


The long presentations featured in Emerge’s recent conference allowed some researchers like Neil McGregor to really dig into their topics.
This is the first in a series of blogs that report on the recent EMERGE conference in Australia. The different conference format – which allowed for long presentations – allowed the presenters to dig more deeply into their topics than usual. Longtime Australian metabolomics researcher Neil McGregor took full advantage of this opportunity to give his grand conception of what’s going on with chronic fatigue syndrome (ME/CFS).
Neil McGregor reported that Ron Davis challenged members of his Working Group (of which McGregor has been a part) to develop a hypothesis. It’s rare in my experience – quite rare – that: a) researchers are challenged to do this; and b) they accept the challenge and then publicly present it.
Most conference presentations consist simply of presenting data from a study. Sometimes researchers even feel the need to inform the audience what ME/CFS is. (Why, why, why?) Much of the talk is then filled with methodology and, their fifteen minutes in the public eye almost up, they squeeze in their results at the end, then leave the platform – the implications of their findings unstated.
Too rarely does anyone climb out onto the skinny branches and say, this is MY conception – my grand idea of what ME/CFS is. At the EMERGE conference in Australia, Neil McGregor did just that. Not only did he attempt to tie everything together that he could but it made for a very interesting presentation.
He started off with a rather astounding assertion – that he’s able to separate infectious from gradual onset patients using metabolites. To be able to show, years after onset, that some fundamental differences persist in these two groups would be a significant breakthrough. Not only would it suggest that gradual and infectious onset patients are different, but the metabolites should, of course, tell us how these two symptomatically very similar presentations of ME/CFS are different biologically.

Out onto the skinny branches Neil McGregor went as he presented his grand conception of what’s gone wrong in ME/CFS.
Then, if I got it right, McGregor also showed that an ME/CFS patient’s metabolites could show whether that person had exercised recently and had post-exertional malaise (PEM) using their metabolites. Those metabolites – which are breakdown products – should point an arrow at where the breakdown during exercise occurred.
It should be noted that metabolomics is a bit twitchy! Metabolites, McGregor pointed out, can be altered by any number of factors (diet, exercise, drugs). A metabolites expert spent most of his talk at the recent NIH ME/CFS Conference explaining how the field was making up for its shortcomings.
The Study
McGregor’s study collected first morning urine – when many patients are at their worst – and then blood when patients found their way to the clinic. That was done in order to remove as many confounding factors as possible (metabolites can be affected by many factors).
McGregor found that fasting morning glucose levels increased and lactate decreased with increasing PEM. (Yes, lactate decreased!)
(Rebound Hyperglycemia or the “Dawn Effect” in ME/CFS – Higher than normal levels of morning glucose can reflect either something called the Somogyi effect or “rebound hyperglycemia” in diabetics or the “dawn effect” in non-diabetics.
Rebound hyperglycemia in the morning occurs when your blood sugar drops too low during the night, In order to “rescue” you from the dangerously low blood sugar levels, hormones are released which tell your liver to dump glucose into your bloodstream. Not too surprisingly, the low glucose levels can play havoc with your sleep, causing nightmares, restless sleep and night sweats. Rebound hyperglycemia is diagnosed by measuring glucose levels around 3 AM.
The “dawn effect”: many people’s glucose levels rise early in the morning. If glucose levels are higher than normal upon wakening, but normal around 3 AM, the dawn effect is occurring.
McGregor’s findings suggested, once again, that glycolysis – that very early step in the process of producing energy – is off kilter in ME/CFS. As glucose is converted to pyruvate (a fairly simple process 🙂 – see diagram), some ATP and a compound called NADH are produced.

Glycolysis – Just another simple pathway… (from Wikimedia: https://en.wikipedia.org/wiki/File:Glycolysis_metabolic_pathway_3_annotated.svg)
All this activity, it should be noted, is taking place in the anaerobic cytoplasm of the cell – not in the mitochondria. Note also that 10 enzymes are involved in glycolysis – providing plenty of opportunity for something to go wrong.
Pyruvate then moves into the mitochondria where things really get rolling, but note that without that pyruvate – if something has gone wrong with glycolysis – nothing happens.
The Last Worst Option
The higher levels of hypoxanthine – a breakdown product of ATP – suggested to McGregor that the ATP in people with ME/CFS was being broken down at a higher rate than normal – leaving less ATP available.
The news was worse than that, though. Our cells want to use glucose to produce energy; if they can’t get glucose they’ll turn to fats – not such a bad substitute, but if the fats aren’t working, they’re forced to turn to their last and least favorite option – breaking down proteins.
The hypoxanthine levels suggested that’s what was happening in ME/CFS, and the urine and blood amino acid tests bore that out. High levels of muscle metabolites suggested that whatever muscles the people with ME/CFS had left were being broken down faster than normal.
Breaking down the muscles of an exercise intolerant, muscle depleted group of people seemed like a rather strange (and cruel) thing to do, but there was a good reason for it. If the body places a premium on any process, it would be energy production. The great majority of energy we use, after all, goes not to exercise or digestion but to simply keeping our body going.
Given that rather essential need, our bodies will apparently do just about anything – including breaking down our muscles – to keep a broken energy production cycle going.
If that’s what’s going on in ME/CFS, we are not alone; McGregor reported that this kind of hyper-catabolic, hypermetabolic response is also seen in burns, post-surgery and sepsis states. McGregor suggests ME/CFS patients have been shifted into an abnormal metabolic state.
Both burn and ME/CFS patients show loss of amino acids, insulin resistance, connective tissue degradation (an interesting finding given the EDS and craniocervical instability problems), altered triglycerides, cortisol, a switch from ATP production to “thermogenic” response, and gut-barrier issues. (Ron Davis, by the way, is finding high levels of connective tissue metabolites in the severely ill patient group he’s studying. Those metabolites could suggest that increased connective tissue degradation is occurring.)
McGregor, then, believes that problems with glucose metabolism are at the core of what is happening in ME/CFS.
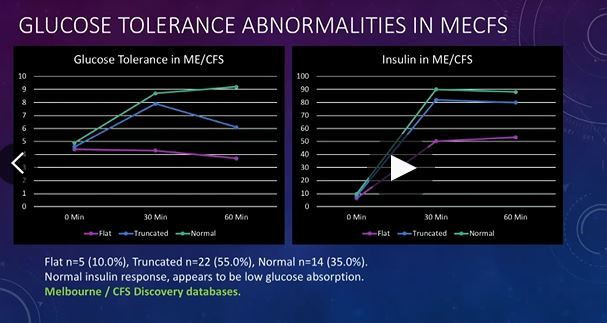
The Glucose Tolerance Test Results
McGregor then turned to a HUGE sample size (n=777) of ME/CFS glucose tolerance test results.
In a glucose tolerance test (GTT), glucose is consumed and blood samples are taken afterwards to determine how quickly glucose is cleared from the blood. GTT’s are usually used to test for diseases and conditions like diabetes, insulin resistance, impaired beta cell function, and reactive hypoglycemia.
Three types of responses show up in ME/CFS. A small group of patients had a normal result (10%), most had a truncated (82%) glucose response, and a small group had a flat (7%) response.
For most, the peak in glucose occurred quickly (30 minutes) after which, as McGregor put it, their glucose levels traveled south (diminished) at a rapid rate. In fact, they traveled south so quickly that almost 30% of the group was hypoglycemic (low glucose levels) by 60 minutes and many others would most likely have been hypoglycemic shortly after that. Glucose did not hang around long in McGregor’s ME/CFS group.
Glucose Tolerance Testing Plus Metabolomic Equals…
McGregor then tied together the GTT results with metabolomics results to see if abnormal metabolic breakdown products were correlated with the altered glucose uptakes.
Flat response (10%) – The metabolite results suggested that the people in this group had very low creatinine levels, and that an autoimmune response was likely occurring.
Truncated Response (82%) – People with the more common truncated response had low urea (low nitrogen) and very low creatinine levels. Because creatinine is an important factor in the production of energy in the muscle, brain and cardiac tissue, the low creatinine levels translated into low energy levels.
As noted earlier, inhibited glycolysis results in an increase in ATP breakdown. Since problems with glycolysis cause the body to scavenge creatinine from the muscles for energy, the low creatinine levels made sense.
Exercise causes a similar result in healthy people, but their creatinine levels get replenished within hours. The low creatinine levels found in this ME/CFS group suggested that their muscles are not getting replenished, even with the pitiful amounts of activity they can generally engage in.
The two-day exercise tests pioneered by the Workwell group suggest something like this must be happening. Some part of the energy production process must be depleted or damaged or interfered with for a short exercise period one day to be able to impair exercise the next day.
Nancy Klimas’s exercise and mega testing regimen suggests exercise is immediately sparking an intense burst of inflammation in ME/CFS, which then goes on to whack the autonomic nervous system, hormones, antioxidant system, etc. McGregor’s findings suggest that depleted muscles may be part of the package as well. If McGregor is right, hopefully that depletion will show up in Nath’s and Tompkins’s muscle biopsy work.
McGregor’s results suggest that people with ME/CFS are in a hypermetabolic (not hypometabolic) state – a state in which the body is pulling out all its stops in an attempt to get energy. (McGregor’s hypermetabolic idea seems to fit with the sympathetic nervous system activation/parasympathetic nervous system inhibition going on in ME/CFS.)
One of the consequences of a hypermetabolic state is, not surprisingly, an elevated heart rate. McGregor found that the metabolites elevated in people with greatest increase in HR upon standing were, indeed, consistent with a hypermetabolic and hypercatabolic (muscle breakdown) state.
McGregor found that the more post-exertional malaise (PEM) a person experienced, the more metabolite dumping (and hence more muscle catabolism or breakdown) was present as well.
That process, unfortunately, puts ME/CFS patients into one of nature’s catch-22s. Breaking muscles down to produce energy causes a raft of metabolites to get dumped into the urine. These are the same metabolites, though, that we need to replenish in our muscles. Some energy, then, is being produced, but at a longterm cost to the muscles.
This dumping/amino acid depletion is, McGregor believes, a key issue in ME/CFS. It can help explain why ATP production is low and why the disease is so darn difficult to get out of. Until the problem with glycolysis is resolved and ME/CFS patients’ cells can get back to primarily using glucose and fats to produce energy, their proteins – and that means their muscles and other tissues, are going to keep getting broken down.
That, McGregor believes, results in problems not just with the muscles but with gut malabsorption, connective tissue issues, difficulty fighting off infections, inflammation, heavy metal absorption, etc.
Gut barrier problems (e.g. leaky gut), for instance, are common in hypermetabolic states. McGregor noted that Shukla showed that exercise – which is simply going to exacerbate the amino acid depletion – results in the gut leaking bacteria into the blood in ME/CFS and spiking inflammation. McGregor believes that gut barrier issues are probably producing many symptoms in ME/CFS.
The Tie That Binds…
McGregor then shifted gears and proposed – using a gene expression exercise study by Whistler et. al. – that the tie that binds all this together may be massive levels of something called histone deacetylation (performed by histone deacetylase enzymes or HDACs). He noted that histone acetylation/deacetylation regulated the top twenty genes found altered in Whistler’s study. A similar outcome was found in a genetic polymorphism study.
That suggested to him that people with ME/CFS are having trouble “acetylating”; i.e. using acetyl groups to regulate our gene expression. Only one study has addressed this issue, and it found HDAC2 and HDAC3 levels three and four times higher in ME/CFS than normal.
McGregor then tied it all together with a nice bow. Bringing us back to square one, he proposed that the HDAC problems in ME/CFS result from the problems with glycolysis.
Glycolytic inhibition, it turns out, increases HDAC activity. Low lactate and pyruvate levels – because they are HDAC inhibitors – allow HDAC levels to rise. HDAC then causes deacetylation and silences the transcription of many genes.
McGregor has found dramatically reduced urea, acetate and allantoin levels in ME/CFS. Acetate, McGregor believes, is a particularly critical factor. Acetate comes into play because it plays a crucial role in the transcription of our DNA – and the subsequent production of protein in the cell. The histone deacetylation he believes is present in ME/CFS inhibits DNA transcription, thus reducing protein production in the cell – which makes the cells go all twitchy when faced with a challenge.
The big question in ME/CFS, then, in McGregor’s mind is what the heck is whacking that very first stage in energy production – glycolysis. That’s where the key to this disease, he believes, will be found. He reported that he’s now searching for the causes of the glycolytic problems in ME/CFS and hopes to have more information on that this fall.
Time will tell. In the meantime, it’s good to see someone put forth a grand hypothesis of ME/CFS.
Conclusion
Neil McGregor swung for the fences (baseball reference) as he proposed that problems with the earliest aspect of energy production – glycolysis – was at the very heart of ME/CFS. His results suggest that difficulty turning glucose and fats into energy is causing people with this disease to turn to their last and worst option – breaking down their own muscles to provide the substrates the energy production process needs.
As their tissues are broken down, the metabolites needed to replenish them are being flushed into the urine, resulting in a chronically depleted system. McGregor believes this chronic depletion of essential metabolites plays a crucial role in ME/CFS and results in numerous issues, including post-exertional malaise, gut malabsorption, connective tissue problems, inflammation and more.
