



Thousands of small alterations in our genes exist. This propensity for genetic experimentation has helped the human race adapt and thrive. Many never have an impact. Others may be helpful in some situations and problematic in other situations, and some, often in combination with other gene variations can significantly impact how our bodies function.
The question the CDC was asking in their most recent study was whether chronic fatigue syndrome patients as a whole, tend to have more variations – more unusual forms – of the genes that produce inflammation, than normal. These variants or polymorphisms are often not rare. They can exist in say 10% or 30% or whatever percent of the population. The point is that they are not the common form of the gene found.

This was the first study to fully assess gene polymorphisms in innate immune and inflammatory genes.
Given the interest in the immune system you might think that this basic question – do ME/CFS patients have a genetic predisposition to an altered immune response – would have been answered long ago, but it hasn’t. Thus far studies have looked at the genetic makeup of only a few immune genes.
In this study The CDC used a chip (Affymetrix Human Immune and Inflammation Chip) that was designed to systematically assess the genetic component of the genes involved in inflammation and immune pathways. How many gene variants are we talking about? 11,000 very small alterations or SNP’s (single nucleotide polymorphisms) in 1000 genes, representing 38 immune sub-pathways.
The most significant finding would probably come from finding increased rates of gene polymorphisms, not in genes scattered around the immune system, but clustered in one section of the immune system. That would suggest enough changes were present in that system to affect how it was functioning.
Was your immune system tweaked from the beginning? Let’s see what they found.
The Study
Pathway-focused genetic evaluation of immune and inflammation related genes with chronic fatigue syndrome. Rajeevan MS, Dimulescu I, Murray J, Falkenberg VR, Unger ER. Hum Immunol. 2015 Jun 24
Please note that because the study used the empirical definition and population sampling to gather its participants it may have corralled an “ME/CFS-lite” group. This group may be different from the kinds of patients that show up in Dr. Lapp’s, Dr. Peterson’s and Dr. Klimas’s offices. The study consisted of 50 CFS and 121 non-fatigued (NF) controls.
Results
Out of the ten thousand polymorphisms in 1,000 genes, a couple of polymorphisms stood out. The CDC categorized polymorphisms according to their type. Check out the branch of the innate immune system that stood out again and again; it wasn’t natural killer cells.
- Non – synonymous changes – First they looked for gene changes that actually changed the form of the protein the gene produced (non-synonymous changes). They found three genes in this category: two of those genes played a role in the complement immune pathway.
- Synonymous gene changes – Then it was onto synonymous gene polymorphisms – ones that don’t change the protein produced by the gene. Four gene variants of this type were unusually common or rare in the ME/CFS group – two of them in the complement pathway.
- Genes in the Untranslated Regions – Then they looked at gene polymorphisms found in the “untranslated regions” (UTR) that often affect gene expression. Again the complement cascade popped up with two genes highlighted.
- Intronic Genes – Finally they looked at gene polymorphisms found in the intronic regions, which also affect gene expression. This was the only group that did not feature genes.
Digging Deeper into the Complement Genes
The CDC took notice and took a deeper look at six more gene variants in the complement pathway. They found aberrations in their prevalence (either unusually high or unusually low) in the ME/CFS group in all of them.
The takeaway: people with chronic fatigue syndrome tend to have higher levels of “unusual” forms of genes regulating the complement system than do healthy controls.
It’s not often that one section of the immune system pops out so consistently. It’s time to take a deeper look at the complement system. We rarely hear much about the complement system but it turns out that it has quite a history in ME/CFS research.
The Complement System and Chronic Fatigue Syndrome: A History
Exercise Induces Complement Activation #1
Activation of the complement pathway is one of the rare measures of immune dysfunction that’s approaching being validated following exercise in ME/CFS. A 2003 CDC study examining levels of complement split products, cell-associated cytokines, and eosinophilic cationic protein found before and after exercise started it off. It found increased C4a levels 6 hours (but not 24 hours) after exercise.
C4a induces the contraction of the smooth muscles lining the blood vessels and increases vascular permeability. It may cause histamine release from mast cells and basophilic leukocytes.
