement) than in the Synergy trial, but the low placebo response (3% improvement) in the 2006 trial made the drug look more effective than it was. The difference between the two allowed the authors to say:
Methylphenidate at a dose of 2 x 10 mg/day is significantly better than placebo in relieving fatigue and concentration disturbances in …. chronic fatigue syndrome patients.
The placebo response in that trial (3%) was so low that the trial ended up shining in the measure the FDA considers most important. In that trial only 17% of the patients were considered to have a clinically significant response.
Such are the vagaries of clinical trials. I tend to hold my breath when clinical trial results are announced. I don’t particularly trust them – by which I mean I wouldn’t choose or not choose a drug based solely on them.
I expect the positive effects of good drugs to be washed out to some extent by the different types of patients in the trial. The high degree of heterogeneity present in the ME/CFS population, after all, is a known barrier to drug development. Drug companies are reluctant to invest in a drug that gets tested in a group of ME/CFS patients. They’re afraid even a good drug will not get the results they need to get FDA approval. In this case that appeared to be true.
I vividly remember Dr. Klimas exclaiming that if they’d just chosen a different endpoint to measure, one trial would have been a success. Instead, the trial was considered a failure.
Additive Effects?
He also suggested that the increased ability of the responders to participate in the normal activities of life could help explain the increasing benefits of the formula over time. Clinical trials do not always produce black and white results.
A Safe Combination
The KPAX 002 treatment was very well tolerated. In fact, it was much better tolerated than expected and far better tolerated than reported in the previous trial using methylphenidate alone. “We believe this may be due to the nutrient supplement’s protective effect on the mitochondria, specifically within the nervous and endocrine systems.”Dr. Kaiser
Central nervous system stimulants like methylphenidate are powerful drugs that can have significant side effects. Dr. Kaiser said considerable concern was expressed regarding the effects of a stimulant on a possibly already stressed nervous system, but the drug combination proved safe. Methylphenidate appeared to be safe in the 2006 trial as well, but significantly fewer side effects occurred in the Synergy trial.
Kaiser’s thesis – that the nutrients would add both protection and enhance the response to methylphenidate – appeared to be accurate.
The Next Trial (The Next Trial?)

Will the K Pax formula become the first FDA approved treatment for ME/CFS? The next trial, if it occurs will be much, much bigger and more expensive.
I asked Dr. Kaiser if he was satisfied with the response rate. He sounded like he was moderately satisfied but felt like they could do better, and listed four ways in which he expected that the next trial, if it is done, would have better results.
- Employing a crossover trial in which the patient goes from placebo to drug or drug to placebo should help reduce the placebo issue that occurred in this trial.
- When used in conjunction with the nutrients methylphenidate was found to be safe at all doses. That suggested a higher and possibly more effective dose would be tolerated.
- The declining benefits of the placebo treatment and the increasing benefits of the Synergy formula over time, suggested a longer trial would have produced better statistical results.
- The addition of ubiquinol (C0Q10) (100mg/day) to the formula should add an extra punch.
The Synergy group is trawling through 2,000 pages of data. We’ll get more results on what percentage of patients had a clinically significant result later.
Wrap Up
All in all it was an odd result. A trial that succeeded on several levels but not on an important one. A trial that appeared, to be honest, to run into a bit of bad luck.
Dr. Kaiser said he was encouraged by the safety and efficacy data but whether the Synergy team will be able to move on and possibly produce the first FDA approved treatment for ME/CFS is unclear. Because no drugs have been approved for ME/CFS, one Phase III trial might be all the CDC requires. The results of this trial, however, suggest the next one will have to be much larger and more expensive to meet the FDA’s requirements.
The Synergy team doesn’t have deep pockets. They’re looking for partners – individuals, pharmaceutical companies – and grants to raise the money for another trial.
Have you tried the Synergy formula or K Pax’s Fatigue and Energy Pack that uses caffeine instead of Ritalin? Tell us how it went here:
- Synergy Formula (Ritalin plus immune and mitochondrial nutrients)
- Fatigue Supplement Pack (caffeine plus immune and mitochondrial nutrients)
John Kaiser on the Synergy Trial
Stimulants for Chronic Fatigue Syndrome (ME/CFS) and fibromyalgia (FM)
Find out more about stimulants and ME/CFS and FM






Did those taking Ritalin crash once they stopped taking it?
I don’t think they checked post trial effectiveness. Kaiser has said that some patients who respond well can go off it entirely after a time and he has some patients who are still on the formula, side-effect free, three years later.
Here’s a piece of it: It’s about dopamine. Or the lack of dopamine. I wish they’d used dextroamphetamine as a stimulant instead of Ritalin. As I understand it, the former contains dopamine; Ritalin does not.