The final straw of the glycolytic inhibition problem results in high levels of histone deacetylation, which impairs DNA transcription on a large scale, resulting in the broad and systemic issues found in ME/CFS.
The fix to all this requires identifying the problems with glycolysis that are setting all this off. McGregor reported he is attempting to do that now and hopes to report back in the fall.
Health Rising
(if you were wondering)
is not a hobby website.
It takes the work of 1 1/2 people to keep it going
and your support keeps it online.









are any off this studys published on pubmed or so? looked for it but could not find anything.
He’s actually participated in a good number of studies – https://www.ncbi.nlm.nih.gov/pubmed/?term=McGregor%20NR%5BAuthor%5D&cauthor=true&cauthor_uid=25344988 – but nothing lately I don’t believe. It sounds like he has some data to get out….
Hi Cort. This article shows why I’m doing so well on Amino Acid replacement. I have amazing improvements in sleep, energy, brain function etc. For those who might be interested in being tested for their Amino Acid status, my Naturopath Doctor contacted Drs. Data Lab. in the USA and they sent him a kit for me to use. It’s only a 24 hour urine collection. My Essential Amino Acids were rock bottom. The extensive report included the Amino Acids that I needed to have replaced, in the exact milligrams. My ND then sent the prescription to be filled by a Compounding Pharmacy that he trusted to do the best job. The Amino Acids come in a powder form and I only have to take I Tsp. 2xaday. I still have ME/CFS/FM, but I now live a pretty normal life compared to before. MDs can also order the test for you. I see that Doctors Data Labs. are in other countries as well. I cannot recommend this prescription enough if you need it. It has been life changing for me!
Hello Cort,
I have been following the research here in Melbourne for a number of years and Neil McGregor is involved in many articles. The most salient one I will post here and you should be able to download it from this site:
https://www.researchgate.net/publication/277979239_Metabolic_profiling_reveals_anomalous_energy_metabolism_and_oxidative_stress_pathways_in_chronic_fatigue_syndrome_patients
Hi Edie,
having blood test and mainly supplying essential amino acids you are low in may well (help) solve the dilemma that ME patients are low in amino acids but many alternative and regular health providers see high amino acid intake as inflammatory.
You solve only the worst and most urgent deficiencies rather then blanket upping all amino acids by eating more protein. I believe that often results in a better ratio of benefits versus side effects.
The main drawbacks I see is price and supplementing “non whole food” as I prefer that one.
I try and solve this dilemma by partly shifting animal based proteins towards vegetarian based ones. They are said to be lower in purines and to generate less uric acid. I also try to find and avoid getting in this emergency state of burst protein consumption. That does not only mean pacing but also trying to get better through the most difficult phase of the day: the night.
@Edie – I wish it was as simple as supplementing my deficient amino acids. My serum tests (5 years back) showed them depleted too (BCAA, lysine, tryptophan, tyrosine, etc) fitting with the findings the Aus group presented in 2016, etc.
But supplementing has only ever given me those kinds of wonderful energy boosts for a few days, at most, before becoming increasingly detrimental to my energy. I’d tried various protein powders (vegy, non-soy), so wondered if it was tripping my histamine intolerance. But it’s the same deal with isolated e.g. BCAA aminos from capsules. Glutamine and glycine (that would be helpful for gut) give me only next day fatigue reactions too (like my dietary intolerances do). Frustrating. Creatine’s effect less noticeable but seemingly became detrimental, too.
@dejurgen – I’m not sure a very small (e.g. 400mg) BCAA intake would be inflammatory, but maybe… I can be crazy sensitive to the neurological effects of tiny sprinkles of tyrosine (and melatonin) for example. I was speculating that maybe whatever process in the body is limiting aerobic metabolism doesn’t want these helpful nutrients around and simply reduces further to compensate…
After I found my dietary intolerances, and went kind of paleo, I was feeling semi-recovered for months, but that tailed off… I’d excluded dairy, wheat yeast and processed meats, etc, and felt I’m probably not gaining full benefit from meat protein. As per “malabsorption”, in the article. Hence supplementing. What veg sources you recommend? My histamine intolerance gets in the way of processed soy products, etc, and I’m already eating porridge, brown rice, hummus plus stopped with lentils recently too.
@Richard:
I was talking eating plenty of high protein meat (and cheese) to have inflammatory potential, not supplementing 400 mg.
“those kinds of wonderful energy boosts for a few days, at most, before becoming increasingly detrimental to my energy.”
I tend to believe that the body willfully (for safety, longvity) shuts down energy production by inhibiting as many energy pathways as possible. Supplying a previously underused pathway (by missing the fuel) will boost energy production until the body notices that “too much” energy production causes a series of problems and is has to inhibit all energy production pathways even more IMO.
However, it may kinda work for some as the bodies aim to reduce energy production may be to crude and some moderate amount of optimization opportunity could be left. One such optimization could be spreading the energy production over more pathways so none is stretched to the fullest (easier temporarily depleting key resources).
“I was feeling semi-recovered for months, but that tailed off…”
Did you have increasingly problems with constipation. A keto diet can improve the gut until constipation due to too few fibers kicks in. Then part of the gain *might* be taken away from the table.
I don’t recommend a vegetarian protein source as many vegetarian foods hold increased risks for triggering intolerances. So it’s an individual search. I personally do well on seeds like sunflower seeds, a variety of nuts, pine seeds, sesame seeds (strong allergenic IMO!) all in small quantities. Quinoa is also a good source for me. It is said that hemp seeds are among the easiest to digest seeds there are and they are full of nutrients. We can legally buy them if they are broken (no ability to grow hemp?). So I’ll have those too. I eat oats too, but better have rolled then steamed ones. Still, it’s said to be a chelating agent so it can reduce minerals in the body.
Konijn,
the research group Neil is part of published a study in 2015 showing depletion of amino acids in the blood – since they were being used for cellular energy production [Chris Armstrong/Neil and others 2015 – https://minerva-access.unimelb.edu.au/bitstream/handle/11343/121955/2015%20Armstrong%20Metabolomics%20CFS.pdf?sequence=5&isAllowed=y%5D.
Chris Armstrong did a webinar in 2016 in which he proposed that people with ME were locked in a negative (stable) metabolic state.
Fluge and Mella published a paper (2016) showing that pyruvate dehydrogenase wasn’t working as normal – so you couldn’t use glucose for cellular energy production (you used amino acids instead of glucose). The finding that really brought the paper to attention was the discovery that something in the blood was causing the switch to using amino acids – when you filtered the blood then energy production returned to normal (i.e. utilising glucose). That something is now proposed to be exosomes (Bhupresh Prusty/Ron Davis – NIH Conference April).
You can find most of this stuff online [Google search – I’ve given the web address for Chris/Neil’s 2015 paper above].
Cort – is the key question where the exosomes are coming from and what they contain? E.g. the genetic switch, Neil has identified, need a signal – presumably that’s in the exosomes. Also, can the source of the exosomes be switched off? As per previous posts; Chris proposed reduced stomach acid as the driver. Weird disease, how do you achieve a (negative) stable steady state?
Thanks for the article – I watched the presentation but there was so much material that I found it difficult to follow. Great research group; working quietly for years – I think they relied on private donations; from 2015 paper:
“Financial Support
This work was supported by grants from the Judith Jane Mason & Harold Stannett Williams
Memorial Foundation (The Mason Foundation) and equipment grants from the Rowden White
foundation and State of Victoria”
Thanks for the link to the paper :). They are a great research group and they have worked quite quietly for years – not getting much attention but plugging away. They have not gotten the funding that others have but hopefully that will pick up.
Ron Davis I’m sure has helped. Armstrong and McGregor both attended his Working Groups and now Armstrong is working with Davis at Stanford.
Cort, this is extremely interesting. (1) Is the prescription in the meantime to avoid carbs as sugar and eat a high protein diet? Years ago, I was advised to do that by alternative and complementary medicine doctors. (2) I kept wondering as I read through it if this theory is consistent with or conflicts with David Systrom’s theory and the Rx of Mestinon. Thanks.
correction of typo: I meant to write “to avoid carbs and sugar”
I have tried low carb high fat high protein before. It gave me some benefit with pain, but that was it regarding ME.
It slashed triglyceride levels however that were way too high.
Later on I learned that I am fructose intolerant (quite likely due to fructose malabsorption).
Now I strongly restrict fructose intake (including foods that break down into more fructose then glucose as that ratio is important in fructose malabsorption) and avoid quick sugars. That is refined sugars but also things like honey and fruit (a bit less quick) (fructose is a huge problem for me).
I returned to a diet with a fairly “normal” portion of carbs, but near all of them are slow digesting starch that breaks down to glucose plus some (quicker) lactose as I’m not having problems with dairy.
For me avoiding carbs isn’t the key, avoiding too much fluctuation in blood sugar (mainly glucose but also fructose) is.
The reason is clear: when bursts of sugar enter the blood it becomes very thick and our body goes “all in” to try and reduce them. That’s producing copies amounts of insuline, burning sugar inefficiently (to quick going to into anaerobe metabolism), converting it to glycogen… and if that isn’t enough produce fat from it with plenty of triglycerides and LDL cholesterol as a result. That fat production consumes NADPH, a key resource to fuel the immune system and to recycle glutathione, needed for fighting oxidative stress. Much of the above is inflammatory and also so much conversion from one energy source to another does cost energy, something we lack.
At night, the digestive track contains very few sources of glucose so blood levels drop really low. Our already overused hormone system has to produce plenty of cortisol, nor-adrenaline, adrenaline… to convert glycogen to glucose.
That probably is why eating a few rice crackers (those very light easy to digest things) before sleep helps me sleeping and restoring during sleep (a bit) better. Won’t work for everyone, but Phil stated it even doesn’t kick him out of ketosis. Note however that the idea here is NOT to eat more carbs (compared to normal) but to eat easy but slowly digesting carbs and spread their consumption more around the clock.
The following quote once more highlights the problem with fluctuating blood sugar levels:
“Rebound hyperglycemia in the morning occurs when your blood sugar drops too low during the night, In order to “rescue” you from the dangerously low blood sugar levels, hormones are released which tell your liver to dump glucose into your bloodstream. Not too surprisingly, the low glucose levels can play havoc with your sleep, causing nightmares, restless sleep and night sweats.”
Note: some people have intolerance versus sources of starch like potatoes (nightshades) or wheat and some other grains (gluten, inuline).
Going with a low carb diet often eliminates one or more food intolerances that are hard to detect. But detecting them and avoiding those specific sources works just as well and leaves space for further optimization.
Interesting that fructose is metabolized quite differently from glucose. You may have problems with glycolysis but you have an extra added metabolic issue with fructose.
I do think the idea of limiting carbs really does fit in ME/CFS. Some people get a lot out of it – others not so much – and it plain does not work for some but I’ve seen it suggested so many times that I think, in general, its a good idea to try it out.
Trying to get over this lingering cold I am trying out this diet – https://www.amazon.com/gp/product/B00W22IKBK/ref=ppx_yo_dt_b_d_asin_title_o02?ie=UTF8&psc=1 – recommended by my twin brother who has lost 30 lbs on it.
It’s quite severe, at least in the beginning, – protein, fats and limited carbs (no carrots, sweet potatoes, grains) and it involves a caffeine (for me) aided morning fast – but I must say my craving for sweets has disappeared, I no longer need an energy bar to get me through the afternoon, and I am eating much less.
I’ve been on it for about 3 weeks.
Thanks for comments re glucose and fructose. I generally don’t feel well when eating many carbs (other than, like dejurgen, brown rice crackers, which are a mainstay for me for nausea).
But again, what about how this theory meshes or conflicts with David Systom’s theory and his Rx for Mestinon. I’ve started taking Mestinon, extremely slowly working my way up, and don’t want to be working against myself. As with all of us, can’t afford to be working against myself!
Hi Cort
Can you let us know if you have benefits from this diet in terms of your energy levels and recovery from infections?
Great article, don’t understand the science but at least it’s providing a more coherent emerging picture.
I believe Dr Davis suggested that there would be individualized suggestions in the future, but not general suggestions for the entire patient population.
Thank you for this report, Cort. So many possibilities…so many theories. Some day, some how I hope it will all come together so that there are some viable and affordable treatments for us.
At 51 I should be gaining pre-menopausal weight. Instead my weight has dropped down to about 110…and has been there for over a year. It is remarkable to me. Even early on with the MECFS I still had my runner’s legs for a while. But, in the past couple of years my legs and arms are very skinny (compared to what I used to be like – at a steady 125). Even my neck is getting skinny. And, 2 weeks ago I have had my 3rd concussion … perhaps loss of neck muscles are part of why. (I also had a concussion last summer. Good times.)
So, just looking at what has happened to my own muscle mass (esp legs and buttocks), I could “buy into” this theory. My friends who have known me for a long time are surprised at how tiny I’ve become. (A couple of years ago I did hit 140 from eating way too many tortilla chips at night during 1-2 years of difficult IBS. Since that time the chips are gone …and I started to eat more “paleo”). Anyway.
The connective tissue is an interesting angle too. I have thought of trying collagen supplements, but am not sure about toxicity/heavy metals…because my skin is losing elasticity. It’s a given for my age. But, in conjunction w/low weight and much less muscle mass, it’s just really noticeable to me.