Increased C4a levels have been associated with Lyme disease. Richie Shoemaker also asserts that C4a levels increase after exposure to mold. One website stated that C4a elevations are found in acute pancreatitis, systemic lupus erythematosus and rheumatoid arthritis, Lyme disease, HIV/AIDS and immune complex diseases such as serum sickness.
Dr. Nathan has stated that Procrit can lower C4a levels.
Exercise Induces Complement Activation #2
The findings of a 2005 CDC gene expression exercise study provided more validation. The results were so unusual that they bear repeating. Out of 3800 genes analyzed, it found that in healthy controls the expression of 21 genes changed during and after exercise.
Eleven of those genes were activating normally in ME/CFS patients but almost half – ten – were hardly being activated at all. (Eight of those genes were associated with metabolism.). The study suggested that many of the genes that rev up during exercise in healthy people, more or less flatline in people with ME/CFS. The authors (one of which was Suzanne Vernon) had this to say about the significant differences in gene expression seen between the healthy controls and the ME/CFS patients.
“Because this difference in gene expression is so dramatic, it implicates a fundamental perturbation in the biochemical activity of lymphocyte and monocyte peripheral blood fractions from CFS subjects, compared with control subjects”.
The authors noted that this decline in immune expression would probably not show up classical markers of immune dysfunction – which are generally normal in ME/CFS.
The results of a “GO” biological pathways analysis nailed ion transport and voltage gated ion channel genes in ME/CFS patients. It suggested that differences in the activity of these genes exist prior to exercise, in people with ME/CFS, and are exacerbated by exercise. Recent studies have implicated ion channel problems in chronic fatigue syndrome and in chronic pain disorders.
Note the prevalence of autoimmune or suspected autoimmune disorders, in a list of other fatiguing disorders the authors reported are associated with ion channel problems: multiple sclerosis, myasthenic syndromes, neuromyotonia and polyneuropathies. All of these disorders are suspected of having an autoimmune component. All affect the nerves and all cause symptoms often found in ME/CFS and FM. Polyneuropathies, for instance, typically cause weakness, numbness, pins-and-needles, and burning pain and may affect the autonomic nervous system.
The GO analysis also implicated genes in the complement system.
The next steps, the authors declared were to examine the gene expression of larger numbers of individuals, during and after exercise. That work is going on right now in the big ME/CFS experts multi-center study.
Turning the Complement System On
Clearly impressed by having the complement system pop up in both a blood and a gene expression study the CDC next did a more detailed analysis of the complement system after exercise. A 2008 study examining the “transcriptional control of complement activation” after exercise, looked at which genes might be turning on the complement system in ME/CFS, during exercise.
The study found increased expression of the mannan-binding lectin serine protease 2 (MASP2) gene, one hour post-exercise in ME/CFS patients. It appears that MASP2 shoots up in both healthy controls and ME/CFS patients during exercise, but then is immediately downregulated in healthy controls, but not ME/CFS patients. The authors suggested that the inability to tamp down the expression of the MASP2 gene could set the stage for “localized and uncontrollable inflammation-mediated tissue damage”.
What might be increasing MASP2 levels (and thus the complement system) in ME/CFS? Leaky gut syndrome, infection, injury, vaccination and a variety of autoimmune diseases could all increase MASP2 levels. Because exercise induces cortisol production – which inhibits MASP2 activation – the low cortisol levels seen ME/CFS could result in increased MASP2 activation as well. Cortisol is also being measured in the CDC ME/CFS experts study.
A Case Study
Next we turn to a case study. After developing infectious mononucleosis in 1989 a 39 year old female became bedridden and unable to work. Her symptoms included severe fatigue, muscle and joint pain, cognitive problems and shortness of breath during exercise. In 1991 she was referred to the NIH where she underwent additional testing and got an ME/CFS diagnosis.
A long series of tests were either normal or negative. (They included pathogens: Lyme disease, toxoplasmosis, cytomegalovirus, hepatitis C, brucellosis, histoplasmosis, blastomycosis, coccidioidomycosis and Mycoplasma pneumoniae, C-reactive protein, lupus panels, antimicrosomal and antithyroglobulin antibodies, cosyntropin stimulation, CA antigen 125, HIV antibodies, immunoglobulins IgG, IgM, IgA and IgE, and IgG sub-classes, total C4 and C3, tumour necrosis factor, interferon α, interferon γ, interleukin (IL)1β, IL2, IL3, IL4, cutaneous immunoflourescence antibodies, lymphocyte enumeration, total lymphocytes, natural killer cells, natural killer cell activity and immune Raji cell activity).