I think most of us on this giant ship are now convinced that repeated elevation of cytokines is part of the process of this disease. Studies show that this will eventually either deplete dopamine levels or disrupt the dopaminergic system, with much the same results.
And, yes, you’re going to crash when you quit dextroamphetamines or Ritalin.
Ritalin increase dopamine levels.
Nico: Any activity (eg exercise), or drug (eg ritalin) that requires a source of dopamine to work, will only produce positive results if sufficient dopamine stores are available. Many with ME/CFS are running-on-empty in this department. You will eventually crash on ritalin, just like we crash after exercise, alcohol, drugs, stress etc because dopamine reserves are not adequate in most ME/CFS sufferers.
I have little faith in these mitochondrial trials. I thought the Heparin trial or treatment was more interesting and perhaps more efficacious, for a subset at least.
The doctor TY Vincent (in Alaska) approach using LDI low dose immune therapy is very intriguing. He postulates it is a form of Lyme that causes the Autoimmune reaction in CFS!
I have always believed that to be the case in the GUT!! RP
Did they say how people with POTS did with it? It doesn’t seem like Ritalin and tachycardia would mix very well.
No word on if anyone with POTS was in the trial or how they did.
Does anyone know what is included in the nutrient part of the K Pax??
You can go the kpax website and see or order it
PamJacobs, here’s the link.
http://www.thesynergytrial.org/study-treatment.html
I wrote a comment about this further down in general comments.
thank you!
Sounds like a treatment that doesn’t correct underlying abnormalities in pathophysiology
Thank you Cort. This opens my eyes to many issues of medication trials and approved medications that are described as “safe”. I was in this trial. I had horrible side effects. (and I had a HORRIBLE 8 days of withdrawal after the trial. Someone should have thought of that possibility and not allowed patients to experience that.)
“Safe” does not really mean safe for everyone. We need to remember that. I do sincerely hope it will be helpful to some, but I have as documented ME/CFS as one can have these days (test on cytokines, virus’, NK cells etc) and it’s not safe for me.
Wow, Mp, sorry to hear about your bad reactions. I agree patients should know about severe possible side effects, thanks for telling.
Mp: I take Ritalin for certified ADD/ADHD. I will tell you from my own experience that 10 mg 2xs a day is a pretty big dose of Ritalin to start on. Esp. if you might be sensitive to meds.
Ritalin is physically and psychologically habit forming (believe me). It is a drug with a fast kick in time versus something like SSRI’s. I have had to stop Concerta cold turkey, and it is not fun. I think it was negligent that the doctors didn’t warn you of this possibility, and taper you down. It actually seems irresponsible, because one can fall into a deep depression (even though it’s not an anti-depressant as such) if Ritalin/Concerta is suddenly stopped.
Dopamine, which is increased with Ritalin, is basically “happy hormones”, (like super duper happy hormone) – and if you have a sudden stop to increased dopamine… well… yeah… withdrawal. Yes, the withdrawal takes about a week in my experience.
I’m sorry you experienced that. And, hope you feel balanced out and better by now.
And more patients. I think longer time, switching them and more patients (300) might have revealed more.
That was the understanding I got from Dr. Kaiser – a longer, larger trial would have reached statistical significance relative to the placebo controls.
My daughter has been on Ritalin for energy per Dr. Peter Rowe for four years. She has POTS, OI, and CFS/SEID. She’s on 30 mg of Ritalin and it’s been the only way she can attend school full time. It has been a life saver. It helps increase her blood volume, less brain fog and gives her energy. She takes a beta blocker at night for the POTS. She says it makes her feel normal and she doesn’t crash as much, but that may be the beta blocker helping.
These results are not very impressive. The placebo effect is the same effect you see in CBT/GET. Also a subgroup of patiënt getting ‘better’ without doing nothing.
I’m glad the results were null, because the trial wasn’t designed properly anyway – there should have been Ritalin-only and supplement-only treatment arms.
I’m sorry, Snow Leopard, but why anyone would want null results from one of the few larger-sized ME/CFS trials done – using a relatively inexpensive drug and nutrients is beyond me. How often do trials like this come along? And you would rather it fail because of study design issues? I just don’t get it. The trial was OK’ed by the FDA, by the way, and Montoya, Klimas and others went along. I’d rather have another tool in ME/CFS doctors toolkits.
Call me a cynic, but the design of the trial looked like an attempt for this pharma company to try and sell their supplements (even in the absence of Ritalin), with ‘evidence’ from this trial.
But for them to have proof that the supplements made a difference, there has to be Ritalin-only and supplement-only treatment arms for comparison. Bad trial design means more questions than answers and ultimately more cost for the patient.