Nico, the same thing happened to me 2 1/2 years ago (when I was 51) in terms of wasting. I’m a little over 5’6″ and weighed 135 (had a lot of musculature) after first marathon many many moons ago. I got down to 106 when it all spiraled in ’17. Now I’m between 111-115.
I do wish there were a few basic directives with this article, and am wondering the same thing as Cameron. When I cut out most inflammatory foods for stretches of time – sugar, dairy, grains etc. – my weight really suffers and I don’t notice a huge difference in symptoms (but I’ll keep doing it if necessary).
I wish there were more directives too. McGregor, a researcher, is probably shy about getting into that, and while he thinks he has a handle on the general cause, there appears, at least to me, to be quite a few ways for glycolysis to fail – if that is indeed what is happening.
(Another Emerge talk is going to focus on mitochondrial issues!)..
There seem to be no dearth of possibilities – which, honestly is much better than the reverse – and one would really, really like a clear direction. That said, it seems that problems with energy production could be that main theme we’ve been looking for. Time will tell.
Nico,
Did you have a lot of pain in legs/butt when first sick? That was one of my first symptoms, intense symmetric pain (and facilitations). I was in good shape, had strong legs (that’s not meant to be weird). I haven’t lost nearly the muscle mass in the rest of my body, but hips-down, lost a lot.
Thanks
Hi Michael,
When I have pain events after exertion it is usually only in the thigh muscles, and was also one of the first pain symptoms when I really got sick for the long haul 13 years ago. I don’t really get pain in the buttock that I can recall that has been “myalgic”. (I’ve had new pain in the past months, and am not sure what it is, it feels more like bone/joint inflammation. But, it’s in the hips, low back, and has radiated down my outer thighs a few months ago. But, it is a different kind of pain compared to the “myalgic” type. I started taking part of the Pridgen Protocol last November…and I’m not sure if it’s something with that which is setting off this kind of inflammation. Anyway, that was a total aside to the main topic.). And, not weird about saying you have/had strong or muscular legs. I think many of us were once very active.
Fall? Ugh. I wonder how many more of us will have given up and opted out permanently by then?
I’m in the same boat as you, it’s getting frustrating to hear about a hypothesis and then having to wait months to find out if it’s worth pursuing. Personally I rather have to wait till the fall for the right information about the possibility of treatment or a cure vs. vague information and grand claims like the metabolic trap that turned out to be nothing.
They disproved the trap?!?! When?!?! So sad that Dr. Davis and Dr. Phair put in so much and time and effort into the metabolic trap and it didn’t pan out. I hope Dr. McGregor is on to something here, we could really use some good news for this horrible disease.
It did hit a stumble but from what I heard things are moving forward.
I don’t believe the metabolic trap theory has been dismissed.
From this short video it would seem like the metabolic trap hypothesis is alive and well: https://www.youtube.com/watch?v=0qYBQAiekPk
When I first saw that video posted by Ron Davis I was really excited to see that there was meaningful progress towards the theory. A few days later at the Harvard Convention Ron said that he had 66 ME/CFS patients tested and all of them were positive for at least 1 mutation. He then went on to say that he did not trust the data and that the IDO mutation is not the cause of ME/CFS, but just a piece of the ME/CFS puzzle. There was also a tweet that has been since deleted on the OMF’s twitter page stating that there are now putting their attention towards the thyrosine trap.
The metabolic trap will not work, we already have a cure for cfs for years, but the FDA, as usual has their head buried in sand. Fecal Transplants have put me in temporary remission. It’s all in the quality of the donor, if I could find a high quality donor to do daily transplants for a few weeks i’d be cured.
Susan, I can definitely see where you are coming from. I have been skeptical about about Dr. Davis saying how we will be able to go any pharmacy and purchase something over-the-counter and we will be cured. Having this disease for 15 years I have personally tried everything under the sun from countless kinds of medications to vitamins, supplements, and herbs. I hope were both wrong, and were cured by the end of the summer!
I do have some good news about about the Cortene trial! This email was sent out about 2 months ago.
Dear Friends of Cortene, We are writing to let you know we have just completed our clinical trail in ME/CFS earlier this week. As you may already be aware, the trial was based upon our theory that a single receptor was up-regulated in the brains of ME/CFS patients. It proposed a novel therapeutic approach, in which a short exposure to a novel peptide (CT38) was intended to down-regulate the receptor, leading to symptom improvement. Our small (n=14) trial utilized 3 treatments of CT38, at 4 different dose-levels (no placebo). While we understand you are anxious to hear about our observations, it would be premature to share these until the data has been thoroughly reviewed and analyzed. We anticipate it will take 1-2 months to complete our analysis and write up our findings. That said, we have found the data encouraging and believe they support moving toward a randomized, double blind, placebo controlled trial. This will require significant funding and we are currently pursuing various channels to support continued development. We look forward to sharing more details soon and thank you for your continued support and patience. Regards, Michael
There are SO many great things about this article! Here are a few:
First, Ron Davis’s hypothesis challenge – I would love to hear lots more of these from researchers. I imagine they would accelerate the science because they would stir debate.
Second, I couldn’t agree more about this superior conference presentation structure – I frequently feel they end prematurely and opportunity for debate is lost.
Third, you have – again – explained it all and put it into context beautifully. Thank you.
Interesting article and research. There’s one huge problem and caveat to this research. They have not distinguished effects of disuse atrophy and all the metabolic changes that come with this versus something that is specific to ME/CFS. There is extensive research with respect to disuse atrophy. There are still so many unanswered questions even with this research focused on disuse atrophy not confounded by any disease process. Disuse atrophy has been extensively researched for decades for the benefit of astronauts, because of the implications on their health with disuse atrophy, including maximizing their diet, being able to maximize their ability to maintain muscle mass and respond to emergencies if needed. Their metabolic pathways are very different and require very different energy reserves and reconstitution. I point your readers to this very good review article (though even now, a bit outdated) of the metabolic changes in disuse atrophy that are, in fact similar to what McGregor is finding. Disuse atrophy starts within 24 hrs of bedrest or disuse and the significant metabolic changes occurs in less than one week. As the linked article points out, “The shift toward increased activity of the glycolytic enzymes in atrophied muscle is accommodated by a shift toward increasing glucose availability in the liver. It appears that there is a coupling between muscle and liver glucose metabolism in this model, suggesting that the change in muscle glucose metabolism is driven by substrate availability via an increase in hepatic glucogeneis.” So, yes, we see more glucose in the blood irrespective of any other potential disease process. It might be fruitful for ME/CFS researchers to look at this literature and collaborate with those who study the effects of disuse atrophy. https://academic.oup.com/jn/article/135/7/1824S/4743254
Thanks Neilly. Because disuse or deconditioning must be present in ME/CFS and because it can cause so many similar issues it must be handled. Hopefully McGregor did account for activity levels but most researchers do not. In fact, its rare that anyone does. Until it is definitely decided that monkey on our backs is going to keep causing problems.
Some studies suggest that while deconditioning is, of course, – present it is an add-on rather than being a critical factor.
https://www.healthrising.org/blog/2018/12/16/deconditioning-denied-chronic-fatigue-syndrome-deconditioning-myth/
It’s odd though, when I tried to learn how little activity it takes to cause deconditioning or vice versa, how little activity it would take to stave it off – I found no information on that at all. It seems kind of like this nebulous entity that’s hanging over us.
Personally I am VERY clear that deconditioning does not play a role in my case. I make sure that I get out for my limited walks and walking too far – which doesn’t take long – leaves me in agony in the following days.
I pushed and I pushed when I first came down with this – I tried to exercise again and again and again. As avid exerciser prior to ME/CFS I flogged myself probably to a ridiculous degree trying to get back on my beloved exercise routine – and failed every time. There is no way deconditioning contributes an ounce to my ME/CFS.
My guess is that many people in these studies are kind of like me; they get enough activity to stave off deconditioning but until that is shown we just don’t know.
I would love to get this issue definitely dealt with….
Re disuse atrophy, I personally saw how extreme that effect can be when I had two herniated cervical disks a couple of years ago, and the associated arm (right, and I’m right-handed) shrivelled up within 3 months since I couldn’t use it due to the very extreme pain. However, once I was able to do just normal everyday things (like washing the dishes and brushing my teeth), the arm muscles came back (and I was past age 50 at that point). I second the notion that disuse atrophy in those of us who had any possibility of trying to maintain somewhat normal physical activities, if on a greatly reduced level, can’t really be an issue. I was lucky when I first got sick that my doctor, a CFS specialist, ordered me to stay as physically and mentally active as I could, and try to develop a sense of what is too much. It has been 25 years, and I still fail sometimes in recognizing the boundaries, and fall into PEM, but I’m extremely grateful for that doctor’s advice. I never was presented “bedrest” as a good option. Lie downs, yes, but interspersed with as much physical activity as possible. Just in case that’s helpful to someone newly diagnosed.
This is fantastic. All makes sense, and is clearly getting closer to the bone… literally, of what’s going on….
And again Cory you translate these studies, and cross reference beautifully for us.
What would be an amazing addition, is not what, but why???
Personally, I am convinced that this proposed glycolysis failure is caused by a polyvagal metabolic freeze response. This freeze option resulting from a damaged nervous system – stress/infection/injury – is ultimately the flight/fight/freeze response closing the body down at the energy level, by cutting its legs out from under itself, in order to simulate death… as it can actually seem like.
The easiest way for the brain to induce such a death like state? Would be to cut the fuel line as close to source as possible….
I think these 2 theories completely stack up together…
It’s marvellous the strides in understanding which are being made. But we do need to push these theories together and get to the why to accelerate treatment options for the millions affected.
Thank you researchers, commissioners and Cort?
Nice information and good write-up Cort!
This is fairly in line with some of my ideas, including the “pillaging of all glucose sources including amino acids” during time of dire need as I call it.
Allow me to add a few ideas:
“The big question in ME/CFS, then, in McGregor’s mind is what the heck is whacking that very first stage in energy production – glycolysis.”
My guess: massive oxidative stress. That whacks both glycolysis (anaerobe and aerobe metabolism) and the Krebbs cycle (pyruvate and fat, aerobe metabolism).
Let me correct that:
It shifts glucose metabolism from glycolysis producing a bit of energy (NADH that can produce ATP) and pyruvate towards the Pentose Phosphate Pathway producing even less energy (NADH) and pyruvate but produces also much needed NADPH and ribose (sugar that helps in ATP construction, needed according to this blog).
The Krebbs cycle isn’t entirely blocked neither, but converted from producing mainly energy (NADH) to producing significantly less NADH then usual and much more NADPH.
Benefit of both? Less energy production = less oxidative stress; more NADPH production = stronger immune capacities (if not too strong already…) and better anti-oxidant fighting capacities plus maybe some better ATP production (and with it likely less phosphor loss, something we are short on).
So far I did found well above a dozen relatively detailed pathways that support this shift from NADH to NADPH production and the ramping down of ATP production that is the logical consequence of it.
@Cort: if you’re up to it and have the time and energy to spare, I have much information waiting to get into a blog onto the topic; can’t do it however without some help as structuring a sizeable project is whacked by my ME a lot. Don’t worry, you’ll won’t need to go very deep into detail, just use the same skills you have when writing your great blogs: sort and present things orderly ;-).
“McGregor found that fasting morning glucose levels increased and lactate decreased with increasing PEM. (Yes, lactate decreased!)”
Now that is something that would hint to shifting form glycolysis to the PPP too:
The PPP needs plenty of glucose and, if my morning levels of “anxiety = stress hormones” is a sign, then these hormone raise it’s levels.
The PPP produces less “excess” pyruvate then glycolysis, so that less pyruvate needs to be converted to lactate.
Strong use of the PPP has however plenty of similarities with strong use of glycolysis. They even share most parts of their pathways. So it’s easy to mistake high PPP usage for high glycolysis. I’d love to see a metabolic research specifically looking for metabolites that can distinguish between them. And I’d love to see a metabolic measure of oxidative stress in the morning and during PEM.
“The higher levels of hypoxanthine – a breakdown product of ATP – suggested to McGregor that the ATP in people with ME/CFS was being broken down at a higher rate than normal”
From en.wikipedia.org/wiki/Creatine_phosphate
“Conversely, excess ATP can be used during a period of low effort to convert creatine to phosphocreatine. The reversible phosphorylation of creatine… …The cell’s ability to generate phosphocreatine from excess ATP during rest…”
=> Now that may be a major problem in restoring ATP then:
Creatine can use EXCESS ATP during rest. With it, it recycles phosphor. Note that when consuming energy ATP is used and conderted to ADP.
BUT: ATP stands for A…TRI…Phosphate and ADP stand for A…DI…Phosphate.
