

This is the third in a series of blog covering the IACFS/ME Zoom conference.
- Oral Rehydration Solution More Effective than Saline IV at Improving Orthostatic Intolerance Pt. I
- Starving for Energy? and The Immune Hole That Produced ME/CFS?- The IACFS/ME Conference Reports Pt. II
Back to the B-Cells: “Immune Dysregulation with Deviated B-Cell Receptor Repertoire in ME/CFS”
It was great to see the IACFS/ME reach across the ocean and get a Japanese researcher, Dr. Wakiro Sato, to present. Japan, which has done a ton of work on chronic fatigue syndrome (ME/CFS), is best known for Nakatomi’s groundbreaking work on neuroinflammation, but it’s done a lot of work on the brain in general.
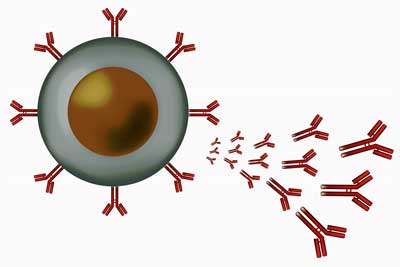
A plasmablast (B-cell) emitting antibodies.
Sato has co-authored two studies on the brain in ME/CFS in the past two years – one of which found that increased levels of antibodies to adrenergic receptors were associated with changes in the brain. All in all, he’s co-authored eight studies that have been cited in PubMed and has probably co-authored dozens more that have been published in the Japanese medical literature.
You might have thought that B-cell research in chronic fatigue syndrome (ME/CFS) was done after the Rituximab study – Rituximab being a B-cell inhibitor – failed, but no. So far as I can tell the B-cell findings in ME/CFS have been up and down, but Sato appears to have produced a pretty strong result.
First he found increased expression of two IGH genes that are involved in antibody production. The genes evidenced something rather rare in ME/CFS: high sensitivity and specificity; i.e. they were great at correctly identifying who had ME/CFS and who didn’t. That’s the kind of precise finding that could, if it’s validated, ultimately result in a biomarker.
Then, an “unbiased comprehensive analysis of B-cell repertoires” revealed oligoclonal expansion; i.e. the B-cells in some patients had started producing large numbers of clones of themselves – presumably to fight off a pathogen. Right off we had two signs of increased B-cell activity, and therefore, possibly a tendency towards autoimmunity.
Sato then found increases in antibody secreting cells or plasmablasts, which was associated with illness severity; i.e. the more severely ill the patient was, the more plasmablasts were present.
That suggested their B-cells might be flooding their systems with antibodies – another possible setup for an autoimmune disease. The more antibodies that are present, the better chance that something will go wrong and our tissues will get attacked.
Next, Sato used a gene expression study to see if the plasmablasts were “tuned” to producing an autoimmune reaction. The answer to that study was yes, the upregulated genes found have been associated with autoimmune reactions.
Finally, Sato reported that he’d found increased levels of the same adrenergic autoantibodies Loebel and Scheibenbogen reported they’d found in some patients back in 2016.
It’s certainly not clear that an autoimmune reaction involving these autoantibodies is happening, but this is the third or fourth study, I believe, to have found that. Sato and colleagues recently found that increased levels of beta adrenergic antibodies were associated with alterations in the prefrontal cortex of the brain.
Japanese studies have suggested that the failure of this center of “executive functioning” in the brain to rein in the limbic system may have profound implications for ME/CFS.
Sato hypothesized that plasmablast expansion in ME/CFS is producing antibodies that are whacking the adrenergic receptors in the brain. Given that, he believes that treatments which knock down B-cells may be helpful. Rituximab is one such treatment which didn’t work out, but others apparently exist.
Could Happiness Help? “Why Patients Improve. Why They Get Worse”
Friedberg’s was more a coping or life situation study than a treatment study. It simply asked what, if any, activities in daily life made ME/CFS better or worse. One of the study’s strengths was that it included a physiological component: along with many questionnaires, etc. it actually measured heart rate variability (HRV).
Studies suggest that HRV in ME/CFS and FM tends to be low – which means the autonomic nervous system is kind of stuck and is not responding flexibly to stressors. Various wearables such as the Oura ring use HRV to advise the wearers whether to rest more, for instance. (It’s not clear how accurate devices like the Oura are …)
Check out how one person with ME/CFS utilized heart rate variability to improve her health.
- Your Crash in a Graph? How Heart Rate Variability Testing Could Help You Improve Your Health
- Heart Rate Variability (HRV) An Underused ME/CFS/FM Management Tool: PT II – Surveying the Landscape
Friedberg hypothesized that maladaptive activity patterns (such as engaging in a ‘push-crash’/ ‘boom and bust’ cycle or severely limiting activity), or higher levels of stressful events or major negative life events (loss of job, a death) would likely reduce HRV and increase symptoms.

Friedberg’s study suggested that having more uplifting experiences might help.
Likewise, things like pacing, uplifting pleasant events (such as positive social interactions or fun/joyful activities) or major positive life events (a marriage, a birth, a new job, a holiday) would increase HRV and reduce symptoms.
It was a large, 6-month study of 125 women, most with long-term ME/CFS (average illness – 16 years). Questionnaires, weekly diaries (charting fatigue, pain, activity levels, and negative or positive events), activity monitoring (using an accelerometer to measure steps) and HRV monitoring, and an interview at the end of 6 months were done.
The results were shocking. I expected pacing, push-crash problems, negative events to get highlighted but no … none of those things were different between the improved group and the non-improved or the worsened groups. Limiting activity didn’t improve symptoms and too much activity didn’t make one more likely to be in the non-improved group.
Instead it was the frequency and/or intensity of positive/ uplifting events that stood out (!). Those patients with a significantly higher frequency and/or intensity of positive or uplifting events tended to be the ones who said they were improved at the end of six months.
People who reported more “uplifts” – very positive activities in the day – tended to be the ones who improved the most. That’s one result I never saw coming …
Some people will undoubtedly be upset to find a study linking something like “uplifts” to improved health. It’s too emotional, too behavioral. Note, though, that Friedberg is not claiming he’s found a cure – the patients were just doing better. At the end of the study, they were still long-term ME/CFS patients.
This finding may make sense in a couple of ways. The hypotheses put forward regarding neuroinflammation and glial activation in ME/CFS suggest our immune systems are on a tripwire – responding to the slightest stressor by pumping out damaging cytokines that produce fatigue, pain, flu-like symptoms, etc. Andrew Miller has demonstrated how an infectious event can sensitize the neurons in parts of the brain that have been associated with ME/CFS.