Regardless, it is all academic now as efficacy was not demonstrated.
Cort in the interest of full disclosure –
How did you obtain the KPax products (purchase? given to you? if so by whom? under what conditions?)?
Are you still using KPax products?
How did your experience with them influence your interpretation of this study?
I bought them from the website (no freebies!). I’m still using them. I’m kind of an odd patient: I tend to respond pretty well to stuff but then fall apart when my energy levels rise. I’ve been taking it very slow with KPAX – taking very low levels of it. My “bad reaction” has started to kick in but I reduced my use and began it again and it helped again – so I’m sticking with it.
I’m hopeful about KPAX. I’ve heard stories of people who’ve done quite well on it. I don’t think any one medication is going to be effective for everyone but I think there’s considerable reason for hope for some people with the formula. The question is how many.
Hi Cort, thank you for writing up the results. I’m already on Ritalin 5mg tid so I was reading this with bated breath. After one of your other write ups, I actually contacted the company to see if I could buy the formula used in the Trial. I got no response back.
The formula on sale is different in that it also contains l-glutamic acid/l-glutamine. To combine this with Ritalin can be very problematic neurologically/neuro-psychologically. I presume that is why glutamine is not in the Trial formula — and why I wanted to get my hands on the Trial formula. Also, it is tricky to add caffeine and Ritalin. Although they are both stimulants, their action combined can: 1. cancel each other out, and 2. cause heaps of irritability and/or hypo-mania. This is from my experience, and my doctor also suggested to stop caffeine with Ritalin. (Although I take in a very small amt. in the morning). So, not to be contradictory, but to ask how the formula that one can buy freely versus the one in the trial is apples and oranges. It’s my opinion that the l-glutamine/glutamic acid is a game changer.
If anyone knows of a way I can buy the formula used in the Trial, I would be very interested. As it is, I’ve been taking a powerful multi-vitamin which was as close I could get to the Trial formula — without tipping scales into a crashy energy push. (I wouldn’t say this current multi is overtly helpful, btw.)
It’s very unclear to me why Kpax will not sell the Trial supplement. My suspicion is that it has to do with the broader goal of marketing as a prescribed medicine. If that’s the case, that is frustrating: withholding something which might help a bunch of people.
Thanks for letting me share my thoughts on this, and thank you for keeping us abreast of all the current events!
What is the multi-vitamin that you use?
Interesting!
I got Tramadol from my doc. And I have CFS/POTS..But when I take Tramadol I’m almost free from my symptoms.I Wonder if that medication contained Dopamin? One tab. of 50 mg. kept me in action the whole day! But I crashed the day after,if I did not took another tab..
Thanks, but I’m still waiting for news of something effective. When it comes, I suspect it will be accompanied by some insight into the cause of the disease. This remedy sounds like a bandaid over a deep cut. For now, I’ll stick to a little coffee and a healthy lifestyle.
Did anyone else get sleepy from Ritalin? I did, and wondered how this was possible?
I had a similar effect with Provigil, a drug given me for sleep disorders that increases dopamine. It was an eyeopener. After I started the MeCbl and added in the AdoCbl the Provigil ceased providing any additional benefit and I discontinued it and went on to finish healing after adding the other factors. I don’t have FMS, CFS, congestive heart failure or any of that stuff any more, just the demyelinations from decades of insufficient MeCbl/AdoCbl and a few slowly healing things. I have been recovered from the CFS/FMS for 8 years or so now and recovered from congestive heart failure about 3 years ago. My kidney damage apppears to be improving, at least by test results. The Metafolin appears big in that.
Thanks.. What is MeCbl/AdoCbl?
MeCbl – Methylcobalamin, Methyl B12, Mecob and about half a dozen more variations. It is the form of B12 that is immediately active in the human body. There are multiple undistinguished isomers with at least one being extremely active and at least one being virtually inactive. It is the form of B12 that is involved in single carbon transactions, methylation, and rapidly affects cell reproduction and nervous system functions and does a lot of detoxification which often destroys the MeCbl in the process. The methylation reactions also require ATP, the output from AdoCbl. A lack of ATP can deadlock methylation
AdoCbl – Adenosylcobalamin, AdnCbl, Adenosyl B12, Dibencozide, coenzyme b12, cobalamide and more variations. It is a form of B12 tghat is immediately active in the human body. It is best known for sitting in mitochondria involved in making ATP from fats in the Krebs (Citric acid) cycle. It also processes fatty acids for use in myelin synthesis and acts powerfully on inflammation. The things AdoCbl participates in need the methylation resulting from MeCbl or they are deadlocked.