=> So using energy “creates” buffers of “free-ish” phosphor (ATP contains 3 atoms of phosphor, ADP 2 so one is “freed”) that need to be recycled before they are lost. That happens during REST, when we produce EXCESS ATP. => We ME patients have very very few excess ATP to use to recycle this phosphor during rest. So we lose it (there are several reports showing we are low on phosphor); lose ATP; lose energy and excess ATP; can even recycle less…
Interesting Dejurgen. I have posted above but I think your comments are very relevant to McGregors et al. original paper published in Metabolomics 11(6) · May 2015.
https://www.researchgate.net/publication/277979239_Metabolic_profiling_reveals_anomalous_energy_metabolism_and_oxidative_stress_pathways_in_chronic_fatigue_syndrome_patients
Thanks for bringing it to my attention again.
When reading it, I see that part of the mentioned problem with is with glucose uptake (and insulin).
“The increase of glucose in the blood of ME/CFS is
trending towards hyperglycemia, which is linked to…
and the development of insulin resistance.”
There do follow other pathways too, but it may be part of the problem.
If I recall well other research indicated borderline insulin resistance problems with ME. The low carb diet may help there. But avoiding blood sugar peaks can go a long way too here IMO.
There is another strongly overlooked potential source of energy here IMO: citrate. Citrate can bypass insuline resistance and could do comparable things to supplementing specific amino acids IMO *IF* oxidative stress does not block it’s use in the Krebbs cycle.
Aconitase is (very) strongly inhibited during massive oxidative stress. When looking at the Krebbs cycle en.wikipedia.org/wiki/Citric_acid_cycle one can see that citrate can just as well fuel the Krebbs cycle and bypass many of the mentioned potential problems including poor glycolysis.
If it were that simple, eating more citrus fruit with lemon being highest in citrate but others high too and citrate fruit being (surprisingly) alkali and containing plenty of vitamins and minerals would be (a big part of ) “the solution to ME” IMO. If it were, it would be known wide and afar I suppose.
That IMO points to massive oxidative stress inhibiting essential steps of the Krebbs cycle itself; inhibition of glycolysis is not a key breaking point IMO.
I’ll read the paper in more depth later (again, difficult stuff).
Also in the linked paper:
“Importantly, in ME/CFS patients, blood glutamate is negatively correlated with urine creatinine and blood glucose with urine creatine, which highlights the use
of creatine to form creatinine and ATP in these patients.”
Now going back to the original comment I posted:
INCREASED reliance on creatine to generate ATP in ME patients?
Note: would that be short for creatine phosphate as creatine is an intermediate step for that???
And “Creatine can use EXCESS ATP during rest. With it, it recycles phosphor.” (quote my original comment).
=> then we consume more of it and can only recycle it “at rest, when producing EXCESS ATP”. Nice :-(.
Maybe that is were a post on the forum on sleeping colder and brown fat comes into play:
Cort wrtoe that sleeping colder produces brown fat. Brown fat contains mitochondria, were “normal” fat cells don’t.
That may be some of the few cells that can relatively easy create excess ATP as there own needs will be very low. By having minimal “settings” of energy production they could produce it in a relatively clean way and be a resource of excess ATP to both give to the bloodstream (for use of RBS, WBC and starving cells down the line) and for creatine recycling.
Brown fat namely produces ATP just to produce heat; can just as well do something usefull with it as all produced energy tends to produce heat finally anyways.
“The hypoxanthine levels suggested that’s what was happening in ME/CFS… …that whatever muscles the people with ME/CFS had left were being broken down faster than normal.”
For an exercise study I can see that. But ME is said to be a Dauer state by Naviaux and Davis. And Dauer does decrease cell division rates a lot IMO. So very few repair is happening.
For patients having plenty decades of ME, having plenty of daily muscle breakdown and few repair can’t go on long. So for long time patients there is very few capacity left for daily muscle breakdown IMO.
The few muscles that are left must have found a sort of way to protect them from further scavenging too much proteins from muscle breakdown.
So this could be the result of an excessive stress test, or (some of) the hypoxanthine could be the product from xanthine oxidase breaking down ATP, all sorts of “protein rubble”…
With all sorts of protein rubble I mean that the xanthine oxidase (a chemical creating massive oxidative stress) tries and breaks down misfolded proteins, viral RNA, bacteria and mold digested by the immune system, poorly digested proteins entering the bloodstream through a leaky gut…
My money so far is on the “breaking down protein rubble” so far as the dominant source (as long as no exceptional exertion happened).
Note that en.wikipedia.org/wiki/Xanthine_oxidase also breaks down “purines” en.wikipedia.org/wiki/Purine and those come from plenty of foods rich in proteins.
So while we are short on amino acids in our blood, consuming plenty of them could cause plenty of oxidative stress (due to xanthine oxidase needed to break down the purines) and (local, in the capillaries) uric acid problems. It’s like another catch 22.
In classic “nature health”, high protein consumption is considered to be highly inflammatory… :-(.
@ dejurgen: Because you have such a grasp of the biochemistry involved in McGregor’s theory I wonder if you could take a moment to speculate upon any therapies that might some day be employed to address the metabolic breakdown he describes. To the layman it’s just a big black box. Thanks.
I do believe McGregor digs deep and his findings are genuine. But I’m far from convinced a hammered glycolysis is the main culprit.
If I recall well, Naviaux did found pyruvate to be elevated in ME patients. If so, glycolysis does produce sufficient pyruvate.
Also from his own paper http://www.researchgate.net/publication/277979239_Metabolic_profiling_reveals_anomalous_energy_metabolism_and_oxidative_stress_pathways_in_chronic_fatigue_syndrome_patients:
“Blood glucose was elevated while blood lactate, urine pyruvate, and urine alanine were reduced indicating an inhibition of glycolysis that may potentially reduce the provision of adequate acetyl-CoA for the citric acid cycle.”
=> I guess if pyruvate were low in the blood he would mention it here as it would strengthen his claim significantly. So there seems to be no real lack of it (in the blood).
That leaves only one final step, converting pyruvate to acetyl-CoA vulnerable. According to Cort’s schematic that is even no longer part of glycolysis. The key enzyme here is pyruvate dehydrogenase. That is something we are short on, but I think it’s just part of the bigger picture that the body wants to inhibit (near) all energy producing pathways.
Acetyl-CoA can just as well be made from fatty acid B-oxidation, so that should be hammered too. And citrate from citrus fruit and several proteins can enter the Krebbs cycle too. Granted, when entering they do no allow for a “full” cycle going round and round, but they can sure contribute to energy production to some extend.
To me it has (for now) “inhibition due to excess oxidative stress and high needs for NADPH” written all over it.
And unfortunately if it combines with poor blood flow and potential immune and infection problems there is no simple fix for that. But I’ll try and work on describing my progress in the “Me current improvement series” on the forum as a mean of making my ideas more easy to follow. Due to some complications I haven’t been able to continue it but hope to be able soon. No promises.
The creatine angle may provide a small insight. But it’s nothing new.
When doing an activity follow the
PEM provoking potential = intensity*intensity*time
rule.
Any exertion intensity going above a certain threshold seems to eat in our (creatine) buffers and those buffers are important to keep having sufficient ATP. The bigger the intensity, the faster this buffer drains. The longer the exertion, the more it drains.
But when you have to do a task, I’d say prefer half intensity but double duration over the opposite. Fast action is harsh on our brain for coordination anyways.
Nothing new, but that goes strongly against the “when exercising we should go for short burst of strong anaerobic exercise” some doctors suggest.
Also, breaking up a difficult action into (a few) smaller parts and rest in between should be more kind to our buffers. But then again starting an activity anew each time does cost brain power too.
Completely untested, *small* doses of ribose before/during/after exercise and during the night *MAY* help reduce phosphor loss, helping keeping our buffers up a bit. But it could also backfire!
It’s on my list of things to test. But I only wish to do one change at a time and there is a waiting queue of things to try.
Has anyone seen information about if disuse atrophy affects people with fast twitch muscles any differently than without? Or if people with fast twitch muscles do any better with ME/CFS than without? Since a lot of athletes get ME/CFS, maybe no difference? I have the fast twitch muscles and wonder if that’s why I’m not bed bound.
My me/cfs started in ’88 and really broke through in ’93. Since ther is inernet I followed an followed every resaerch. Now (the last time I am to ill, but once in a while I read or see a short period off a webinar or conference. I do not know how long I still wil be alive getting worse and worse,but I have “difficultys” whit the millionst research paper and after decades, they still do not know, everyone says something different, there is still no therapy (even the slightest), my life has passed away getting so much older,… I can not see (how do you say it in englisch) the treas anymore through the woulds. All those years I have been reading, and what has it brought me, us???? And they can go on and on and on for 10,20,30 years or much longer just like with cancer. How many sorts of me/cfs would there be? saw recently on a number of &0 patients (do not remember for shure the research, thought whole genome sequencing) in every &0 patient, there was something different wrong. And then the NIH and jarred younger who have to say now, no you do not have cfs , you have that desorder. It makes me all sad, my life is thrown away. but thanks cort! you can not help it! xxx
So. How many of us have test results that show low Creatinine levels? … Not me. Mine are always borderline high.
I never even thought about them before this article from Cort, not knowing what they were. Now I’ll look back. I’ve saved years of my blood tests since sometimes my doctors find it useful to see them.
My creatinine levels have always been on the low side of normal
Sorry I should have written creatine is low and not creatinine.
Just had the lowest level of creatinine on my blood test from 2 weeks ago. It fits with me being extremely unwell recently because I keep getting viruses from my family members plus gut infections which I seem to be predisposed to these days.
I believe my sodium and potassium balance going out of whack previous to this has also contributed big time due to adrenal insufficiency. My energy level has been awful compared to how it is in the summer.
Cort, any more info on how McGregor can separate infectious vs gradual onset of CFS?
Does this difference only show in resulting metabolites? How does the biochemistry between them differ? Does his hypothesis support both types of onset? Is one easier or simple explanation?
I don’t know. I got this from the Emerge Symposium presentation and I’m afraid I started it some time ago and I don’t remember if there are specifics regarding the different metabolites, which my notes suggested were how he differentiated them.
Thanks for asking exactly what I was wondering as a gradual onset patient.
My ME definitely needs more easy sugar the normal. If I drink a can of coca cola every day I get less ME symptoms. And every time I try to avoid any cola then the ME symptoms get worse. And no it has nothing to do with being addicted to sugar. I don’t get any sugar cravings. It’s feels like my body can’t metabolite the glucose efficient enough and need the extra boost of sugar to get the metabolite working. I’ve tried using grape sugar instead but it doesn’t have the needed effect.
I don’t have any deconditioning of the muscels and don’t get fatigue in the muscels. But I have big troubles whenever my pulse raise when I exercise. Especially the heart and lungs struggles with getting any energy if either my pulse goes up or whenever I’m hit with PEM.
I use D-Ribose daily but it only help with energy to the brain and not to the rest of the body. L-carnitine has a positive effect too.
I’ve tried reducing carbohydrates in my diet and add more protein and fat but it doesn’t have any effect on energy levels. Only the coca cola does. Without it I get more hangover symptoms and more aching feeling within the cells like they miss something essential to function properly
I hope my description make sense because I’m convinced that I can reduce my ME symptoms more if I can optimise the metabolism a bit more
What about the caffeine in Cola? Caffeine definitely reduces my ME/CFS symptoms. Sugar will for a short while but then I feel depleted. Whatever it is congratulations on finding something -even if it’s Coca Cola – that helps 🙂
Caffeine is also a vasoconstrictor, so would be expected to improve low blood volume effects in cfs, and act upon orthostatic intolerance in varied ways.
Ha! I didn’t know that….Very interesting. I am remarkably sensitive to caffeine. Just a little bit has significant effects.
Caffeine is also a modest or better bronchodilator, making you breath more easily. We can use better breathing.
And it’s also an anti-oxidant (but don’t expect very much from it as quantities may be fairly low).
Cort have you tried using coffein tablets? I tried it a few times but didn’t test it properly because I didn’t noticed any immediately effect.
I have a feeling that I get an effect when coffein is combined with something else but I can’t seem to figure it out.
The coca cola is a combi of coffein and sugar. A regular soft drink dosn’t give the same effect but not sure if it is because of the coffein or the amount of sugar?
The aspirin I use a lot contains 50 mg coffein + 500 mg acetylsalicyl acid
and sometimes coffee works, also containing coffein
And a mix of the three can get me going if I want to do something to
On top of that I can sometimes generate energy just by doing something like go out dancing. The first 1/2-1 hour it’s quite difficult and then I notice that my body somehow have an hitten energy sorce. It’s not a massive boost but it is notiible. I struggle to do anytimng that raises my pulse but sometimes I can keep on dancing for hours if I keep an eye on the pulse and also get a boost form the music and friends. Not sure if my body is using lactate as an energy sorce? It’s more then just endorphine
As long as I don’t get to sleep. I can come home late at night feeling full of energy, have a clear mind and much less pain. If I go to sleep I sometimes wake up with almost no effect and most times I wake up feeling like being smacked in the head with a hammer. Like the brain and body is toxicated in the sleep.
Depending on what country you live in, the sugar in coca-cola will probably be different.
I heard that in the USA fructose derived from corn is widely used in food and drinks. In Europe, sucrose which is a di-sugar composed of one molecule of glucose and one molecule of fructose, is the dominant sugar used.
=> It can be interesting to search and see what type of sugar works best for you. If it’s fructose, then ripe apples might do well too. If it’s sucrose, then honey may do well.