Plus, many parts of the brain that are associated with ME/CFS and FM – the insula, basal ganglia, limbic system, amygdala and brainstem – regulate both the autonomic nervous and the emotions. It’s likely you don’t get a dysregulation in one without some problems in the other. We also know that the fight/flight system is jacked up in ME/CFS and that the other major stress response – the HPA axis – has problems in ME/CFS as well.
Plus, there’s the lived experience of ME/CFS. For me, having ME/CFS/FM has been associated with difficulty calming down, irritability, difficulty concentrating, lots of fear, etc. My version of ME/CFS/FM is also accompanied by quite a bit of pain – a sure fight/flight inducer.
This is a bear of a disease. Being occupied with it, thinking about it, worrying about it, etc. – that is a heavy load to put on any stress response system – let alone one that’s already been damaged.
Maybe finding ways to bring more joy or happiness or “uplifts” to our daily lives could be helpful. Friedberg’s study suggests that it’s worth a try. .
We have after all seen stuff like this work in autoimmunity. Donna Jackson Nakazawa has severe autoimmune disease – one that twice left her paralyzed for periods of time. She was on heavy drugs, exhausted and miserable.
She was able to dramatically decrease her symptoms, increase her energy, and improve her immune results through a year of mindfulness, meditation, yoga, etc. She still had her autoimmune disease but she was much happier, more functional and healthier.
Mitochondria Maladies: “The Dysregulation of Mitochondrial Function and Fuel Preference in ME/CFS Lymphoblasts”
Talking about stress – Daniel Missailidis’s Australian study suggested that “a persistent mitochondrial stress response” was present. Missailidis is a young, obviously whip smart PhD candidate in Paul Fisher’s group at the University of La Trobe. Still a candidate, he’s already been the lead author in two ME/CFS studies and one review. Let’s hope he sticks around ME/CFS.
At the last EMERGE conference, Paul Fisher, a longtime mitochondrial researcher, reported that he’s been wanting to study ME/CFS for ten years. When he finally got the funds, he made the most of it.
In February of this year, Missailidis and Fisher published a complex study, “An Isolated Complex V Inefficiency and Dysregulated Mitochondrial Function in Immortalized Lymphocytes from ME/CFS Patients“, that will be covered further.
Missailidis and his team put cell lines from 65 ME/CFS patients and 37 healthy controls through the Seahorse metabolic torture chamber. (Beside measuring the oxygen consumption and other metabolic parameters of the cells, one of the things the Seahorse does is metabolically stress cells in different ways to uncover where, metabolically, they don’t measure up.)
As has been seen in other studies, they didn’t find any differences in ATP production when the cells were unstressed.

Among other things Missailidis found was that Complex V was inhibited in ME/CFS – and Complex I were trying to compensate. The end result – a likely inability to respond to energy demands.
The Gist
- A B-cell study from Japan produced several findings (oligoclonal expansion, increased plasmablasts, altered gene expression) which could be associated with autoimmunity. Plus, the study author also recently linked a possible autoimmunity (adrenergic autoantibodies) to prefrontal cortex problems in the brain.
- Fred Friedberg’s large heart rate variability and “life situations” study suggested that having more positive life events might play a larger role than pacing in determining who improves or doesn’t.
- An Australian study found more evidence of problems in mitochondrial energy production. Complex V of the energy production pathway in the mitochondria was inhibited, plus, in what was apparently a compensatory reaction driven by the TORC-1 pathway, activity in Complex 1 was upregulated. Several other problems (non-mitochondrial oxygen consumption, overall oxygen consumption, and proton leaks were found).
- The Australian study also found evidence – as other studies have – that ME/CFS patients’ cells are turning to amino acids for fuel rather than glucose – thus adding another inefficiency to the energy metabolism equation in this disease.
That suggested that Complex I was racheting up its activity – flooding the next complexes with electrons – in order make up for poor performance of Complex V. Plus, non-mitochondrial sources of oxygen consumption were elevated as well – suggesting that the cells were trying to metabolize oxygen in any way they could. All told, the cells were actually using more oxygen than normal (as a proportion of basal oxygen consumption rate) – another sign of inefficiency and stress. Plus, the ME/CFS patients cells were leaking protons.
Further analyses pinned the TORC-1 pathway – one of the regulators of cellular respiration – as the source of the Complex I upregulation.
A look at glycolysis – the part of the ATP production cycle which does not depend on oxygen – found everything normal. However, a shift towards fatty acid utilization (instead of glucose) as a source of energy was found. This was highlighted by the increased degradation of branched chain amino acids or BCAAs. Because BCAAs break down ammonia, one of the potential consequences of increased degradation of the amino acids could be increased ammonia levels.
- See more on the possible ammonia issue – Is Ron Davis’s ME/CFS Collaborative Research Center at Stanford Poised for a Breakthrough? – Health Rising
This metabolic shift – from glucose to amino acids – as a source of “food” appears to be pretty consistently found in ME/CFS. That shift, plus the problems with Complex V, could leave ME/CFS patients’ cells less able to respond to demands for more energy – resulting in the mitochondria being in a state of post-exertional malaise.
Conclusion
In the third in a series of blogs from the IACFS/ME Zoom conference we find more evidence of immune dysregulation and problems with energy production.
In what looks to be a strong B-cell study from Japan produced several findings (oligoclonal expansion, increased plasmablasts, altered gene expression) which could be associated with autoimmunity. Plus Dr. Sato recently co-authored a study suggested that autoantibodies to adrenergic receptors was impacted the prefrontal cortex of the brain. We might keep in mind as well a recent study which possibly linked B-cell problems with impaired energy metabolism…
Fred Friedberg’s large heart rate variability and life situation study suggested – probably to everyone’s surprise – that it wasn’t a pacing (or the lack of it), or the number of negative life events that differentiated people who didn’t improve over 6 months from those who did – it was the frequency of positive or uplifting life events that did.
Daniel Missailidis and Paul Fisher’s study from Australia found more evidence that energy metabolism is off. In fact, using cultured cells the Fisher group may have come closer to figuring out what that is than anyone else.
Missailidis found significant inhibitions in Complex V of the mitochondria and other problems and -in an apparently compensatory reaction – Complex 1 was upregulated. (Complex 1 is one of the complexes that CoQ10 (ubiquinone) plays a role in.)The TORC-1 pathway was determined to be the source of the Complex I upregulation.