Further tests, however, indicate consistently increased levels of C4A and other complement products were present. The case study didn’t say how her complement levels returned to normal but a year later they did. Within two months of that occurring she had a complete resolution of all her symptoms and returned to health. She’s been healthy ever since.
The Complement System
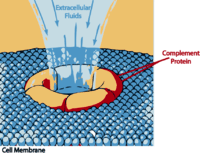
“The complement system has the potential to be extremely damaging to host tissues, meaning its activation must be tightly regulated.” Wikipedia
The complement system is part of the innate immune response that “complements” antibodies and phagocytic cells such as macrophages, in their efforts to remove pathogens. It consists of 30 or so inactive proteins floating through the blood. Most are produced by the liver but a good number are also produced by monocyctes and macrophages and epithelial cells, of the genitourinal tract and gastrointestinal tracts.
During times of pathogen invasion or inflammation these proteins get cleaved by enzymes to release cytokines and quickly upregulate the immune response. The complement system may play a role in many immune mediated diseases and is increasingly being thought to play a role in central nervous system diseases as well. Polymorphisms in several complement genes have been linked with several disorders, including macular degeneration.
The Future
One small exercise study is not enough to validate the CDC’s complement findings. We’ll hopefully get a much better idea of how important the complement system is in “true” ME/CFS patients soon. The exercise portion of the CDC’s ME/CFS experts studies includes a gene expression analysis. Given the CDC’s findings thus far, it’s hard to imagine them not doing a full-bore analysis of the impact of exercise on complement gene expression and complement blood levels. The results of a huge gene expression study at Stanford should be released shortly as well.
If the complement system pops up in the ME/CFS experts study that would be a very big deal. Alterations in all three legs of the triad: the gene level (gene polymorphisms), gene expression and blood levels would be validated. I don’t know that that’s been done for any other substance. The basis for an immune cause of PEM would be laid.








Thank you for yet another interesting post!
I’ve had much lower than normal both C3a and C4a during three years of treatment by Dr De Meirleir. Slowly normalizing during the course of treatment, which have made me go from about 50 to about 80 on dr Bell’s scale. From what i hear it’s not unusal among his ME-patients to have low values also for C4a, although the raised levels probably is more common. Stuff is going on with the immune system, but sometimes seem to go both ways. I also had lowered sCD14 at the start of treatment instead of raised levels which seems to be the most common in ME.
Thanks.
Reduced C4a is common in lupus I think is was…
C4 is not nearly as well known as the other complement factor C3..
Gosh, I could really use a simple boiled-down explanation of this!
This is a “boiled-down explanation!” Imagine if we were looking at it in its original form. Thank you Cort for taking something that is a completely foreign language to most of us and breaking it down so that we can, at least, get a gist of the content.
I’m trying to get a gist of it too 🙂
Here’s the bottom line as I see it. Problems with a specific part of the immune system that could cause inflammation and pain during exercise have been found in three places:
(1) there signs in the blood that the activity of this inflammatory system is abnormally increased in ME/CFS after exercise
(2) it appears that expression or activity of complement system genes may be increased during exercise as well
(3) of all the immune genes, there appear to be more genetic irregularities in those involved the complement system than any others.
This is just from a couple of studies but what’s exciting is that a problem in the complement system appears to be showing its face at different levels – at the genetic and in the gene expression level and in the blood. We definitely need more studies but these findings spread across multiple areas are encouraging and suggest its real. The next studies will probably tell the tale. If it keeps showing up then researchers are really onto something.
Interesting Cort, but what turns the complement system on as a reaction on exercise? 🙂
One possibility would be nanometer-scale biotoxins that have settled into tissues, including the muscles, of people with ME/CFS. These toxins could be from molds, bacteria, viruses, parasites, etc. As the muscles get used and blood flow shunts to supply oxygen for aerobic energy production, biotoxins are “pulled” into the bloodstream. This would activate the mannin-binding lectin pathway. That would activate MASPs 1 and 2. Once that happens, C4a levels would shoot up and voila! – you have exercise-induced inflammation that makes you feel beaten up.