Do you by chance spread your cola intake well over the day? That *could* to some extent reduce high blood sugar spikes.
As Cort says, caffeine can do a lot too. I even make coffee to drink before sleep and when I wake up. Makes me sleep better :-). If sucrose is the thing you thrive one, you can even add half a coffee spoon of honey to it. I did so a long time before I learned full rice crackers work even better for me.
If it’s fructose you thrive on then a bit of agave or ahorn syrup might help; those are mainly fructose if I recall well. Could help you better through the night. Just be modest in amounts, half a coffee spoon can do a lot!
Forgot to mention: I suggest honey or agave syrup dissolved in water because it will contain more minerals, enzymes and vitamins compared to cola.
The very high acidity of cola may help with digestion though, but then again the gas in it may or may not be troublesome.
OMG Coffee before sleep!
Just another instance in which ME/CFS breaks the mold. 🙂
I didn’t use to be able to handle caffeine – it would get me going but would cause gut cramping and jitteriness later. Something must be going right as that doesn’t happen anymore.
Yes, but low doses of coffee in a large amount of water. I use about 4 gr of coffee per cup of 200 ml. But I also tolerate stronger doses before sleep.
I seem to have a high tolerance for the negative effects of stimulants like coffee (and adrenaline) so I can have higher doses before side effects overwhelm beneficial effects.
An important effect of coffee at night is improved breathing IMO, something that easily gets (very) troublesome with me. In fact, it may well help reduce needs of my body producing adrenaline (an even stronger bronchodilator) so it does in fact sometimes calm me (at low-ish doses).
I live in Denmark/Europe so the cola must contain of mainly Sucrose. It’s quite interesting about the sugars you mention. I’ve noticed that I have a tendency to eat more ahorn syrup and honey and still get no sugar spikes from them. So if I eat/drink cola, ahorn syrup and honey I don’t get blood sugar spikes but if I eat candy/chocolate I often get effected. And they don’t effect my digestion like other sugars do.
I usally drink the can of cola at 3-4 pm, in one go. I try every day not to but mostly the achiness won’t go away without it and I get more out of the day after the drink. The time of day can be influenced by the LDN I take in the morning. It lowered my blood sugar massively in the beginning that I almost fainted several times because of the drop. I take LDN twice a day (9 am + 9 pm) but I sleep to deeply to notice any blood sugar effect in my sleep. The cola I drink 4-5 hours after LDN so it could be related.
The cola is an easy way to get both coffein and a masive amount of sugar. Won’t it be quite difficult to get the same amount from honey?
Same with coffeine. I’m almost immune to coffeine except in the morning. Can’t tolerate coffee in the morning well but after noon I can start drink coffee and the later the better. I can easily drink 4 cups of coffee at 3 o’clock in the night just before getting to bed with no coffeine effect and sleep very well and deep. Sometimes in the evening it feels like the cells in my body is sucking up the coffee. That said the coffee is instant coffee that I think contain less coffeine then regular coffee.
I somethimes make my own energy drink before going out from drinking a can of cola, a cup of coffee and add a headache pill that contains 50 mg coffein and 500 mg aspirin (acetylsalicyl acid). Again I get no blood sugar spikes and very rarely any coffein effect but it helps my have a night out. A few times I got a bit of shaky hands, rapid heart because of the coffein but it’s very rarely.
@Tove: my fault,
Most but not all agave syrup is dominant fructose en.wikipedia.org/wiki/Agave_nectar:
“The carbohydrate composition in agave syrup depends on the species……”
Ahorn syrup is more properly called maple syrup in the US and that is dominantly glucose en.wikipedia.org/wiki/Fructose, see table: 1 fructose to 4 glucose.
“I eat candy/chocolate I often get effected. And they don’t effect my digestion like other sugars do.”
Candy is made to be sweet and fructose is almost twice as sweet as sucrose so it may be high in fructose.
“And they don’t effect my digestion like other sugars do.”
=> It seems to read candy and chocolate don’t effect your digestion, but I suspect you meant to say they do effect it more, right?
If so: fructose and chocolate (having sort of “prebiotic components”) might trigger bacterial problems. If those are bad for digestion: investigate fructose + FODMAP intolerance.
Tove, do you drink the Coca Cola before exercising? Or when? What about getting the specific amino acids Edie talks about above? Edie, what is the name of the compounding pharmacy? Dejurgen, I did catch that you think there would be some kind of overload with taking amino acids. I have a good Super 8 Aminos and it doesn’t seem to help at all.
I think a diet high on animal protein like plenty of meat and cheese may create additional nasty waste products from high protein consumption. Having a small amount of supplements is another story.
I believe the body actively tries to reduce energy production. So trying to “force” another pathway into higher gear may just cause the body to work even harder to reduce energy production. See my comment to ZeroGravitas / Richard Lewis above on it.
Tracey I drink it almost every day just to get less ME achiness but I also use it in combination with coffee and coffein aspirin to make a pre activity energy drink as way to get out if I have to even if it is a bad day.
By exercise I mean “exercise” because I can’t realy do any but because I live on my own and in a top floor apartment without a lift I have a lot of stairs.
I sometime choose to do activities that I know I’ll crash from but they bring positive life quality and keep my mind positive too.
A few times I go out, do way to much activity and don’t crash like expected.
I normally meet ME patiens who are intolerant to sugar and coffein but I use both as a sort of daily supplement
Interesting; Coca Cola helps me too, but I try not to depend on it. Indeed, hoping the placebo effect would work, I switched to Caffeine Free Diet Coke — only when desperate — and it helps with overall sick, weak, nauseated, dizzy and headache; but not with back or muscle or neck pain.
I wish someone in the research of all this, would add a test for heavy metals in our cells. Because having these (H/M) can cause alot of the problems. I know in our case, it is. (3 in family with this)
Shouldn’t researchers be looking more closely at the CCI/brainstem hypothesis given the recent and complete recoveries of Jen and Jeff?
I agree with Steve. Stopping glycolysis at the start sounds intentional. Like a control mechanism that would be part of the freeze response. Our bodies could be playing possum. Consider the shallow breathing problem of CFS patients. That is part of the freeze response. Along with a shift towards anaerobic metabolism perhaps. And in a long-term situation with low blood volume could lead to a chronic hypoxic state at the cellular level. With lower oxygen the metabolism would adjust, and muscles weaken, making strong breathing more difficult. That would reinforce chronic hypoxia and possibly handicap the CORI cycle. Which means less glucose can be recycled. Across the long term, if this logic is correct, the freeze response could be self reinforcing, rapidly leading to dauer state perhaps.
Of course this model does not address the cause of a chronic freeze response. But that would be the next mystery to solve.
I think chronic freeze is a reasonable hypothesis. It seems to match many recent findings.
Oops I meant to put that prior comment under Steve’s comment.
Hopefully they will bur not Neil McGregor. McGregor is a metabolomics researcher – so that will be his area of contribution.
You bring up an important point, though. Because most researchers don’t read patient produced blogs most will NOT know about the CCI/AAI issue and the remarkable recoveries.
The only way they’re going to find out about them is if someone writes them us in a case report and submits it to a journal as Peter Rowe did with spinal stenosis and ME/CFS or alternatively does a study. The case report is the easiest and quickest way to get the information out to the research world. Let’s hope one of the doctors associated with their cases is doing that.
The doctor that treated Jen for the central apnea / partial Ondine’s would be ideally suited for this – but who can say if he would do it? Seems that it could get published easily enough given the dramatic reversal of symptoms and novel nature of the “cure”.
All this is nonsense. ME is autoimmune or infectious brain disease. It has nothing to do with mitochondria or ATP in the cells.
Nice, care to present some evidence so we all can attain this knowledge?
I am skeptical like Radomil. It’s like major depression used to be confused with CFS. I wonder if Jen never had CFS, if all her symptoms were eradicated with the surgery.
“The fix to all this requires identifying the problems with glycolysis that are setting all this off. McGregor reported he is attempting to do that now and hopes to report back in the fall.”
He might find various “fixes,” but unless he goes straight for the jugular and stares into the vaccine injury/poisoning rabbithole he’s likely not going to have anything authoritative in the fall.
No wonder Dr McGregor scowled at me when I told him about “Mold at Ground Zero for CFS” at the Open Medicine Foundation symposium last September.
He’s got his own ideas and doesn’t want any “actual evidence” to interfere with them.
This is why so-called “CFS researchers” are so confused. They want to make sure that nothing gets solved if it isn’t their own theory.
https://www.youtube.com/watch?v=-EXxnhRm4CA&feature=youtu.be
Mystery Disease hits three teachers
I would like to thank you all for your comments, both positive and negative. In research we must follow the evidence. Amino acid depletion is a secondary response and not Primary. It occurs as a result of the underlying disease issues. Taking an amino acid supplement will only work for those who have that issue. Connective tissue turnover (collagen, hydroxyproline and P5C) are simply part the bodies mechanisms to provide amino acids. Those subjects with Joint hypermobility syndromes such as Ehlers danlos syndrome have defects in the ability to provide this amino acids from this source. They have their own metabolome signature with it own biomarkers. Fall is only 2 months away.
Thanks for taking the time to communicate Neil. Looking forward to September and finding, hopefully, another puzzle piece that fits. It does feel like things are starting to come together. May it be so!
I greatly appreciate your research and I greatly appreciate your reporting it, Cort. If possible, I would like to have the same testing done on myself that you are doing in your research, at my expense. Please contact me at suekmurphy@cox.net if this is possible. Thank you
Thanks doctor for all your good work for our community. It is very much appreciated!
Your work provides insights in what was hidden so far and allows us to better base our opinions on facts then on assumptions.
Thanks for your research, Dr. McGregor.
Will you be presenting information specific to Hypermobility and metabolism in the fall? Is there any existing literature that points to this that you can direct us to in the meantime?
Looking forward to following more of your work.
Dr. McGregor/Cort- just wanted to check in on the Fall 2019 event that was mentioned in Dr. McGregor’s above comment. Looking back, I didn’t find any presentations by Dr. McGregor at the 2019 conference and I’m looking for more info specifically on collagen breakdown/EDS related to these metabolic issues. Any links or references are appreciated. Thank you.
ALL the pathogens/injuries/trauma that are hypothesized to be the trigger for our illnesses, WOULD NOT be the triggers if we weren’t being experimented on with toxic injections of poisonous sewage at birth.
We’ve got ZERO chance of EVER uncovering this reality if nobody will shout this from the rooftops and stop the fully operational Genocidal Eugenics Agenda that’s supported by every human being from the Deep State spooks, to the entire population of the unwashed masses.
It’s a foolproof Total-Control strategy that will forever foreclose Truth from ever seeing the light of day to inject every human being with a crapshoot roll-of-the-dice concoction of poisonous sludge from the moment we are born.
It’s diabolically brilliant: there’s objectively ZERO control subjects, and there never will be:
WHEN IT IS IMPOSSIBLE FOR CONDITIONS TO EXIST TO CONDUCT EXISTENTIALLY USEFUL & MEANINGFUL SCIENTIFIC INVESTIGATION, OBJECTIVE TRUTH WILL REMAIN FOREVER ELUSIVE IN THE ABYSS.
Fantastic quote.
WHEN IT IS IMPOSSIBLE FOR CONDITIONS TO EXIST TO CONDUCT EXISTENTIALLY USEFUL & MEANINGFUL SCIENTIFIC INVESTIGATION, OBJECTIVE TRUTH WILL REMAIN FOREVER ELUSIVE IN THE ABYSS.
Nice attempt, could hardly produce a more conspiracy theorist writing even if I tried.
Thanks for the information on the exosomes. Ron Davis has suggested they could be it and Maureen Hanson is taking a deep look at them as well. I had never heard of them until last year.
I have often wondered whether vaccinations have been a cause of my ongoing health issues, ones that have plagued me throughout my life. I was finally diagnosed with CFS in 2000. I was in the cohort that was probably injected with the tainted polio vaccine between 1955 and 1963. And as there are no control groups in this “universal vaccine experiment”, I am astounded that researchers across the board do not take such things into account when devising their theories and hypotheses for CFS/ME/FM, etc. I agree with Smelly Melee that this should be shouted from the rooftops!!! It is hardly a conspiracy theory. Mercury? Aluminium? Formaldehyde? etc, etc…….
Are you serious??!!
I have never been more so!
Thanks Cort for such a clear explanation, yet again, of a very complicated issue.
This fits exactly with what I have experienced. Twelve years ago, and then again 4 years ago, I had my metabolites from Krebs Cycle and others tested at lab we have in Ottawa, Canada called Nutrichem. The owner and head honcho of this place is a Biochemist who is way ahead of current research. He believed ME/CFS to be an issue with glycolysis years ago and was able to demonstrate it in his Body Biochemistry Analysis in which 40 plus metabolites in the urine are measured.
My results, both times ,showed extremely abnormal amounts of metabolites of glycolysis. Either very low or very high amounts of various metabolites in the Krebs Cycle and other cycles such as Urea. So each time I read reports of the latest research and hypothesis I get very excited since I personally can relate.
I am so eager to find out the fix, as are we all. For now I take supplements that alleviate the deficits but it is very costly.