While glycolysis was normal, Missailidis found, as others have before, evidence of an increased use of amino acids for fuel. All in all his findings suggested that cells in people with ME/CFS are more likely to became exhausted quickly when presented with increased demands for energy.
More blogs from the 2020 IACFS/ME Zoom conference
Congratulations to Lily Chu, Fred Friedberg and the others at the IACFS/ME who adjusted to the coronavirus pandemic, moved the conference (at least part of it) online, and provided some intriguing findings for us to chew on.
- Oral Rehydration Solution More Effective than Saline IV at Improving Orthostatic Intolerance
- Starving for Energy? and The Immune Hole That Produced ME/CFS?- The IACFS/ME Conference Reports Pt. II
- Bad B-cells, Malfunctioning Mitochondria and “Could Happiness Help?” The IACFS/ME ME/CFS Zoom Conference #3
- Coming up – Simmaron checks out the autoantibodies in ME/CFS.






I had 4 doses of Rituxan to kill B cells that seemed to help. I went from not being able to work. To earning my Nursing degree. Now I work part time as a nurse. I do think the Rituxan and a few other things helped.
I’m sure it did!
Is there any natural agents that would replicate Rituxan via the down regulating of B cells? My Th2 cells are very overactive.
I’m wondering if not EDS and Other connective tissue syndromes are connected or the underlying cause of MS, CFS, ME.
Genes mitochondria autoimmunitet, defective immunesystem etc. Ben wondering if thiamine deficiency is a problem for us
And what’s wrong with our genes?
Don’t forget the key scientific principle: correlation is not causation. Those well enough to actually engage in meaningful positive events, who are not experiencing depression, and are well enough to participate in a research study, are at the most mild end of the ME/CFS spectrum, and most likely to be well enough to engage in activities that will help them recover or improve. I’m sure this research will be incorrectly applied to those with more severe ME/CFS and they will be told to just do more happy things, which is a kick in the guts to those who are actually unable to do so. I’m all for being happy, but nothing helps the depression ME/CFS combination LESS than being pushed to do what you know is well beyond your capabilities. Then if you refuse because you’re managing your condition within your energy envelope, being blamed for not doing enough to get better (ie. participating in more “uplifting” activities when you’re severely ill). This research must only be applied to those with mild / high-functioning forms of the illness, as that’s all who were studied.
Friedberg’s study probably did not include the really severely ill – few studies do. It does not suggest, to my ears, though, that you go out and do more. To me it suggests that within the realm of what you can do, that having more uplifting activities might be helpful. I get that that’s a lot harder for the severely ill to do. I would hope that most people by now understand how damaging it is, particularly for the very ill, to go beyond your personal healthy activity zone.
Although the study didn’t appear to bring this out, I don’t think it’s necessary to “do more or even different things”. I’m sure that most everyone will interpret this study that way – and it’s certainly one avenue but there is another way and I think it’s ultimately more powerful – to bring happiness or however you call it to whatever you’re doing. I’ve been doing this in the very short term and it’s been helpful. Time will tell how long this lasts. I typically experience short pickups from mind/body work that don’t last that long. Still, it’s been pretty good. I’ve never tried that before.
I know anything about the emotions is a very touchy subject. For me this disease has come with a boatload of fear and dread. I’ve never understood that. I don’t know why it’s there but underneath the surface it’s there in spades. I can punch it down but that takes energy as well. At least for me I think the fear is a huge energy drain which does not help my immune system at all.
Nice you bring this up fact check: correlation is no causation.
To illustrate it with my experiences:
When I feel less “inflamed” I often spontaniously start to hum tunes all by myself without thinking about it.
I even consider it as an indicator health is good that day. Most often, I spontanuously hum at the peak of my well being. So I just as well could conclude “Humming, a clearly uplifting activity for me, causes the deterioration of my health in the following days.”
Why? Because after the peak, it gets worse. That’s why it’s called a peak. And I only start humming when reaching an extra high peak. So the day after starting to hum chances are great I’m at lower health then at the peak day or the day I hummed. See, it’s easy to come to wrong conclusions this way.
So my conclusion:
Less stress helps to less deplete us. I can see that helping as long as you don’t have to overexert to get less stress to start with.
Positive emotions: a double edged sword. Those can increase hormones like oxytocin and oxytocin is found to be helpfull on average in FM. But producing extra oxytocin by practicing stronger positive emotions does cost energy as ALL emotions cost energy and do cost more of it the stronger they are.
Do all emotions cost energy? Does love? I don’t know. That’s a good question. Certainly some do. I have a lot of problems with excitement. It’s a positive emotion but it’s too much and it throws me off…System definitely has problems handling it.
Some are draining us , some are filling us up I would say.
If it’s brain / neuronal activity, then it does cost energy. The more intense this activity is, the more energy it costs.
From own experience: I could crash myself in less then 10 minutes by just feeling myself moderately intense genuine happy. That doesn’t involve the feeling of having lost the ability to be happy as I was that in that state. The crash was near identical to what would have happened when trying to do difficult multiplications and divisions for some time.
I did found a blog on theverge.com with title “For the first time, scientists can identify your emotions based on brain activity” saying:
“We noticed that there were distinct patterns of activity in the brain that separated positive and negative emotions.”
There is no brain activity (strong enough to flare up on an MRI) that does not cost energy.
The main difference with negative emotions IMO is that possitive emotions are far less likely to trap you in a ongoing negative spiral of keep feeling those for very long times. In such, it’s “easier to break free from the high energy cost that emotions IMO pose” when they are possitive. Just stop doing effort and they’ll often vanish by themselves. The same can not be said from negative emotions.
@Cort, you asked does the emotions connected to love cost energy. I would say most definitely. Love is not passive. It requires effort to maintain and equal effort between those involved in having and being in love. Be it love with a partner or love with a friend. There is a lot of giving involved for it to be successful and remain strong and hold true. So does it require energy…..yes, but it is well worth the effort.?
And on a science note……the emotions attached to love can also trigger not only oxytocin but more dopamine, and even more histamine from mast cell response. Someone with an over response/sensitivity to any of these things will feel a high/good to start with and then as they subside a low comes with it. Or if its a mast cell degranulation from intense strong emotion there are all the things that go with MCAS. Its well known by us with MCAS that strong emotion, happiness, sadness, feelings of romance, getting over hot, over tired, etc. Can trigger that response. So emotions do cause release of different hormones and then full body responses occur. And that definitely takes energy.
So yes LOVE takes energy, determination and preserverance. But that brings JOY?