Thanks!
That makes sense…. PEM is a reaction to toxins being stirred-up by any sort of exertion. Thanks for putting this in terms my adled brain could understand. 🙂
It’s truly exciting that the CDC is looking at this. The same CDC that insisted this wasn’t a real disease in the past. The Klimas clinic has been looking at proinflammatory cytokines for years and the new Alabama clinic headed by Dr Younger is dedicated to inflammation. A lot of the terms here are over my biological head but I hope these studies replicate and that some treatment grows out of the results.
I researched this years ago when I was trying to get Mayo to address MCAS for us POTS people. I think I still have my research. The connection to the complement system plays a big role with this. I felt like it was playing a part in all my autoimmune issues as I have hypogammaglobulinemia. The immunologist poured over the books of info I took him. (He had it tagged and underlined. So I know he was studying it.). Not too much longer and we were getting meds for MCAS, those treatments address the autoimmune system in the gut area. I feel this was one of my best meds. But is very expensive. Called GastroCrom. It is also a mild calcium channel blocker. (I’m off all my meds right now. So far so good. Hope my healing and new protocol will continue to work.). I did not know this was connected to Lyme. So double whammy for me. Interesting how things start to add up as knowledge increases.
I’ll see if I can find it. Cort good job explaining this. It is very complex when you go into the science of it.
Issie
What kind of medicine do u take?
I’m off all my meds right now. I was on pain meds and muscle relaxers for EDS and POTS. Meds for MCAS (H1 and H2 and GastroCrom). Lots of supplements that I’m not taking. And meds for Lyme co infections and another Protozoa. (Doxycyciline and herbals). Not on any meds now for 3 weeks.
Trying new protocol for inflammation and autoimmune system. Working as well maybe better. Still in trial phase. Many times I will get results to start and then it phases out. So far so good.
With the complement system, addressing inflammation and immune system will swing in the right direction. There are herbals to do this. And believe it or not —–zinc. (You can look on Cort’s forum – I’ve been writing about something I recently found out about that zinc, B6 or P5P and EPO addresses.)
Issie
After 15 years of looking zinc was the first thing that ever worked for me.
Cool! Glad it’s helping!!!
Issie
Yes! This is so great. They are finally barking up the right tree.
Unfortunately this study uses the Reeves Empiric crtieria, so not sure it tells us very much, sadly. Yes, they say it’s Fukuda, but the implementation was Empiric (Reeves 2005).
Yes it does. That’s why the ME/CFS experts study will be so important. I’m not willing to throw the baby out with the bathwater with the Empirical definition. I assume that the people in ED studies probably mostly do have ME/CFS – I just think – as I noted in the blog – that they may be more ME/CFS lite than in other studies and more non-ME/CFS diagnoses may be present. It’s possible that the results may still apply. (None of the definitions put forward is perfectly tight – all are leaky to some extent)
We might also ask if Empirical criteria studies are having different results than expected from studies using Fukuda criteria. The answer to that is that it’s hard to tell – there are just too few studies. In general they appear to, though.
None of the three earlier studies finding evidence of complement activation in ME/CFS used the Empirical Definition. The findings in this study track with those – so the ED doesn’t appear to have much of a difference with complement.
Of the two gynecological studies one used the empirical definition and the other did not – both came up with similar findings.
The findings from an NK cell study using the definition were not all that exciting but it’s findings did suggest reasons for the NK cell dysfunction in ME/CFS and it found a blunted NK cell response as expected.
“In conclusion, we have documented an increase in PRF1 expression that parallels an increase in NK cells in response to acute psychosocial stress where patients with CFS had a blunted response compared to NF controls. Blunted expression by CFS may be related to high baseline PRF1 promoter methylation that was found to be an important epigenetic determinant of inter-individual differences in PRF1 expression.”
It’s a small list but thus far the ED does not appear to be producing unusual findings.
CDC got a laughable prevalence of 2.5% with Empiric, which uses hopelessly lax criteria (10x what they got with well-implemented Fukuda, which also tallies with an excellent recent UK study; 1 in 40 is not mecfs). I also took a good look at this sample before (it’s from the same hospital sample as used in the Pharmacogenomics papers). All bathwater, no baby in my view: whether the results ‘fit’ or not, the sample is unreliable.