Hi Claire,
I live in belgium. Would it be possible to send my urine or other needed specimens to send it to canada to as I understodd it well nutrichem?
are you feling (can do more, sleep, lymphnodes, etc) really a lot better with the supplements?
thanks!
What supplements do you take Claire, and you do find them helpful?
Claire, are the supplements sold by the same lab that does the tests? Are they willing to let you get an Rx to go to an independent compounding pharmacy to get them?
Hi Claire,
I did google Nutrichem and found a clinic/pharmacy in Ottawa. Is this the place that did the testing for you?
This test that you had done sounds like it would be of help to me given some of the symptoms I struggle with and I would like to ask my naturopath about it.
Any input would be appreciated.
I think your talk of “hypermetabolic” states may be a little confusing for some. Given Naviaux described ME/CFS as hypometabolic and dauer like.
I don’t *think* there’s any contradiction here, is there? It’s *just* the anaerobic metabolism that is hyper-active. Reduced glucose metabolism causing (presumably?) the observed amino acid depletion. And all the other types of lowered metabolites too…?
Ha! It’s confusing to me – thanks for providing a possible explanation. 🙂
Being very simplistic but is McGregor trying to actually solve this problem with glycolysis and fix it?
Maybe both are right:
I believe our metabolism to vary far more then that of healthy people.
That is a lot more variation in time (day versus night, morning versus evening, after rest or after exercise / PEM) and a lot more variation inside the body itself.
Cells as close as a few mm apart or less could see vastly different conditions to live and work in depending on where they are connected to the blood flow. Being located at the beginning of a wide capillary would see very different blood flow, oxygenation, blood sugar and protein levels, amounts of hormones and signal chemicals, waste levels, oxidative stress… compared to cells at the end of a narrow capillary IMO.
Also, different organs seem to have vastly different activity levels. Our livers for example seem to go at 200% of normal metabolism all of the time, while our muscles…
Both Naviaux and McGregor can be right at the same time because metabolism is the sum of anabolism (building up) and catabolism (breaking down). I believe Naviaux describes a hypo-anabolic state and McGregor describes an hyper-catabolic state. Combining the two would spell disaster (or ME/CFS)
@Moira – Yeah, thanks, what I’m thinking. 🙂
@David – Well, it sounds like various researchers are looking for the serum factor knocking out our energy metabolism. I think cort was talking about Naviaux (and Ron) looking at ‘exosomes’ (intercellular signaling envelopes of cell contents), which sound very promising to me. It’s a new and rapidly developing field for all of medical diagnostics/treatment.
@dejurgen – Interesting thinking about the degree of cellular variability. You get this from any particular source?
For sure our bodies are performing a kind of triage with nutrients and energy allocation to organs, this has been the thinking of ME/CFS practitioners (like Dr Myhill), etc, for some time.
But on a cellular level… Maybe it *is* important to move beyond thinking of our bodies/tissues as a kind of uniform soup. In a similar that it was essential to chemistry understanding for the ‘plum pudding’ model of the atom to be superseded by thinking of electron shells.
Cort was writing about tissue anaemia with reduced cellular iron uptake/content, right? And small blood vessel pathologies have been implicated for various symptoms in the past, too…
“You get this from any particular source?”
No, from the combination of:
A) Many / ?most? ME patients have reduced blood volumes
B) Most / ?near all? ME patients have reduced blood flow
C) Blood is a fluid where smaller speeds lead to a greater viscosity. In difficult speak: blood is a non-Newtonian fluid (due to RBC being quite big compared to the capillaries they have to flow through, it is like trying to pump soup with meatballs through a small pipe).
A) and B) lead to reduced blood flow in all blood vessels.
C) decreases the speed of blood a lot further in the tinniest blood vessels.
=> This leads to a disproportional reduction in volumetric flow in the tiniest blood vessels compared to the bigger ones due to physics IMO.
=> This in turn leads to the cells provided by the tiniest capillaries to get a very big portion of the reduced blood flow (nutrient supply and waste removal) seen in ME, while the cells provided by bigger blood vessels are far less effected IMO.
I’ve written a few times on this topic somewhere on Healthrising before. The concept is me applying physics and knowledge of blood being worse then water in that respect.
(Physics is heavily underrated in this systemic disease IMO).
So many interesting possibilities. My understanding is that Maureen Hanson, Prusty and the Canadian dynamo – forget his name – are also looking at exosomes.
Did McGregor use the word “malaise?” I believe that it is a huge mistake to connect that word to this disease. Malaise–as most people understand it— is “feeling of general discomfort, uneasiness…(Wikipedia).” That’s trivial compared to the very real symptoms of muscle weakness, pain, etc., described in your wonderful blog. Prefixing an “M” to the “PE”, as in “PEM,” doesn’t change that.
This is a good point. PEM should really be rebranded. Perhaps postexertional flareup. PE-FU.
How about adopting the same language as MS: viz., relapse. Or does that have too many pejorative connotations.
Cort, your site is a breath of fresh air in the muck of life with CFS. The more physical activity I try to do, the more I lose muscle weight. Not one person in my life understands this, and most don’t believe it, even though they know I was a successful multi-sport athlete before illness and this crap dragged me down. I am physically active every day. I push to the edge of my limits, in pain and “spatial discomfort”. I get relatively little done compared to my former self, but I will never believe for a moment that deconditioning causes the “self-cannibalism” that is going on.
Another multi-sport athlete taken down. (I know what its like.)
If you had told me decades ago that I, a very athletic (if I don’t mind my saying so) and physically fit person, would spend most of the rest of my life unable to engage in even minimal exercise, I just wouldn’t have believed it!
Stories like yours Debs are why I think if only people really knew what people were like pre-ME/CFS and then afterwards they would get it! This disease is going to shake up the medical world some day…I truly think it is going re-arrange the medical world’s thinking – not just about ME/CFS but about how the body works.
Hopefully that will come sooner rather than later 🙂
Thanks!
I’m not sure trouble with sugar is the problem. If I don’t eat for over 3 hours, I start to get shaky. But if I eat sugar (e.g. an apple) I am back to my normal20 minutes later. I think I have trouble with fats though, as that does not seem to help too much. Protein either, for that matter. If I eat only nuts, I still seem to need more, namely carbs.
I am losing muscle mass tho. Not sure if wasting from not moving, or if my body is using muscle as a source after all.
FWIW
the sugar “restores” you because its giving you a spike in sugar again. eating an apple, rather than drinking apple juice, is a good idea, though, because the “bulk” of the apple (sorry, I’ve temporarily forgotten the word) helps spread the effect of the sugar out a little longer than if you just drank the juice.
but doesn’t that go against the theory here that we can’t handle sugar metabolically? I don’t need to turn to muscle, as the sugar is utilized. And when I am low, muscle utilization does not get me feeling my normal CFS-like.
Fiber is the word that escaped me.
From amino-acids biochemistry and from experimenting on myself:
a) Positive effects when supplementing in BALANCED amounts:
glycine, serine, methionine (or preferably SAMe), tyrosine, phenylalanine, tryptophan (or preferably 5HTP).
These aminoacids (or metabolites) taken together in a balanced amount support lipidmetabolism, methylation and synthesis of creatine, heme, taurine, glutathione. Get the balance wrong and side effects will occur depending on where the balance goes wrong (crankiness, somnolence, nausea, jitters etc). B-vitamins ( in their active form) and Minerals need to be in place for supplementation with these amino-acids to work.
b) Negative effects in the long run so AVOID supplementing:
Threonine, BCAA (Valine, Isoleucine, Leucine), Glutamine, Glutamate, Cysteine, Arginine.
The body may be low on some of these aminoacids, however supplementing with threonine and BCAA interfere negatively with the Krebs cycle in long run. Supplementing with arginine interferes with the Urea Cycles and increases iNOS activity (too much ‘bad’ Nitric Oxide). Supplementing with cysteine increases production of H2S. Try to get these aminoacids from whole foods (not protein shakes!!). Glutamine is quickly metabolised to glutamate which the body may have too much of already.
c) Undecided about Aspartate and Asparagine. These will speed up the Urea Cycle which, depending on the level if other amino-acids in the body, can be either detrimental or beneficial.
Thanks Moira for sharing your insights and experiences.
I read “however supplementing with threonine and BCAA interfere negatively with the Krebs cycle in long run.” and wonder what makes you think this.
When looking at figure 6 in http://www.researchgate.net/publication/277979239_Metabolic_profiling_reveals_anomalous_energy_metabolism_and_oxidative_stress_pathways_in_chronic_fatigue_syndrome_patients I see
Threonine, Valine and Isoleucine “injecting” at the succinyl coa point. But so does methionine that is recommended; probably being both essential and needed for glutathione production (if cysteine intake is reduced).
=> Is the perceived problem chances of increased oxidative stress or do you see something else?
Why do you think leucine interferes negatively with the Krebbs cycle? In figure 6 I see it injecting at the same point as lysine, phenylalanine, tyrosine and tryptophan but the latter three you do recommend. Is it because leucine can be converted to glutamate?
Kind regards,
dejurgen
1)Why avoid threonine, isoleucine and valine supplements
Ha! I knew you would zoom in on that sentence as I should have written “Threonine and the BCAAs isoleucine and valine”. Leucine is a whole different story. Propionyl CoA (a metabolite of threonine, valine and isoleucine) inhibits the Krebs Cycle according to the article linked below.
Once the Krebs cycle is thrown out of balance (via inhibition of the enzymes aconitase and alpha-ketoglutarate dehydrogenase by free radicals running rampant) the body would turn to its second option for making succinyl-CoA: valine, isoleucine or threonine. This would deplete these aminoacids and increase propionyl-CoA levels. And according to the article linked below, Propionyl-CoA inhibits the Krebs-cycle even further.
https://www.sciencedirect.com/science/article/pii/S1096719217305322?via%3Dihub
2)Why I do recommend methionine (or SAMe), phenylalanine and tyrosine:
The body still needs succinyl-CoA (to make heme ) and would turn to the 3rd option for making it. It would turn to fumarate as a source for succinyl-CoA via ‘reverse Krebs cycle reactions’. This would deplete GTP and deplete tyrosine/phenylalanine (as these aminoacids can be metabolised to fumarate) which in turn would deplete dopamine. GTP can, if I’m correct, be replenished via ATP so oxidative phosphorylation would be ramped up** which increases the need for NADH which increases fatty acid oxidation (as the Krebs cycle is now partly running in reverse and is therefore not producing NADH). Increased fatty acid oxidation would deplete carnitine/SAMe/methionine. SAMe depletion in turn would deplete creatine.
To maintain proper dopamine, creatine and succinyl-CoA levels in presence of of crappy Krebs cycle (regardless of the source of that inhibition which can be anything including oxidative stress), I combine tyrosine/phenylalanine with SAMe (or carnitine or methionine) when at the same time avoiding threonine, valine and isoleucine in order to avoid increased propionyl-CoA levels.
3)Words of caution
Note that the strategy above puts pressure on GTP levels. Once GTP drops too low the cofactor (BH4) for metabolising phenylalanine/tyrosine becomes depleted and fumarate/succinylCoA production via this route becomes compromised. BH4 depletion will also interfere with tryptophan/serotonine/kynurenine metabolism and to circumvent imbalances in those pathways I take 5HTP, Ribose and Nicotinamide Riboside.
BH4 depletion due to increased GTP utilisation due to ‘reverse Krebs cycle acitivities’ puts pressure on oxidative phosphorylation** (ATP production) and thereby on membrane polarisation. That’s where electrolyte and pH balances become important I believe. I’m currently reading up on the electrolyte channels and exchangers.
**Prof Fisher had an interesting talk at the Emerge conference about finding an INCREASE in oxidative phosphorylation which did not translate in higher ATP levels. I hope Cort’s next blog is on that topic.
“Ha! I knew you would zoom in on that sentence”
I like to learn from the best, and on this topic you sure dig deep!
Thanks for pointing at propionyl-CoA. Read about it before but having different angles and repetitions helps to better grasp the importance, let it sink in and make it active knowledge.
I was only able to scan the paper for contents right now, I’ll have to do a few more extensive reads. However I did find a few things (you probably already know) by using my search skills:
A) Vit B12, a vitamin helping plenty ME patients, is vital to “detoxify” or transform propionyl-CoA to something the Krebbs cycle can use.
B) Odd chain fatty acids are another main source of propionyl-CoA. And that is mainly found in ruminant fat and milk.
C) the amino acids you mentioned of coarse ;-).
B) may be another reason why some people have problems with dairy, next to lactose allergy or intolerance and dairy protein intolerance. But this one would suggest that people who do poor on dairy FAT would also do poor on fat of cow, goat, sheep…
Maybe those are the people that do well on high doses of vit B-12?
Refs:
A) en.wikipedia.org/wiki/Propionic_acid
” In most vertebrates, propionyl-CoA is carboxylated to D-methylmalonyl-CoA, which is isomerised to L-methylmalonyl-CoA. A vitamin B12-dependent enzyme catalyzes rearrangement of L-methylmalonyl-CoA to succinyl-CoA, which is an intermediate of the citric acid cycle and can be readily incorporated there.”