I think it does require energy and if you can manage it it can lift you into a calmer less energetic state. Of course we’re already in a some sort of emotional state. Think about the state we or at least I am already in – racing mind, anxious, worried – a very high energy state.
Maybe the state we should aim for is calmness.
Yes! And thank you for bringing this very valid point up.
I agree that this information/research has the potential to be misused by a doctor or others who think more simple-mindedly—a simple jump from “if” to “then.” My previous doctor was a pill-pusher, even though I told her repeatedly about my extreme sensitivity to medications. Her solution to CFS was antidepressants, even though I told her many times I was not depressed, nor was I in denial (I reminded her I was a counselor with an advanced mental health degree and that I’d be the first to admit to depression).
When I switched to a doctor with a more holistic approach, I looked at my medical records and noticed something interesting: The times when CFS was written in my records had coincided with a series of rather long-term emotional periods (not necessarily depression, but sometimes more likely anger or a sense of feeling “trapped”). The body is well-adapted to handle short-term stress, but not long-term.
The first bout of CFS was after 16 months of being a caregiver to my father (emotionally challenging in a “grrrrr” kind of way), but not during the caregiving. That was right after I retired (loss of purpose) and our kids moved away, too (we were very close).
The second time was after my husband retired and we excitedly embarked on the hiking adventures I’d dreamed of…but 8 months later he had a debilitating stroke. The CFS was not there in my first year of caregiving, as I was busy and hopeful because his prognosis was very good (even though he only regained about half his abilities). The CFS hit after that year and lasted about a year. I was surprised because we had begun traveling again (with adaptations) and I felt like I was coping well, feeling positive.
But then, in 2019 when I had a 3-month CFS reaction to the Shingrix vaccine and again to the higher-intensify flu shot 2 months ago, the irregular heartbeat returned, along with PEM (post-exertional malaise), blacking out on standing, shortness of breath, etc. and I realized 2 things:
1) When I had the Shingrix vaccine, I had had a prolonged sense of loss (depression) regarding something that happened to one of our children, and the reaction 2 months ago (and counting…) happened after a prolonged depression over how the quarantine had taken away the coping mechanisms I’d developed after my husband’s stroke—that is, we could no longer travel, and that meant not seeing the beloved relatives that have been my “happy pill.” The other thing I discovered was:
2) Feeling “trapped” or tired has always in the past been resolved by exercise. It was my cure-all. So, CFS and my emotions looped into a vicious cycle. If I felt “trapped,” I’d try to cope by exercising, my last domain of control and personal power, but when I exercised and ended up bed-ridden for the next few days, I felt weak, both physically and emotionally and, therefore, the sense of feeling “trapped” by my limitations was furthered.
Having worked as a counselor in a school, too, I noticed an interesting pattern in some of the “regulars” who came to me to talk about their problems. I noticed that a surprising number of those dealing with anxiety and school/social phobias had had Mononucleosis at some point (they brought it up) and/or a heart or thyroid condition. I had read that the heart is not just an organ but a gland as well that secretes a certain hormone, and that those with heart-related issues often suffer from anxiety, though my memory on the details is vague.
As for doctors like my previous one who simply want to push antidepressants on people with CFS, I have also read that such medicines can make symptoms much worse. It’s clear that mood and emotions do affect the immune system based on research dating back to dogs in electrified cages, some who had an out (a pad to jump onto) and some who had no “out.” In those studies, it was the theme of feeling “trapped” that stood out, and the impaired immune responses at the cellular level that were studied in those animals.
I recall a coworker who had been diagnosed with MS (multiple sclerosis), an autoimmune-related ailment. I knew that when she was a child her mother died (not of MS) and she once told me that she always thought she herself would have a short life because of that, and yet there she was at 40, having surpassed her mother’s death age.
I asked her how old she was when she was diagnosed with MS. She said, “38.” I then asked her how old her mother was when she died. The coworker got wide-eyed and stared for a good bit before answering. “38,” she said somberly, as if she’d just realized the connection. Was it that she was destined to die around the same age because early deaths were in her genes? I think it was very possible that she had strong emotions about dying young that may have triggered a sharp autoimmune response, especially given the additional PTSD emotions surrounding her mother’s death.
The thing is, emotions are so individual to the person feeling and interpreting them, and very hard for a scientist to analyze. Besides, with all ailments, there are genetic predispositions. I even read recently that Ashkenazi Jews from Eastern Europe are more prone to genetically based “familial disautonomia.” Add that predisposition to the cultural/historical trauma of suffering the Holocaust, and there isn’t a more lethal combination for autoimmune disease, all which can be passed on to offspring both genetically and psychologically.
In closing, I just want to thank Cort for bringing us all this data, and to those of you who post here. It’s affirming as well as eye-opening and enlightening.
Very well expressed d and i was thoughtful of the exact same ideas you expressed here- can be frustrating as well as depressing if your body is simply not allowing you to do the activities that wouod make you happy / they’d where i am at and it’s not a pretty place to be/ physically as well as emotionally
Oh! Friedberg’s “Happiness” results are music to my ears! And I’m not surprised by them.
Years back when I moved to the countryside in an attempt to get my health back, I instinctively knew I needed some more feel-good in my life, not just less stress. I prioritised walks in nature, yoga and qigong, making yummy food, swimming in the sea, socialising as much as I could manage. Unfortunately though, I’d become so depleted that everything I used to enjoy had become an “it’ll be good for you” thing I needed to willpower myself through, rather than an “oh wow this feels good” thing that propelled me along under its own momentum. Life became all, and only, about doing the right things that might hopefully give me a livable life back. (Even my reading preferences graduated from entertaining literature to gripping medical research papers — Cort, I’m sure you relate!) And on top of that I felt guilty that I was prioritising pleasurable things and rest, in a world that had brought me up to believe that that’s the most despicably selfish and lazy behaviour. (That crazy internalised voice said I should have been working — not in the way I was, which was kind of as a full-time self-carer, but rather in the conventional sense of performing a service for other people in exchange for money which I could then use to pay other people to perform the services I was trying to do for myself!)
Stress is a definite flare-trigger for me, and conversely, I’ve found that while true feel-good (like a delicious swim in the sea, an authentic conversation with a friend, a good meditation practice), doesn’t provide a cure, as Friedberg says, it seems to provide the groundwork that enables all the other stuff to be effective.
I’m so glad to read about Friedberg’s study because it means I can chuck the energy-gobbling guilt and feel vindicated that my pleasurable pursuits are not purely heinously hedonistic!