No definition is perfect, as you say. But the Empiric, like the Oxford, are largely useless. Studying this illness is hard enough without adding confusion from such poorly-defined samples. And I’m not sure any of those other studies you mention amount to much so far – they are all hopelessly underpowered.
We need large studies, with reasonable diagnosis. Otherwise we will continue to go nowhere and the ‘evidence’ will continue to support almost any speculation. With work from Columbia, Stanford & Norway amongst other places, I think we are beginning to see what’s needed. And I’m hopefull the UK will soon come to the party too.
What isn’t underpowered? We have what we have and if thus far the evidence fits I wouldn’t kick it to the side of the road. I wouldn’t call studies finding NK dysfunction, high rates of gynecological problems and complement issues bathwater either. You would expect to find that in any sampling? (I didn’t know natural killer cell dysfunction was so widespread.) There is also a study finding reduced information processing and working memory – another good fit for ME/CFS – a study finding increased rates of inflammation from that time (not clear on the definitions used).
Yes we need better definitions and larger studies and I would rather not have the ED being used but I disagree with automatically discarding any study using it. ME/CFS patients are surely in there- the CDC may not be right but they’re not stupid either. They want to be successful. Why produce a definition that results in nonsense?
I’m sure the CDC will switch their definition and soon and at least we won’t have this problem to worry about – at least not so much…:)
“What isn’t underpowered? We have what we have and if thus far the evidence fits I wouldn’t kick it to the side of the road. ”
I’ll have to disagree there. We’ve twenty years (of my illness) running on weak studies getting findings that go nowhere. Just because the finding fits, is no reason to endorse the study. These studies are just as likely to throw up generic markers of ill health. More blind alleys.
“Yes we need better definitions and larger studies and I would rather not have the ED being used but I disagree with automatically discarding any study using it. ME/CFS patients are surely in there- the CDC may not be right but they’re not stupid either. They want to be successful. Why produce a definition that results in nonsense?”Don’t know, but they did (the CDC may not be stupid, but the Empiric criteria are: have you read Lenny Jason on them; powerful stuff). If 80%+ of your patients in a study don’t have mecfs – as the prevalence rates indicate – you have no chance of learning anything useful about our illness, more about generic ill health (NK activity can be down in other illnesses too, I gather, which is why the IOM and others stress the importance of using sick controls as well as healthy ones; that rarely happens).
There are thousands of studies on mecfs, and little progress to date. It’s important to view the current evidence with a critical eye. mecfs is surely a tough nut to crack, else we’d have got somewhere by now. So lets focus on the best of what we have, and look to new, bigger and better studies to help move the field forward.
I’m sure we both want the same thing: progress in understanding and treating mecfs. For me, critically evaluating research is an important part of that, with some attempt to sift the wheat from the chaff. And I can’t envisage a definition of chaff that doesn’t include the Empiric criteria.
I don’t know Simon….I don’t know if complement and NK cell problems are “generic markers of ill health”. I don’t think the study needs to be “endorsed”. I think it needs to be seen for what it is – it presents a possibility – and that possibility which showed up in other studies not using the ED – should not be discarded because this study used it. I noted in the blog that the bigger study – hopefully ME/CFS experts study – will tell the tale.
I’ll stand by my statement that the ED is not nonsense….When it was created I said we’ll see what the studies show. I don’t think their results have generally been consonant with what other studies not using the ED have found. That should be figured in the mix as well.
This idea that ME/CFS can be signaled out from other idiopathic fatigue states biologically is not clear either. Nobody knows where the cutoff is biologically. You’re assuming because the ED adds more patients into the mix that it’s completely invalid. Only time and study will tell if that’s true.
In the mean time Jason found that the ED was less effective than the CCC at accurately identifying people with ME/CFS but it wasn’t THAT much less effective. It accurately identified 79% of the people with ME/CFS while the CCC accurately identified 87%. With regards to new cases the suggested it would have identified only 68% correctly, but guess what the CCC only identified 73% correctly…It was off by 5%…
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3228898/
This article contrasts two case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). We compared the empiric CFS case definition (Reeves et al., 2005) and the Canadian ME/CFS clinical case definition (Carruthers et al., 2003) with a sample of individuals with CFS versus those without. Data mining with decision trees was used to identify the best items to identify patients with CFS. Data mining is a statistical technique that was used to help determine which of the survey questions were most effective for accurately classifying cases. The empiric criteria identified about 79% of patients with CFS and the Canadian criteria identified 87% of patients.