B) en.wikipedia.org/wiki/Odd-chain_fatty_acid
“However, propionyl-CoA instead of acetyl-CoA is used as the primer for the synthesis of long-chain fatty acids with an odd number of carbon atoms, which are found particularly in ruminant fat and milk”
“Prof Fisher had an interesting talk at the Emerge conference about finding an INCREASE in oxidative phosphorylation which did not translate in higher ATP levels.”
Now that research I’d LOVE to see!
Inhibition of the Krebbs cycle and an increase in oxidative phosphorylation (which did not translate in higher ATP levels)?
=> not impossible! smells like the en.wikipedia.org/wiki/Pentose_phosphate_pathway going full overdrive.
The pentose phosphate pathway has two oxidative steps, and the PPP has the name PHOSPHATE in it, so maybe it might be observed as oxidative phosphorylation by Dr. Fischer depending on the applied research method.
Interestingly enough, these 2 steps produce NADPH rather then NADH. This results in no ATP production from these two steps, but essential “resources” for the immune system, gluthathion recycling (and fat synthesis from glucose and fructose, to be avoided when having ME IMO).
A strong increase of use of the PPP alone could make for more oxidative phosphorylation (if observable as such) observed and less ATP produced. Add in inhibition of the Krebbs cycle and IMO a shift in the Krebbs cycle from producing dominantly NADH towards producing some NADH and some NADPH would be in line with these observations and my line of thinking.
To continue my hammering /?nagging? on the importance of the PPP and NADPH in ME, if the body wishes to go flat out on the PPP (at a rate never seen or expected before) if should inhibit the parallel path of glycolysis.
That could yield the following confusion: some people / ?researchers? believe we go way too fast in anaerobic metabolism, others like McGregor here believe glycolysis is inhibited.
Both observations could be accurate enough as shifting from glycolysis to PPP is / requires an inhibition of glycolysis and looks a lot like going into anaerobe metabolism. It requires anticipating it (or going very broad on metabolites) to see the differences in key metabolites IMO.
I don’t expect our NADPH levels to be high as they likely are “eaten away” due to high demand on the spot.
Maybe we start to have enough data from these great researchers to sew the pieces together in a convincing and helpful way?
@Cort if you still read this: you ever asked if metabolics could back up my ideas about oxidative stress shutting down the Krebbs cycle, shifting it towards producing NADPH and fueling up the PPP a whole lot while inhibiting glycolysis; all to reduce oxidative stress and increase much needed NADPH.
Maybe this combo plus the work that Moira and I do on oxidative stress inhibiting the Krebbs cycle (and glycolysis) starts to be your answer?
@Moira: if convinced it has sufficient potential, care to combine efforts to work this out?
Before avoiding all BCFA, here is a paper on the potential benefit of having them http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4381348/:
“These data all point to previously neglected nutritional properties of BCFA that may be important for development and maintenance of microbiota, enterocyte health, skin, and possibly other functions.”
“Note that the strategy above puts pressure on GTP levels.”
I’v largely avoided (the topic of) GTP so far. From en.wikipedia.org/wiki/Guanosine_triphosphate:
“GTP is readily converted to ATP with nucleoside-diphosphate kinase (NDK)”
As we seem to be short on ATP, that will help drain GTP.
But from en.wikipedia.org/wiki/Glycolysis converting oxaloacetate back to pyruvate requires GTP.
And from en.wikipedia.org/wiki/Citric_acid_cycle and the blockage of aconitase in the case of high oxidative stress we see that the Krebbs cycle becomes quite blocked / cut open and more becomes a roundabout with a large concrete block limiting traffic at one point.
So if we would wish to use proteins that enter at the alfa-ketoglutarate or the fumarate point the we would wish there is a good way to get there end products out of this Krebbs-blocked-roundabout. GTP seems to be in need here too.
While I in general am not found of “forcing” the mitochondria to produce energy if the body wishes to block them, both these steps seem to be able to produce anti-oxidants so I’m undecided so far.
That (production of “excess” pyruvate as it cannot enter the Krebbs cycle easily due to an aconitase blockage may lead to normal to high blood pyruvate as observed by McGregor and others while still having poor glycolysis throughput.
In an unlikely but possible way from en.wikipedia.org/wiki/Glycolysis an excessive buildup of pyruvate could be prevented by having (part of) oxaloacetate convert “upwards” consuming ATP and NADH to glucose-6-phosphate for the PPP or even glucose itself to dump in the blood. Don’t know if other cells then liver cells could actually do this.
Hi Dejurgen,
I’m not entirely sure I follow your line of reasoning as maybe I should have clarified that with ‘oxidative phosphorylation’ I mean OXPHOS or cellular respiration.
But I agree that NAD(P)(H) imbalances could be key in ME/CFS as Naviaux’s data pointed towards NADPH and Maureen Hanson’s metabolomics data pointed to a redox imbalance.
The introduction of this linked paper explains how a redox imbalance caused by improperly regulated NAD(H) levels leads to oxidative stress. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4869616/ and the following article explains how oxidative stress impacts alpha-ketoglutarate dehydrogenase and in turn the Krebbs cycle https://www.ncbi.nlm.nih.gov/m/pubmed/16321804/?i=2&from=/23378250/related
The question becomes how to rebalance NAD(P)(H) levels. Would increasing NAD levels while simultaneously reducing NADPH production have a beneficial effect? I think it would but maybe that is a discussion for another time.
Hi Moira,
I likely overreached. With my brain working optimal in flow mode I extended the idea that when PPP usage easily could be mistaken for glycolysis, then that maybe the oxidative steps in the PPP could be attributed to oxidative phosphorylation.
Thereby I assumed some similar trafficking in basic phosphor chemicals would happen. Didn’t check as I’m better at being creative then at being structured, and have better ideas when thinking unstructured. Sometimes helps, sometimes works against me. But checking again there are no identical chemicals used in both pathways. Just oxygen uptake by cells.
But I made a reasoning that would hold for “general oxygen cell uptake without increase of mitochondrial activity. And after some searching I did find Prof Fisher’s presentation: non-mitochondrial O2 uptake was higher in ME lymphoblasts. I think the PPP oxidative steps are a valid and logical source of that one. So maybe still a partial hit?
As to the mitochondria itself: the presentation delivered plenty of new and unexpected data. Fascinating! I’ll have to chew on it time and again.
As to NADH/NADPH I try to up NADPH production and further “imbalance”
it as I see a strong need to it, but yes that’ll be for another time. Thanks for sharing your knowledge!
Just curious Moira – what is your background? Very impressive stuff! 🙂 🙂 🙂
and Yes! Dr. Fisher’s presentation is coming up.
A muscle biopsy paper years ago concluded that there was no muscle abnormality in CFS patients after exercise. It was done to compare PEM to DOMS, but it probably would’ve shown if there was metabolic muscle muscle breakdown. I’ll post it if I locate the paper. Any case, the muscle biopsy should either prove or disprove this theory of McGregors, I would think.
On personal note, I don’t seem to have problem generating enough energy when I’m rested/excited. I got carried away last winter and skied on an intermediate slope for hours. Then I paid for it with 3 weeks of PEM. Now I’m back to the steady state struggling to take 35000 steps a week..
Decreased lactate is a significant problem.
Lactate is formed and utilized continuously in diverse cells under fully aerobic conditions and not just a consequence of oxygen lack in contracting skeletal muscle. ‘Cell–cell’ and ‘intracellular lactate shuttle’ concepts describe the roles of lactate in delivery of oxidative and gluconeogenic substrates as well as in cell signalling. Examples of the cell–cell shuttles include lactate exchanges between between white-glycolytic and red-oxidative fibres within a working muscle bed, and between working skeletal muscle and heart, brain, liver and kidneys.
Two processes are important here: lactate uptake by mitochondria and pyruvate for lactate exchange in peroxisomes. Lactate for pyruvate exchanges affect cell redox state, and by itself lactate is a ROS generator. In vivo, lactate is a preferred substrate and high blood lactate levels down-regulate the use of glucose and free fatty acids (FFA). As well, lactate binding may affect metabolic regulation, for instance binding to G-protein receptors in adipocytes inhibiting lipolysis, and thus decreasing plasma FFA availability. In vitro lactate accumulation upregulates expression of MCT1 (monocarboxylate transporter-1) and genes coding for other components of the mitochondrial reticulum in skeletal muscle. The mitochondrial reticulum in muscle and mitochondrial networks in other aerobic tissues function to establish concentration and proton gradients necessary for cells with high mitochondrial densities to oxidize lactate. The presence of lactate shuttles gives rise to the realization that glycolytic and oxidative pathways should be viewed as linked, as opposed to alternative, processes, because lactate, the product of one pathway, is the substrate for the other.
There is little or no difference in lactate production in trained and untrained men but in trained men the arterial clearance of lactate is greater/increased as a result of training.
When arterial lactate rises during exercise, it becomes the predominate fuel for the heart, decreasing relative use of other energy substrates. If lactate does not rise during exercise or during increased activity then the response can be increased heart rate. In this sense lactate is also a signalling molecule.
Interesting!
Hi Ian,
You have some interesting points on lactate. I’ve been wondering about something you discussed for a long time however:
“lactate uptake by mitochondria”
=> Do you have any clear pathway as to how lactate is metabolized by the mitochondria? It is often discussed that mitochondria can metabolize it but I have never seen a specific complete pathway. If you could point to a reference with a schematic and further information that would be great.
Kind regards,
dejurgen
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0002915
I dont’ think it is known where in the mitochondria lactate oxidation is occurring. One possibility is that lactate is oxidized by LDHB located in the intermembrane space. Such compartmentalization of LDHB may facilitate substrate channeling. If LDHB is positioned near a mitochondrial pyruvate transporter for example, this could decrease local concentrations of pyruvate and therefore help promote lactate oxidation. This has been proposed to constitute a “lactate-malate-aspartate shuttle” in which lactate oxidation is coupled to the malate-aspartate shuttle in the intermembrane space to translocate reducing power to the mitochondria.
An alternative possibility is that lactate is directly transported across the mitochondrial inner membrane and oxidized by LDHB in the matrix. But you probably know this. Sorry I cannot provide you with a detailed biochemical pathway, it is just that evidence for its occurrence in many cell types is available.
This is enlightening. I knew lactate is formed under all conditions. I didn’t know that it was a preferred substrate ! Or that it can play a positive role in mitochondrial muscle functioning. It does make sense given that dose makes the poison in so many instances: at the appropriate levels a substance can be beneficial while at higher levels it can be problematic. The body seems to strive to make use of every substance it can.
“gives rise to the realization that glycolytic and oxidative pathways should be viewed as linked, as opposed to alternative, processes, because lactate, the product of one pathway, is the substrate for the other.”
I learned that
“lactate exchanges between between white-glycolytic and red-oxidative fibres within a working muscle bed”
should mean:
Fast muscle fibers produce excess lactate and donate it to slow muscle fibers that can use it as fuel.
Now that presents a problem to some extent IMO:
The most easy way to transport lactate from one muscle cell to another would be for the fast cells to dump it in the bloodstream and for the slow fibers to pick it up.
But that poses a problem: with blood flowing relatively fast in one direction that would require the fast cells to lie upstream from the slow cells in order to get the lactate in the wright direction. But cells downstream the finest capillaries may see poor blood conditions with plenty of metabolic waste and less oxygen. So these cells may not be the best positioned to oxidize the lactate.
My gut feeling says the cells closer to the hart (in the direction of flow, upstream) would be in a better position to use lactate as a source for oxidative ATP production, while the cells downstream seem to be better located for anaerobic pyruvate consumption.
Moving lactate between adjacent cells may seem an option, but that would be limited in range a lot as per cell traveling 2 cell borders must be crossed and diffusion within the cytosol must happen for each “hop”.
=> Would the fascia with it’s liquid take that role as transport medium? If so, fascia quality, density, structure and connective tissue disorders would impact flow and distribution of lactate as an energy source quite a lot.
=> The same concept could apply to the brain: would the cerebral spinal fluid help to distribute lactate? journals.plos.org/plosone/article?id=10.1371/journal.pone.0002915
“so the presence of both MCT isoforms could provide metabolic flexibility across the physiological range of arterial and CSF lactate concentrations.” seem to say so.
=> If so, flexibility and adequate pressure in the spinal cord and spinal fluid brain bag plus optimal position and flexibility of spine and neck plus the appropriate breathing techniques should optimize the distribution / stirring of lactate in the CSF quite a bit IMO.
If lactate would be that good a source of energy then that would clearly hint to why reducing blood pooling is quite important in FM/ME.
Pooled blood would likely be full of lactate but poor of oxygen. So the lactate couldn’t be used as a fuel.
While reducing blood pooling has already shown it’s importance in reducing hart prefill problems and reducing too high acid accumulation in tissue with poor blood flow, it now seems it would also hold potential to provide a boost in fuel for large parts of the body receiving sufficient oxygen.
And ME research shows that “we have the oxygen (at rest) but our cells can’t seem to use it”.
As written in this blog, McGregor sees problems with too few of glucose during the night (and too high in the morning, rebound hyperglycemia), too high levels of stress hormones during this shortage and “pillaging” of proteins / muscle tissue, too few lactate in the morning (as measured by tapping blood from a main vein)…
Those nightly problems seem all to be eased by doing some nightly exercises to reduce blood pooling (and sitting upright for some time a few times a night). It not only improves blood flow, but mobilizes a “stuck” fuel, lactate, that can easily supplement the shortage of glucose at night while at the same time reduce local problems of lactic acid acidosis.