Go for it Andrea! Have some fun! Have some hedonistic fun. 🙂
That voice in our head that says we must work, work, work and be productive – that voice was never a particularly good one to pay attention to anyway. It’s driven a lot of healthy people to work, work, work their entire adult lives – and miss out on a lot of life. Then you retire, exhausted, and wonder where it all went.
And that’s for healthy people.
“a full-time self-carer”
Nice one Andrea. I have to remember this for explaining my professional occupation ?.
Since I can’t work any more I list my job title as a “Domestic Engineer”. I feel I need disability leave from that job too. LoL. Wonder if my doc would write a letter for that one? ? SMILE, after all the blog is to find cheer where we can…..it will improve our health.
I managed to let go of that guilt about three months after I had to stop working. I’ve always liked non-conventional approaches to stuff, so the novelty of turning my back on guilt made it an even more rewarding action to take.
And that ‘pushing through things’ mentality, that was actually much harder for me to recognise as being a problem. I remember watching lots of TV shows, stand up comedy specials, hanging out with family/friends and just being flummoxed by how unfunny everything had become. I was convinced I was fine and doing the right things, and it just never really gelled for me, that I might need to take a different tack.
I think we really just need to be able to rest and be free from internal and external stress for long enough, and we finally get back to being somewhat ourselves. With ME we’re never exactly the same as before, but with enough freedom from stress we can get back to somewhat near the correct ratios that make up our personalities/beings.
Anyway, just wanted to share my thoughts, as your post resonated quite a bit with my retrospective view of my own journey through hell and out the other side.
This is interesting to me. I recently had my mitochondrial genome sequenced. The results showed a mutation in the TMEM70 gene which encodes ATP synthesis in Mitcohondial Complex 5.
Whoa! That would fit. Now that we have this Complex 5 research, I imagine one of the next steps will be looking for mutations like that. Thanks for sharing that.
The Doctors Lights in Utah did that study already. Both my sister and I were in that study. It looked at family genes and also Mitrochondria function. They looked at all 5 complex and how it was functioning. Both my sister and I have ME, but her Mitrochondria was functioning and my function put me as one if the lowest functioning in the study (she said 20%) Mitrochondria of all of the 5 Complexes. So my Complex 1 did not upregulate to compensate for my Complex 5. They looked at other genes to try to determine other assistor genes for function and dysfunction. My sister and I shared a lot of the same polymorphisms, but mine were dysfunctional and hers were not. The conclusion was it could had been other things I had been exposed to (Lyme, fungus, virus) or that I was really tired that day from traveling to be in the study. So there was many possible reasons for my poor results. They had wanted me to go to a geneticist, but we moved, and I haven’t been able to attempt that – with the world pandemic going on.
As for being positive. I think it is VERY important. I always say to try to have an hour of joy every day. If nothing more, sit in a chair and watch the clouds pass. Watch a butterfly. Find a flower and count its petals. Get your phone and find a bug and see how many good pictures you can take of it. The small, little things, take notice of. Feel the wind and notice the smells and be aware of what “IS”. To take notice of the small things in life and find their beauty and notice the things we have forgotten to pay attention to……its easy to find an hour of joy a day. Then give THANKS for what gifts we have been given. ☀️??
@ D Johnson,
The Complex V is interesting to me too. Thanks for the TMOM70 heads-up. I took a quick look at mine and noticed that I have some detrimental looking SNPs like a noncoding transcript exon variant and two 5 prime UTR premature start codon gain variant/5 prime UTR variants.
My ID for those last two are NM_017866.5:c.-23C>T and
NM_001040613.2:c.-C>T
Care to share what your SNP was?
I don’t have my mitochondrial DNA results but did look to see that there are over 1000 genetic SNPs which can influence mitochondrial function. There is also a long list of genes in the ATP family involved in the various Complexes and having Ehlers-Danlos I was surprised to find that some of them also were involved in things like skin structure (Cutis Laxis and Wrinkly Skin Syndrome–what?!) Other factors influenced by these genes (labeled a-h if I remember correctly) were involved in Complex V production and electron splitting.
I know just enough to know I am in deep water here–just shy of being an idiot. Regardless, looking down my list I did find two ATP genes with a number of missense SNPs which have a high likelihood of being detrimental.
Anyway, getting on to ‘feelings’ sometimes I think it would be better if I never looked at or thought about anything ME/CFS and just carried on with my life as best as I can. Ah sweet ignorance! Knowing myself that’s not going to happen, curse the Internet. As far as ‘happiness’ is concerned I think a better word would be ‘contentment’ as opposed to frustration, which is the other side of the equation for me.
I can see how this study might upset some whose doctors might gaslight with diagnosis’s of ‘depression’ rather than an organic disorder. But hey, the body and mind are so intertwined sometimes it is hard to say where one ends and the other begins…
Does Karmin from the HRV article have contact info?
This is probably the most encouraging research I’ve read in 20 years. I pray the funding and research continue. It would literally be a miracle to so many sufferers if a breakthrough occurs from these findings and yes I hope these young brilliant medical professionals push forward.
FULL SPEED AHEAD…..would be wonderful. Looking forward to future updates.
It seems to me that the continuing problems that are being found with energy production are good news indeed.
Should this read?: “increase HRV and [reduced] symptoms.”
Also, on my phone, after “The Gist” insert, there’s some words chopped off before: ” complexes” make up ATP production in the mitochondria.”
Interesting findings there, though. 🙂
Yes – REDUCE symptoms :). Thanks. I will check out the phone boo boo too.
For severe and severest ME Sufferer love can be to intense and they depend on mercy.
This article is cynical because it’s impossible to be frequently happy with severe ME. Exactly this Frequency is not possible with ME.
Thanks for not deleting again my comment.
I agree it’s a tough proposition, a really tough proposition. I don’t see being frequently happy at all. That would be really hard even for healthy people but as Issie put it so well – finding ways to be joyful sometimes during the day – even for a little while – can provide a balm.
Jamison has a nice blog on the Battle against Bitterness – https://jamisonwrites.com/2020/11/23/the-battle-against-bitterness/
What’s happening with the Cortene project? Haven’t heard anything….