It’s not as good as the CCC and I’m sure it won’t be in the final definition, but it’s not nothing either. Therefore I suggest that we be wary but not automatically reject findings from studies using the ED.
Hi Cort
Thanks for the thoughtful reply
“This idea that ME/CFS can be signaled out from other idiopathic fatigue states biologically is not clear either. Nobody knows where the cutoff is biologically. …”
Yup, that’s the nub of the matter – distinguishing ‘true’ mecfs from idiopathic fatigue.
“In the mean time Jason found that the ED was less effective than the CCC at accurately identifying people with ME/CFS but it wasn’t THAT much less effective. It accurately identified 79% of the people with ME/CFS while the CCC accurately identified 87%. With regards to new cases the suggested it would have identified only 68% correctly, but guess what the CCC only identified 73% correctly…It was off by 5%…”
Now, if EC was only different from CCC by 5% it would indeed be an irrelevant difference. But you can’t conclude that from this study. They started with Fukuda-diagnosed patients and the sensitivity of EC vs CCC has never been the issue with EC. The problem, has Jason has pointed out so eloquently, is that it pulls in a whole bunch of non-Fukuda-CFS cases, including those with depression instead. These patients were not included in the study you cite (they were excluded before the EC v CCC contest was performed).
However, the patients in the study you blogged about ultimately came from a CDC population study that found a 2.5% prevalence of EC-defined CFS.
http://www.pophealthmetrics.com/content/5/1/5
That’s the problem. And no one outside the CDC thinks the dividing line between Idiopathic fatigue and mecfs is drawn anywhere near 2.5% prevalence.
The previous CDC study found a prevalence over 10x smaller, using Fukuda, of 0.235%
http://www.ncbi.nlm.nih.gov/pubmed/12860574
An impressive UK study found a prevalence of 0.19% for Fukuda
http://www.biomedcentral.com/1741-7015/9/91
On that basis, up to 90% of EC patients don’t have Fukuda, hence my concern about placing any weight on findings from such studies.
Good points. I would note some mood disorders were surely ferreted out. (However ordinary depression are allowed in the ED it’s possible that depression was not. )
the significance of Jason’s study that found that 38% of patients with major depressive disorder were missclassified as having ME/CFS can be overstated. It simply compared Fukuda identified CFS patients with patients with major depression. A random sampling study is going to pick all sorts of patients – of which people with depression will be a small percentage (national rates – 3.4%). A significant number of them will have melancholic depression and they would be excluded from these studies. Some will end up in the study but probably not many. That could explain why the ED was just 5% less effective at labeling new patients than the CCC….
More importantly, the Jason data mining study did not start with Fukuda diagnosed patients. It included a wide variety of patients including patients with CFS-like illnesses. It stated ” In this study of Wave 2 participants, we had 24 individuals with a CFS diagnosis compared to 80 without a CFS diagnosis.” It appeared to be put together in much the same way as the CDC studies. That’s apparently why Jason was able to assess the effectiveness of both criteria at assessing “new patients”.
Again I assert! 🙂 that if it looks like duck, etc. it could be a duck or at least a subspecies of one :)..Yes, the prevalence figures for the ED are very but the study findings using it appear to mostly fit. Again I’m not arguing that we should be using the ED but I don’t think we should completely disregard results from studies using it.
Very interesting stuff.
“the Jason data mining study did not start with Fukuda diagnosed patients. It included a wide variety of patients including patients with CFS-like illnesses.”
Thanks, I hadn’t spotted that (though some non-CFS patients were healthy controls, it seems), and does seem to strengthen the case for the Empiric criteria.
However.
“the significance of Jason’s study that found that 38% of patients with major depressive disorder were missclassified as having ME/CFS can be overstated… A random sampling study is going to pick all sorts of patients – of which people with depression will be a small percentage (national rates – 3.4%).”
38% of 3.4%= 1.3%, and even half of that would be more than the amount of Fukuda patients – let alone other ill-health cases swept up.