These are the two major forms of cobalamin found in liver and other meats. There are more tha a dozen plant cobalamins not active in humans or other animals.. It is estimated that more than 100 other forms of cobalamin are created temporarily in the human body during metabolism. HTC3 transports these waste or junk or done with processing cobalamins to the liver for disposal in the bile.
Freddd, are you homozygous for the MTHFR C677T (T/T)? I am. I am currently taking metafolin supplement. I have tried the B12 injections, but don’t think they were the right type of B12. What brand/type of MeCbl and AdoCbl do you take and at what dose? This would be very helpful for me. Also, how long after starting these supplements did you notice improvements.
Thanks!
Jil
I would have liked to see a group of supplement-only patients in this study. It may be that some benefit as much or more from those alone than the supplement-drug combo. But echoing what others have written, if that were the case, pharma would not be able to patent it and make money and these trials would not be taking place. Such a shame that it’s that way.
I’ve been disabled for eleven years but believe that the supplements I take are among the things that have me homebound/low activity instead of bedridden. Is it a cure? No, but it’s a welcome difference. Would such a difference be enough for FDA approval? I compared my supplement protocols (figured out between my research on sites such as this and Prohealth, my acupuncturist, and my doctor) and find that my multivitamin is 98% the same as this combo. It’s Source Naturals Life Force Multiple without Iron. I take some additional single supplements, and because they are all grounded in science my doctor includes them in my chart as treatment so that I can use them toward the medical tax deduction. But boy would I have preferred that she could prescribe the supplements and have it covered by insurance!
One last thought: the supplement combo in the study includes iron. That could be unhelpful for some, so I was surprised to see it.
I’m curious to know if any of the supplements useful for ME/CFS/FM are whole food based vs chemically formulated. I find that I do much better on food based products. Like Cort, I may benefit from chemically formulated sups up to a certain dose and then have to back off due to side effects, paradoxical reactions (opposite), or some other system upset is triggered. I’m extremely sensitive to drugs, supplements, herbs etc. I still tolerate natural, whole substances and herbs, etc the best.
I was dx’d FM in early 90s after a cycling accident, CFS in 80s after major chemical exposure at work in the 80s. I’m living a fairly regular life yet live with high pain days at times and cyclic fatigue episodes esp. when I overdue. I also had a 2 1/2 year Major Depressive Disorder and severe anxiety with agoraphobia and insomnia lasting almost 3 years. I was helped not by meds as I don’t metabolize most meds like “normal” people. All meds for sleep wire me, for instance. Paradoxical reactions to all antidepressants became my MO. I tried about 20 different antidepressants and anti-anxiety meds. I did get better after 5 ECT, Electroconvulsive Therapy treatments and was in recovery as of June 2014 after tapering down ECT sessions. Anecdotely, ECT help the Fibro pain immensely and repaired sleep architecture 100%. Additionally, it is known by clinical trials that ECT improves cognition and brain fog has been greatly reduced. My cognitive abilities and an MRI of my brain were done before and after the EVT seties as part of a study at The Mind Institute at University of New Mexico. Food and alcohol triggers still cause some occasional brain fog.
Sorry, I digress. And I’m not advocating ECT as an answer for FM, ME/CFS. Just mentioning it.
I would very much like to see studies with high potency food derived supplements. If anyone knows of any, please post a link. Thank you.
Thanks for mentioning ECT. If the central nervous system is involved I see no reason why it would not be a viable option (??)
Cort, do you use both the Energy and Immune formulas of KPAX? If just one, which one and what dose are you at?
Thanks!
Yes, I am moslty using the KPAX immune supplement. Sometimes I add a bit of the energy tablets…I take much, much less than they suggest. I only take about one tablet a day – I’m very sensitive and I have to be careful no t to boost my energy up very much to be honest…(lol)
I have taken 30mg Medikinet adult (Time released metylphenidate) a day for years and it has been a life saver for me. Im currently taking a break (which is hard). Withdrawal was much easier for me than SSRI’s. Was able to study and have some joy in life with in combination with a Mitochondria tuned life style.
Congratulations Fabian – and thanks for sharing your experience.
If you’re interested in putting a review section – I encourage you to do so! You can find methylphenidate here – http://www.cortjohnson.org/forums/reviews/treatments/energy/ritalin-methylphenidate.264/
I was just coming off a bad crash when I started the K-Pax Immune. Took one tablet the first day and a few hours later I crashed hard. The next morning I also took one tablet when waking. Went to get up and couldn’t walk! I am in the worst state I have ever been at the moment. I can’t walk at all. I didn’t take any k pax this am. Anyone else have this happen or is it just a coincidence?. I usually tolerate most supplements/meds.