They are moving forward – fundraising…
Thank you for all of your efforts and research. I have had ME, FMS, MCS among other issues i.e thyroid, adreal, empty sella syndrome for 30 years. Several doctors have been intrumental with holistc approaches. That is not the case now. Can you provide me with names of doctors knowledgeable in the field within a 60 mile radius from Boca Raton, Fl and accepts medicare. Before Covid 19, our symptoms seemed weird and the old psychological labels applied. How do you address the need to isolate as well as know people will confuse our symptoms with Covid 19. Thanks
Hi Harry,
I don’t know about doctors around Boca Raton but we hope to have a program up by the end of the year that may help. It will be interesting to see if doctors start to respond to ME/CFS differently given the attention given the long haulers. I suspect some will. As to the need to isolate that’s unfortunate as isolation has its costs as well. Alex Howard has talked about “environmental fatigue” – the fatigue that occurs from being in the same environment doing the same things time and time again – if I got that right. I’m sure that doesn’t help. I know that my occasional trips to the coffee shop did actually pick me. Just a little human contact can be helpful.
When I saw the Fred Friedberg piece I thought – there’s going to be trouble with this one. When I was at my worst – Autumn 2016-March/April 2019 – I really had to batten down the hatches. I’m a well practised ‘freezer’ (as in fight, flight or freeze) and can cut myself off, if I’m in a position that I feel I really need to. This time in my life was definitely a time to retreat deep inside. In short, at times, I had become so completely exhausted I felt I didn’t really have enough energy to keep myself alive and think/feel etc., at the same time. Thoughts/feelings could be dangerous – too costly, in energy terms. By Christmas of 2018, I was aware that I couldn’t even ‘afford’ to think about writing Christmas cards. Not because they were tiring to write – but because thinking about the people I was going to write them to, I felt I was in danger of using up more energy than I had available to stay alive. Pure terror. There were times that my legs started to collapse under me, or where I just had the ominous feelings that I was on the point of total collapse – I was about to stop. I just had to live within an emotional fortress – nothing threatening could be allowed to enter or leave – zero. No thoughts of family or the medical profession were allowed free access. My son and my dog Murphy were fine. My home-care clients were fine. Anybody else was a threat to my well being. I had no margin for error. I had absolutely no idea how I was going to get out of this extremely precarious position – I couldn’t think about it. I just survived one day and then another and so on.
By chance, probably in early 2019, whilst trawling around the internet on random searches I came across a video of a doctor, in a white coat, called Dr Nancy Klimas talking about something called ME/CFS. What’s that? I then looked up ME/CFS and came across a video of Dan Neuffer talking to someone called Brenten, who like me, couldn’t tolerate most food. I couldn’t really think or remember anything at that time but I thought to myself – you just HAVE to CALM down and get better SLEEP. I didn’t weigh up the pros and cons – I couldn’t hold thoughts in my mind long enough to make an evaluation – I just thought do it – I don’t care how you do it – you only have to concentrate on two things… Calm down and get better sleep. I didn’t even really know how it was going to help but I didn’t have any other options – no one would help me and I was getting worse. Much to my surprise, it did actually work. I don’t think I realised how much of an negative effect, being constantly wired and hardly sleeping was having on me. After one night of better sleep, I could eat more food. I had been down to tolerating just a few things.
So since then, March/April 2019 I have been working on further improvements and in this last week, I can actually say that I feel fairly normal. I still, however, have to focus on not setting my immune system off or aggravating my brain. My main issue is getting enough energy to my brain, without raising inflammation.
On the issue of the frequency of positive uplifting events – I feel I had to get out of the danger zone first. I loved your observation Dejurgen (in your three part series) of ‘having standard stock daily boring routines’ – Yes! Boring is great! I focused on then, what I’m still focusing on now – calming down, getting better sleep, getting up at the same time, going to bed at the same time, finding and eating foods that didn’t cause a major health calamity (if possible) and were nourishing, calming down again, relaxing, dealing with issues that needed attention, trying to empower myself to deal with people and issues on my terms and not their’s, tidy bits of the house, supply my son with food and love, mow the lawn, feed and watch the birds, wander down the lane with Murphy, stand and take in the view of the wide green valley, the birds, wander back up the lane with Murphy, chat to the neighbours, do a bit of washing, hang it out and bring it back in. Iron my shirt. Working, chatting (and often laughing) with my homecare clients. I’ve watched Homeland on Netflix, read novels, lay on the trampoline in the warmth of the sun, watching the blue sky, white clouds and green and copper trees swaying above me. I’ve marvelled at the starry sky at night. Everything has been about a normal, calm, life, which had been so crazy for so long.
My sense of humour returned. Sensing a bit of warmth and safety, my freeze melted. I enjoyed the banter on Health Rising and learnt so many things. I empowered myself by writing letters to politicians, getting involved in raising awareness of ME/CFS. I recently began to feel energised by music, which I love. I’m starting to feel like I have a bit of energy to live and enjoy things and am not just focusing on scraping by, dragging myself around, just trying to survive. I’ve recently joined Niki Gratrix’s Healing Circle meditations, which have come just at the right time for me. I’m not sure I would have been able for them before but now I’ve acclimatised, I feel 100% relaxed, safe, warm and grounded – fabulous. I now have enough strength and stamina to open up and not be afraid.
However, I’ve done this on my terms and in my time because I’m the only one who truly knows my own experience, my own perspective, my own reactions and my own history. No one else knows. I believe in myself and it’s a good place to be.
Thoughts and feelings, too costly in energy. I can relate to that.
Man, you came back a long way in such a short time. I’m both happy for you and a bit jealous for me. My path is slower and longer, but bit by bit getting there too.
But above all you represent hope. Hope that much of ME is reversible wihtout too much permanent damage. Hope that, with better ways to try and deal with our disease, we have a chance of coming back a good bit. For some, future drugs will offer that opportunity. For you, improving sleep and reducing energy waste by practicing calmness seems to be vital. For me, a combo of things so far is needed.
So long medicine does not provide clear answers, improving optimal functioning and self healing seems to be important. That is a very individual path to walk.
Thanks for leading the way on you particular path. Tanks for sharing and showing us hope!
Hooray for hope!! Even on the days that I visit this blog hoping for the news of THE breakthrough, and not finding it, there is actually a feeling of hope here that I find in this community, from corts’ reporting and from all of us. This illness is sooo isolating, so the hope in the midst of dispair from community means alot!
Thanks Dejurgen, I was fairly anxious that I was going to get annihilated for writing that…
I do more things like taking a Digestive Aid, with enzymes and betaine hydrochloride to help digestion, psyllium husk and Migrea (by Bio-Kult) – live bacteria to help my gut and brain. Magnesium, Vit C and Vit D and a multivitamin and mineral formula high in B vits. The vitamins are mainly produced by Viridian (UK). I also take an Astragalus, Elderberry and Garlic complex by Terra Nova to help my body fight off any viruses that have over stayed their welcome – I was very taken by Amy Proal PhD and her snowball idea, ie that our health gets knocked off balance and then we start collecting issues, like a snowball gathering snow, as it rolls downhill. Amy Proal PhD did a webinar for Solve M.E. in 2019, possibly Sept or so.