There is also an important difference in methodology between Jason and the CDC. Jason screened initially on fatigue. The CDC screened on ill-health, and swept up more people initially as a result. Some of these low-fatigue cases could still pass the EC’s ‘disability’ threshold of low functioning on the Role Emotional scale that isn’t part of any other defintion. Jason’s study would miss these, making comparison between the two difficult (esp as he only managed to contact half of those he wanted to recruit who had been assessed in the original study – which is a big issue in itself).
We could debate the Jason work forever, but I’m not sure how critical it is to these latest findings (even though they have improved my view of the EC per se).
Going back to your original comment, and the nub of this debate
“I assume that the people in ED studies probably mostly do have ME/CFS”
While the Jason study is interesting we know it’s a different sample. We also know the Empiric patients in the study you blogged about came from the SAME population study that found a prevalence of 2.5%. So this specific cohort is unreliable (unless you think 1 in 40 people have mecfs), whatever the case for the Empiric definition more generally.
“Again I assert! 🙂 that if it looks like duck, etc. it could be a duck or at least a subspecies of one :)..Yes, the prevalence figures for the ED are very but the study findings using it appear to mostly fit”
It looks like a mid-sized bird to me, rather than a duck. The ‘fit’ with other studies (which are hardly brilliant themselves) makes me fear we are more likely to be looking at generic ill-health than mecfs specific findings. To make progress, as well as bigger studies with tighter case definitions, we need routine use of sick controls too.
Hopefully we’ll get a better definition soon that everyone will follow and we can put this definition issue (mostly) to bed (for awhile anyway 🙂
This study used the ED, but if memory serves correctly, I think the first CDC complement study was done with female patients that James Jones had diagnosed years earlier in Colorado or something like that so I think the initial findings were from well-characterized patients. Not sure about subsequent studies. The Experts study should be a good replication effort.
I’ve been waiting and wondering for years why the CDC hasn’t gone and done more in depth work on these findings, just small study after small study that all find the same thing!
Great article Cort! I’ve always felt that there is a genetic component to our problems.
I was a patient of Dr. Jones in 2003 at National Jewish Hospital here in Denver. One of the tests he ordered was a C4a. It came back significantly elevated at 7124 (normal 0-2830). At my follow up appointment his comment to me was along the lines of “wow, yours is very high just walking in the door.” My number was elevated when I came in for the appointment, not as part of his exercise study on C4a. I wonder what mine would have been if I had done the bicycle exercise challenge? Maybe ME/CFS patients don’t need to do an exercise challenge to find an abnormal C4a and that this really could be a bio-marker. At the time of the C4a test I was just beginning to work with Dr. Jones – just before he left National Jewish to start a new job at the CDC.
I’ve always wished that he could have gotten the funding to continue the study of C4a back in 2003. Glad to see that this is being revisited.
Which Dr. Jones was that? I’m looking for experts to ask if there are any known interactions between C4a and mast cells.
The Dr. Jones I am referring to is Dr. James “Jim” Jones, MD. He was a physician (clinician and researcher) at National Jewish Hospital before he accepted a position at the CDC where he now works.
Thanks, Tanja!
Thanks again for important updated information and discussion. There was an important article in Fibromyalgia Frontiers in 2012 ( Vol.20, no.2) “Why You Should Be Thinking About Positional Cervical Cord Compression ,” by Andrew J. Holman, MD., Univ. of Washington.
Some points of this article were:
1. the descriptions and symptoms of FM ( and CFS – from all I have experienced/read) overlap with those of cervical myelopathy – some spinal cord pathology in the neck.
2. Although regular position neck MRIs may be reported as normal, it is possible to have cervical cord compression/ irritation when necks are naturally bent forward or backward. Thus, it is necessary to have MRIs done in flexion and extension of the neck to see the situation.
So, then, a big question: Can constant irritation of the spinal cord in the neck cause immune system changes? I went to my main resource, Spinal Cord Medicine: Principles and Practice, Editor in Chief – Vernon W. Lin, MD, PhD. There is an entire chapter on the immune system and the inflammatory response in people with spinal cord injury.