By doing so, one would need less stress hormones like cortisol and (nor)adrenaline, allowing the adrenals to take a break and heal over time if needed.
Also, the lesser fluctuations needed in insulin (with lesser need to “create” peak glucose by cortisol also comes lesser needs to “create” peak storage or peak insulin) should over time alleviate insulin resistance a bit. Insulin resistance is believed to be increased in ME patients even if non-diabetic.
During the day as well, making lactate better available in the blood stream (and fascia and csf) by reducing its pooling would allow for the combined amount of glucose + lactate fuel to be bigger. That should result in a combination of:
* later onset of glucose shortage / protein pillaging when exercising
* less need to have constant elevated blood glucose levels making blood thinner and increasing blood flow by doing so. Reduced blood flow is common in ME.
@Ian
Thank you for bringing this up! I was aware of the importance of the malate aspartate shuttle but was not aware of the importance of the lactate-malate-interactions. Looking up information about this topic in astrocytes led to finding a link between lactate shuttling, membrane polarisation and calcium shuttling. Article linked below. Calcium channel functioning in ME/CFS is a topic of research in Australia
@dejurgen and @Ian
For a diagram, see figure 2 in this linked article. Dejurgen, is this what you were after? Ian, does this diagram summarise your comments about lactate?
Sorry forgot to add the link. Here it is:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6028533/
Hi Moira,
I tried to read it in several goes, but it’s too much arcane science for me now. My strengths are at the physical and system level.
Biochemistry is very challenging to me but I dab into it because I wish to asses it’s impact on the system behavior.
Having learned that there indeed is solid evidence that many cell types can consume lactate does allow me to better estimate the cost of anaerobic energy production as not all needs to be reconverted to glucose by the liver.
It learned me the importance of freeing up pooled lactate at times of glucose shortage, something of practical consequence.
I also learned that white and red muscle cells play different roles in energy production and this seems to lead to an important role of the fascia in lactate / energy transport. Phil will like the idea ;-).
Something similar seems to happen with the CSF but it seems more limited in capacity. I’ll have to find good estimates on the percentage and distribution of in brain (as opposed too in the CSF bag) extracellular fluid in order to get a better idea of it’s importance, role and potential handles to influence it.
I’ll revisit the topics of MCT, MAS (and it’s potential vulnerability to ROS) and calcium pumps but it’ll take plenty of time and rereading to have sufficient understanding of it in order to put it to use. But please keep pointing me to where it links to other ideas in the future. That’s how I learn: by understanding little link by little link I start to grasp these basic mechanisms (like the role of calcium pumps) little by little.
Thanks Ian and Moira (in order of which the information appeared to me).
When understanding the importance of lactate and the Lactate-Malate-Aspartate shuttle and combining it with McGregor’s research I did find a potential vulnerability to energy production.
It combines following thoughts:
A) McGregor: we seem to have shortage of glucose (availabillity) at times (night, exertion) and our bodies turn massively to proteins for energy during those times.
B) Ian and Moira: Lactate is a key component in oxidative phosporilysation.
C) The lactate-malate-aspartate shuttle is essential to B)
D) McGregor’s paper “Metabolic_profiling_reveals_anomalous_energy_metabolism_and_oxidative_stress_pathways_in_chronic_fatigue_syndrome_patients” (real link will be blocked by WordPress):
“blood metabolites that were significantly decreased, including glutamate”
Fig 6: glutamate can covert into aspartate; aspartate can enter the Krebbs cycle after converision into fumarate.
E) en.wikipedia.org/wiki/File:Malate-aspartate_shuttle.png: aspartate and glutamate are key components of the lactate-malate-aspartate shuttle
F) during “amino acid pillaging” plenty of ammonia will be created, strongly increasing the need to turn up the urea cycle and en.wikipedia.org/wiki/Urea_cycle: the urea cycle consumes 1 amount of aspartate per amount of NH3 to “neutralize”
=> that could consume massive amounts of aspartate / glutamate (to make aspartate) during a “amino acid pillaging action”
=> These often occurring moments of glucose (availability) shortage seem to initiate “amino acid pillaging”
=> These seem to create high peaks in toxic NH3 production
=> These seem to (temporarily or more long term) drain aspartate and glutamate stocks
=> this potential drop seems to impair (speed of) the essential lactate-malate-aspartate shuttle, impairing oxidative respiration speed (a lot)
=> this may further decrease glucose availability to the mitochondria
as a side, interconversion of arginine to glutamine may decrease arginine levels; this in turn may decrease NO produced constricting veins and further reducing tissue glucose (and amino acid and oxygen supply)
=> please share any thoughts on this idea!
Kind regards,
dejurgen
I would like to see a small cohort of researchers:
sending the Krebbs cycle in reverse ( and, separately, other energy cycles too) to flesh out whether commonly held understandings (and/or) past assumptions on how cycles work are fully correct— ——/or if some small additional factor is hiding in plain daylight (that is an additional regulator (would likely need the computational engineering analysis grinding it down to find this)
without metabolomics, these would remain invisible
would be nice if testing could be done on small numbers of still ‘live’ cells ———(a cfs/me) person’s various organ tissues——-to see how eachs’ micro-metabolites function.
akin to work already started with blood cell differences in ‘non-ill plasma vs ill-plasma
Rambling, and perhaps not of use, so thank you for not posting this if so Cort.
wonder if small needle biopsy or flush could provide ‘live’ cells for this.
’
also perhaps unknown relationships to temperature as rate limiting?
may show a difference for cfs/me than in ‘normal’ subjects.
I think one good thing is that this area is being explored so much more now -and everyone seems to find something. I wish we could have a 200 person study which explored every aspect possible of glycolysis and Krebs Cycle…
@Ian:
Thanks for the information. I’ve read some hypothesis but this paper is a clear and founded one.
While I’m still trying to grasp some concepts, my eye caught some potential interesting points:
I learned quite a bit more about the lactate malate aspartate shuttle and the malate-aspartate shuttle.
From the top left diagram picture in en.wikipedia.org/wiki/Malate-aspartate_shuttle I spotted a potential vulnerability:
Oxaloacetate (just like but even more then a-ketoglutarate) is vulnerable to high levels of oxidative stress. http://www.sciencedirect.com/science/article/pii/S0006291X11021644: “Oxaloacetate was approximately as effective as pyruvate in decreasing H2O2 levels and more effective than α-ketoglutarate.”
Looking at the previous quoted diagram and at figure 1 in http://www.researchgate.net/publication/268035126_Lactate_oxidation_at_the_mitochondria_A_lactate-malate-aspartate_shuttle_at_work/download, could that in the case of high oxidative stress in the mitochondria matrix and in the intermembrane space lead to an opposed imbalance in nitrogen/ammonia by creating imbalanced amounts of glutamate and aspartate (by reacting some of the oxaloacetate and α-ketoglutarate away to other stuff in the presence of peroxide)?
Especially in the intermembrane space with fairly small volume that could create quite a meaningful imbalance.
If so, could that decrease the effectiveness / speed of the lactate malate aspartate shuttle?
If so, the IMO likely less vulnerable to oxidative stress en.wikipedia.org/wiki/Glycerol_3-phosphate_shuttle should have to do the shuttling mostly by itself, limiting maximum metabolic rate and decreasing ATP per glucose by 2 ATP. That would combine lost speed with lost efficiency.
It sounds similar to the decreased maximum throughput and decreased efficiency (“ATP lost”) observed by Prof Fisher’s presentation at the Emerge conference (hint from Moira).
Chances are I miss some biochemistry basics, but asking questions is key to further understanding.
Have a look at this recent paper by Mazat. It has some nice pathway diagrams.
https://www.mdpi.com/2218-1989/9/5/81/pdf
Thanks for the link!
It was a difficult read but if I get it correct I learned quite a few things from it:
* amino acid consumption in the tissue leads to ammonia formation, not uric acid
* ammonia can be converted to either urea or aspartate but (near?) only in the liver
* high burst of amino acid consumption should increase ammonia at the tissue site a lot. Hyperammonia inhibits mitochondrial respiration, the ETC and increases oxidative stress
* during periods of high ammonia production, both aspartate and urea production should increase a lot.
* As the urea cycle consumes aspartate, depending on the different rates of aspartate versus urea production either a shortage, normal value or excess of aspartate could be the result.
* According to figure 4b of the paper you linked, producing so much aspartate to detoxify ammonia should cost quite some amount of glucose but yield very few net ATP. Maybe a variant with fat exists. The route of producing aspartate by glutamine does not make sense during these events as it produces ammonia itself.
* This should cause a prolonged tail of increased glucose consumption following an event initiated by shortage of glucose creating a sort of temporary lock-in.
This model seems to hold potential for use in ME modelling IMO in the hands of experienced researchers. An extension with other amino acids, fatty acid and ketones would be really nice.
Cort, thanks so much for writing this up. This is my experience, over 50 years. Seriously, I was given these tests when I was a teen, even, and failed them completely. I knew that the amino acid tests I requested between 2000 and 2005 would be replicated in people with ME, if only someone would do those tests. This is the research that makes me the most hopeful, for my particular case.
Congratulations, you just figured out what Dr George Watson discovered 40 years ago and wrote about in his book ‘Nutrition and Your Mind’. What Watson discovered was that you could test and sort out whether someone had a slow, balanced, or fast metabolism; which he call slow, balanced, or fast oxidizers. Slow oxidizers were very poor at breaking down glucose into pyruvate, thus the low production of pyruvate limited the energy production from the Krebs energy cycle. Fast oxidizers on the other hand broke down glucose into pyruvate too fast and thus the Krebs energy cycle was limited by the breakdown of fat (glucose + fat, or pyruvate + acetate) is required in the Krebs cycle.
Furthermore, Watson went on to find that certain vitamins, minerals, & foods enhanced the breakdown of glucose, while others slowed it down. Hence, metabolism became malleable – it could be measured, it could be sped up or slowed down.
Dr Paul Eck built on the work of Dr Watson (and others) and found that a hair tissue mineral analysis that measured the levels of the major minerals – Ca, Mg, Sodium & potassium, was more accurate and easier to obtain. He put together a program (after thousands of tests) to restore proper metabolism and called it Nutritional Balancing (aka mineral balancing). Many of use, including myself, have used Dr Eck’s program to improve substantially or recover completely from ME/CFS using natural methods – no toxic medicines required.
The main question is why these researchers don’t build on Dr Eck’s work instead of wasting so much time trying to discover what Dr Eck and Dr Watson knew decades ago?!?
Don’t you think patients with ME/CFS have tried going the route of hair and blood mineral tests and correction of their deficiencies based on the results? Heck, that was the first thing I tried a decade or more ago. If this was solvable through common supplements, we would all know by now.
And so this article does not make any suggestion on what to DO about these problems. Awfully long article for people to wade through, only to find that no suggestions are given.
Really, not even a clue is given in the article on what avenue might lead to help.
It would be nice indeed if the current research had an answer to it – and I promise you if it did – it would be there. I caution you not to expect the answer to come so easily or to be so readily available. (There will probably be several answers). The intent of this blog is to demonstrate that researchers are understanding more and more about this condition. While it is not the answer you and everyone one else wants – it is a distinct step up – and will hopefully lead to an answer.
One possible avenue this research could suggest is a ketogenic type diet – which does help some people.
I have been wondering about this for a while myself. I usually have intense cravings for sweets. I have tried high protein and high fat diets and those haven’t worked for me. May it depends on what the source/ cause of the problem is. Searching for sugar and viruses and stumbled into this.
https://www.theatlantic.com/science/archive/2016/09/glucose-inflammation/498965/
This scientist demonstrated that if the source of the infection then sugar is beneficial and deadly if the infection is bacterial.
Anecdotally I feel better the morning after I have had some alcohol the night before, especially prosseco. But not so much with fruits. I have been experimenting with red bull this week and I do think it helps. I should point out I don’t have a severe version of ME. I have mcas, brain fog, anxiety and if I didn’t cardio I get intense soreness and muscle aches about 48 hrs later but not enough to knock me out. Weights are usually ok.
Other symptoms
Ear ringing
Neck pain and cracking
Food intolerance
Used to have fragrance intolerance but that went away.
Humans lack uricase activity so it is interesting that we can make allantoin. It is made in muscles under stress conditions as a by-product of peroxynitrite* scavenging by uric acid [ PMID 8958141].
*I’m assuming the free radicals mentioned in the abstract are mainly peroxynitrite; I can’t access the article since it is paywalled.
Jan Gruber has been investigating allantoin as it extends lifespan in C elegans worms. Don’t know if would translate to humans since it could be increasing uric acid levels thru product inhibition of uricase, which we don’t have. I’m sure he’s thought of that, though, and still seems interested so there must be more going on there and possibly points some more interesting effects on metabolism. [ Gruber did a recent webinar available on NUS Medicine’s YouTube channel].
I really wish we could get some of these longevity researchers (another would be Brian Kennedy) to look at the metabolomics data for ME/CFS and see if they have any insights.