I also take Macushield for my eyes and have been taking 100g of CoQ10 daily but I’ve run out. I think I’ll get some more. I also pour a bit of apple cider vinegar and extra virgin olive on my food – organic egg, grass fed beef/lamb, kale and spinach and ground linseed/chia seeds. I drink Roiboos tea. I can now eat some nuts again – almonds, walnuts and peanuts. I also eat a bit of dark chocolate and have some rice cakes with dark choc. Trying to avoid the choc chip cookies. Oh yes, I also sprinkle ginger and tumeric on my food. And I eat mackerel and take Omega 3 capsules. I think that’s it but I know I’ve forgotten something – I always do.
Also when I made a bit of an improvement, I was aiming very low! I just thought if I could even make a small improvement over the next year, that was fine – much better than the precipitous decline I had been on. I just wanted to be a mum/mom to my son and I needed to be around to do that.
I think we’re all so different genetically, environmentally etc., I think it’s unwise to compare too closely. I think me calming down – I have to work on it daily – and getting better sleep just gave my body a better chance to regain some health because it’s limited resources were not being diverted to defense duties or run down and lacking maintenance through lack of restorative sleep.
Congratulations Tracey Anne. For anyone wanting to check out Niki Gratrix’s Healing Circle Meditation here’s a link – https://nikigratrix.com/weekly-healing-circle/
It’s a sound healing meditation. It sounds like fun…
Excellent reporting as always, Cort! You explain things so well.
I wonder if researchers do “happiness” studies on conditions and diseases to which ME/CFS has been compared such as MS, Gulf War, sepsis, and narcolepsy. I imagine not. Then we have to ask ourselves, why only ME/CFS? I’m afraid it adds up to just one more claim that at least part of our problem is psychological, which I have a difficult time buying in to. Those who have described what cheers them up would cheer me up too, if I were able to do those things. I find the more debilitated I am, the lower my mood goes, which is simply human nature and not a new discovery. This would be true across all debilitating illnesses, not just ours.
I don’t know if they’re studying “happiness” in these conditions but I checked out if cognitive behavioral therapy studies are being done and they are in MS (https://pubmed.ncbi.nlm.nih.gov/28528567/), many have been done in rheumatoid arthritis (https://pubmed.ncbi.nlm.nih.gov/?term=cognitive+behavioral+therapy+rheumatoid+arthritis), several in Gulf War (https://pubmed.ncbi.nlm.nih.gov/?term=cognitive+behavioral+therapy+gulf+war), none in hypersomnia and astonishingly, one in sepsis…https://pubmed.ncbi.nlm.nih.gov/?term=cognitive+behavioral+therapy+sepsis
It appears that CBT is just about everywhere. Epilepsy? Many studies! (https://pubmed.ncbi.nlm.nih.gov/?term=cognitive+behavioral+therapy+epilepsy); heart failure – believe it or not – yes (https://pubmed.ncbi.nlm.nih.gov/?term=cognitive+behavioral+therapy+heart+disease), Parkinson’s disease )https://pubmed.ncbi.nlm.nih.gov/?term=cognitive+behavioral+therapy+parkinson%27s+) etc. It’s kind of amazing.
Of course the difference between most of these and ME/CFS is that they are characterized as biological diseases and CBT or other kinds of behavioral modification are used to help deal with having a difficult disease.
That’s not how CBT was originally used in ME/CFS: it was posited as an explanation – a very different context.
I agree – the more debilitated you are – the likely it is that your mood is going to be impacted. I don’t see how it could be any other way.
Hooray for hope indeed Crystal! I also know what you mean about hoping for “THE” breakthrough 🙂 Surely, one day?
I would dearly love to be redeemed and validated after decades of getting by with this illness with so little support. All of us are so much stronger, resourceful and brave than the world gives us credit for. After nearly 30 years of being sick and trying everything in my power to escape it, I am now trying to make peace with the possibility that this is as good as it gets, to live within my means, and to make the most of life as it turns up instead of passing time in a waiting room. I have managed to free up some of the energy I previously spent on resistance, negative emotions and commitments / people that do not bring me joy and redirect it to positive emotions, activities and people who do bring me joy. I am a long way from well and some days I grieve and sob but the mindset change has helped in its own way. I am lighter. I will also keep the very real flame of hope alive that better days are ahead.
I still have some questions about the “failed” Rituximab trial too. The Loebel / Scheibenbogan research suggests that only about a third of patients have autoantibodies to beta adrenergic / muscarinic receptors, suggesting that Rituximab should only work for about a third of patients (unless other autoantibodies are at play). I don’t know exactly how the Rituximab trial failed but I wonder if some of the control patients had a strong placebo effect to fake treatment and 2/3 of patients who were treated don’t have autoantibodies and therefore didn’t respond as well to real treatment as hoped? Potentially the results were quite muddy?
I know that if I was in a hospital setting and was treated with the sort of kindness, care and respect normally afforded to someone with a legitimate illness (and I am sure the Fluge / Mella team were kind and respectful to patients in the trial), I would probably experience a placebo effect just from being treated with kindness. There is a lot of relief that comes from being believed and treated humanely and it would free up energy for healing. It takes a lot of energy to cope with this illness on our own and energy is on short supply.
I agree with you debsw. I know I’ve agreed with you before on things – I can’t remember what exactly, I just remember the agreeing! 🙂
I don’t know about Rituximab. The authors seemed pretty clear about the results and did look for subsets but I don’t know about the placebo effect. A strong placebo effect can override a lot and there were those earlier Rituximab studies which were quite promising: some people obviously did quite well on the drug.
Has Friedberg’s study been published yet? I don’t see a link to it. It would be interesting to read the whole thing.
I think it should be noted that the average illness duration of the patients in the study was 16 years and so it is not that surprising to me (being at over 20 years myself) that the patients might be in a more stable state where they had pretty much maximized the benefits of pacing and avoiding negative events that were especially problematic. I’m not sure the same could be said of ME/CFS patients who are in the first few years of illness (physiological differences between new and long term patients have been seen in several studies if I remember correctly).
It also sounds like this was not a study where patients intentionally tired to increase positive events or change any of the things that were being measured (unlike Donna Jackson Nakazawa)? It would also have been very interesting to know how financially well off these people where and how much stress they had in their home life that they could not control. It is hard to have enough positive events to offset fear of poverty and not being able to meet basic needs or living in a home situation with family that are not supportive.