The authors of this chapter state: ” deficits in immune function and the presence of a chronic inflammatory state have been documented in the acute and chronic phases of spinal cord injury.” Also,” Lung, bladder, and gastrointestinal mucosal barriers are rendered vulnerable by the withdrawal of normal neurological innervations. ”
Here are just a few of the research documented issues of the disordered immune and inflammatory responses after spinal cord injury:
1. Elevated serum lepton
2. Reduced NK cell counts, impaired neutrophil phagocytosis
3. Elevated interleukin 6 and IL 2r
4. Depressed T cell function and activation (Hmmm – my comment – how is this influencing B cell behavior?)
5. Reduced natural killer cell function, depressed NK cytotoxicity and lymphocyte transformation
6. Elevated complement and acute phase proteins- don’t have details on which complements.
Well, I remember what happened to the neurosurgeons who were looking closely at this neck problem. Ha – one had his license revoked, the other could not get published in the US , was published in the European spine journal. The National Fibromyalgia Research Association ended up collapsing as a leading thinker in this area. I attended an early conference on this topic in Salem, Oregon. A world renown neurosurgeon and expert on tethered cord syndrome cancelled at the last minute – fearing for his career.
Even though my son developed typical CFS after a viral infection at age 5, I remembered he had been delivered by suction and had some kind of neck trauma.
Make that “leptin” – love this auto correct spelling 🙁
I don’t know About the selection criteria, but for sure I do not have MDD and am not depressed nor ‘fatigued’.
I was diagnosed with “CFS” (remember that “ME” is non-existant, unknown, denied, … in Belgium).
Diagnosis was made in a ‘cfs center’ in a university hospital (fukuda) but also by De Meirleir, in 2002.
PEM is unquestionable present. After 10 yrs i got Some better days … On and off … Nothing points out why or when or how.
But then i crash and almost burn again. Like right now.
Here are Some interesting – repeated – results (De Meirleir) (not exercising btw):
– NK function down
-Perforin down
– b cells cd19 up
– monocytes up
– elastase up
– rnaseL up
– EBV, CMV, hhv1 igG’s off the charts
– HHV6 DNA PCR via stomach biopsie IgG up
– no bacterial Co-infections found till now
– heavy metals off the charts
– cd14 (leaky gut) high
– l lactate and D lactate too high
– calcium high (PTH ok in follow up)
– albumin high
– Cytokines/chemokines: il1, il6, il8, mip, mcp
– pge2 (inflammation marker) up
– CRP never high though
– repeated and single CPET tests bad bad bad
…
And (!)
– c4a high while C3 too low
Low grade chronic inflammation seems to be certain.
Lyme tests where either negative or a few of them borderline (could be cross reaction with EBV antibodies imo).
I don’t know what’s left as a possible cause.
Gut problems are a candidate but that’s the chicken/egg thing.
I have severe CNS problems.
Spine feels like a shortened cord from bottom to neck and head. After sleeping i feel the worst. Undiscribable. Perhaps immune activity during sleep? Don’t know. No Apnoe or anything.
Brain: alpha/delta waves not normal. Alpha intrusions during sleep and peaks while in rest (qEEG).
Heart: HRV several times not normal. General heart examination ok though.
The complement issue has been my own focus for a while bc i try to tie repeated abnormal results together.
Still not getting anywhere, as You might imagine.
Every piece of the puzzle is important.
Wished someone would tie them together for me. For all of us. So fed up with all the self-researching and wondering what ‘definition’ i really fit into.
If there are a substitute, could you still experience kidney failure cure
dialysis? http://kidneyok.tumblr.com/
Is this thread still active? In 2018 my C4a was 6,534. In 2021, my C4a is 3,882. C3a is normal at 148. C4 is 14 and C3 is 92, which are on the low end of normal. I have been worked up for tons of autoimmune stuff and ANA and such are always normal. Past 15 months have been pancreatitis (non-drinker) and chronic pericarditis w/ pericardial effusion. Note- thin, look totally healthy, no red meat, gluten free, low sodium, low sugar diet….in other words I should not be sick.
What doctor do you see for evaluation regarding the Complements? My docs are scratching their heads….
Doctors knowledgeable in mast cell activation syndrome (MCAS). Not sure how to find those but HR has a way to find doctors in your area coming up in the next month or so.
One possibility – go to Pubmed – look up research papers on complement – check out the authors and see if any are in your area and practicing at a academic center that provides health care. Kind of a long shot!