So not to disagree with the conclusion positive events being beneficial (this is also a major part of the “brain retraining” programs) but just the feeling that the conclusions of this study may apply to a more specific group than all ME/CFS patients.
It has not been published yet. Good points. You’ve got to assume, as you noted, that the patients who improved probably did not have significant money issues as they would have probably gotten in the way of their having any uplifting experiences. You have to assume, as well, that they were healthy enough, as others have pointed out, to be able to go out and have uplifting experiences.
It has been ‘proven’ that inflammation in the gut can cause depression . And how do you meassure love? Love is a very special feeling. But feelings are subjective.
So calming down the stressresponse with mindfulness sounds more rational to me.
When you have more energy and less pem , anxiety or positive/negative stress.
Less cytokines and inflammation you will feel more happiness. If feelings where simple as oxytocine etc… we use that and everything would be oke. That is not how it works.
Yes Gijs, it’s definitely not simple. I think so many systems are interconnected and can ricochet off each other – it makes no sense to me to view things in isolation.
HHV6 was first identified in the Gallo lab in 1986. Initially, it was called Human B Lymphotropic Virus (HBLV) because of its adverse effects on B cells. When the researchers discovered that it also affected T cells, they changed the name to HHV-6 and then for unknown reasons, subdivided it into HHV6A and HHV6B.
Re. the happiness factor. Norman Cousins was an American political journalist, author, professor, and world peace advocate. He documented the benefits of the happiness factor in his book “The Anatomy of a Disease”. He recovered from an extremely serious connective tissue disease by checking himself into a nice hotel and convincing a doctor to give him IV high dose vitamin C. He also asked his wife to bring him funny movies which he watched every day. He wrote that having a good laugh helped him have an hour or two of pain free existence. This homegrown protocol cured his mysterious illness.
This is a great discussion and I hope I can add to it. I am recovered (about 80%) from mild CFS and I think I know how I did it. Regardless of the advice I read from the experts, I did whatever pleased me for the first time in my life. I baked bread and ate slabs with piles of butter and jam, I read a book for hours and then took another nap, I drank wine every evening, I did the Norman Cousins funny movie cure, etc. I also practiced biofeedback to retrain my poor exhausted ANS…I stopped being a Type A person after 70 years. I also did Nancy Klimas’s pacing with a huge calander and hourly rating of how I was feeling. One day last December I felt different: that terrible feeling which we call fatigue, but needs a better word, was gone.
I wanted 100% so I did Annie Hopper’s limbic retraining and I think I would have improved, but the visualization requirement brought up emotional issues that I was then forced to work through. Delayed grieving over the death of my father when I was nine was exhausting and put me back in bed for at least two days after each episode. (Annie does not recommend touching painful issues during her training). It is now obvious to me that ANY negative emotional stuff pulls me back into that CFS feeling. So, my plan now is to continue limbic training and biofeedback and refuse to let my mind think negative thoughts (and keep baking). I will let you know if this works ! Yes, happy thoughts do take energy, but I believe they are helping to rebuild my sad, frightened, weak limbic system.
Congratulations Nansy. It’s surprising how hard it is for us to give ourselves the gift of enjoyment. I know that I am really driven….That must just add another load for our poor wounded stress response systems to handle. I’ve often wondered if I could just get my body to slow down and relax – what would happen…
Good luck on your continuing recovery. 🙂
As to the happiness study. The findings can be explained in a different way: upliftig life experiences may well be markers of improved ME/CFS. So the patients studied may have improved for whatever reasons – and therefore are more capable of engaging in and resonating with positive life experiences… So the question may remain: what is the root cause for their increased happiness?
Good point!
Thank You so much Cort for another amazing article! I had another PEM relapse for days, so I just now read it. I have a question. How does the Missailidis results reflect on PDH dysfunction or downregulation? Did anybody talk about that?
Hi Christyne,
If I remember correctly, it does. (Would that be associated with using more amino acids for fuel?)
I did a quick search and did find no connections yet between pyruvate dehydrogenase or pyruvate dehydrogenase kinase (the chemical decreasing pyruvate dehydrogenase) and the “classical” feel better hormones like dopamine, oxytocin and serotonin.
There is a relation between insulin, NE (nor-adrenaline or nor-epinephrine) and pyruvate dehyrgogenase kinase https://e-dmj.org/journal/view.php?doi=10.4093/dmj.2012.36.5.328.
I also searched for “the opposite of pyruvate dehydrogenase kinase”: pyruvate dehydrogenase phosphatase. That chemical increases pyruvate dehydrogenase activity.
Pyruvate dehydrogenase phosphatase activity is also influenced by insulin https://en.wikipedia.org/wiki/Pyruvate_dehydrogenase_phosphatase.
Chances are that getting good control over blood sugar levels will help pyruvate dehydrogenase activity. Being in a better mood preventing mood splurges of food may play a role.
Chances are also fairly high that too high nor-adrenaline levels wont help us for the better. Just a gut feeling. So some more feeling of leisure might help in that department. Nor-adrenaline has a clear influence on blood sugar levels and insulin is the primary hormone to regulate blood sugar levels so it seems fair to think nor-adrenaline will influence insulin and with it pyruvate dehydrogenase.
There are also relationships between dopamine and insuline https://www.sciencedirect.com/science/article/abs/pii/S0891061816300692 saying:
“Dopamine and insulin signaling systems have a reciprocal regulatory relationship.”
So it is very reasonable to assume doing things that change our hormone balnce, to some extend, can and will influence how our bodies opperate. We however have to gain more then what it costs to feel better in order to be better off.
The more important question however is, how much leverage do we have to do so? I often have to shake my head when people tell me “to think possitive and my health will get better”, while they get depressed if they have to stay a whole week at home or are unable to buy that new shiny car while we manage to stay reasonably possitive despite plenty and plenty more challenges.
The same goes when people who can’t get off their cigarettes or leave a piece of pie untouched telling us if we just ate a bit more healthy we would be in better health too. We have to be ten times more strict with food and mild drugs like tobacco and alcohol then they do on a daily basis to still remain “that healthy” and they point to us for a lack of control. Are they kidding or self dellusional?
Dejurgen, they are neither kidding or delusional. They are ignorant. They just don’t know. And the only way they could possibly “KNOW”, is to have it themselves. And I don’t wish any of this on anyone. So we have to just “bite our tongue” or if they are open to learning…..educate them.