This is the second in a series of blogs on the virtual 2021 International IACFS/ME Conference,
Dr. Scheibenbogen shows the impact that a leader in the field can have. Virtually no ME/CFS studies came out of Germany prior prior to her taking on ME/CFS. Since 2010 she’s co-authored 30 papers on ME/CFS and was able to point to a large multidisciplinary team at work on this disease.
In her “Emerging options for Autoimmune ME/CFS” presentation, Scheibenbogen laid out the case for an autoimmune cause of ME/CFS in at least a subset of patients. There’s the infectious trigger (often EBV – a stellar autoimmune disease trigger), evidence of increased family histories of autoimmunity, and study results.

Carmen Scheibenbogen proposes that autoimmunity drives ME/CFS in many people.
She pointed out that a 2020 study from her German lab found that ME/CFS patients with an infectious onset were 2x’s more likely than those with a non-infectious onset, or healthy controls, to carry two gene polymorphisms that are strongly associated with autoimmunity. A 2020 Norwegian study, featuring among others, Fluge and Mella, also found an increased prevalence of MHC II molecules that are associated with autoimmunity.
Scheibenbogen’s been focused on B-adrenergic, M-acetylcholine, ATI and ETA receptor autoantibodies. She hastened to note autoantibodies, including the ones she’s looking at, can have positive effects. These are natural autoantibodies – they’re found in everyone, healthy and non-healthy alike – and at the right levels they’re helpful, but in some diseases, they’ve been shown to produce problems.
They certainly seem handmade for ME/CFS. They can vasoconstrict the blood vessels, causing the release of vasodilators and pain-enhancing subjects such as bradykinin, and ultimately reduced blood flows to the muscles, the brain, as well as leakage from the capillaries and low blood volume.
Her group recently found that the levels of these autoantibodies correlated with the severity of symptoms like fatigue, muscle pain, cognitive impairment, and gut symptoms in ME/CFS patients with infectious onset. Interestingly, some of the autoantibodies were correlated with fatigue and gut symptoms even in non-infectious onset patients.
A recent study assessing endothelial functioning suggested blood vessel problems may be common. Endothelial functioning refers to the ability of the endothelial cells to vasodilate, or enlarge, the blood vessels. Inadequate endothelial functioning potentially means reduced delivery of the blood to the muscles, brain, etc. The study found that was reduced in about half of ME/CFS patients, and people with worsened endothelial function had more severe disease.
The Rituximab Shocker
Then came a shocker. Scheibenbogen went over clinical trials (Rituximab, IgG, Endoxan, Immunoadsorption) that provide support for an autoimmune origin in at least a subset of patients.
Rituximab, of course, was high on the list. Scheibenbogen reviewed all three Rituximab trials – the two early, successful, small Phase II trials, and the big Phase III trial which failed – dooming Rituximab as a potential therapy for ME/CFS. The second Phase II trial followed 28 patients over 15 months and found that 18 responded well to the drug. It seemed we were set for a big success, but then the Phase III trial, following 151 patients over 15 months, failed with more responders in the placebo arm than the drug arm.
The problem was not that some people didn’t get better. About a third of the study responded. The problem was that the placebo group responded to a greater degree (35%) than did the Rituximab group (26%). The fact that 35% of the people in the placebo group did well enough to meet the criteria to be a “responder” demonstrates just how strong the placebo response can be.
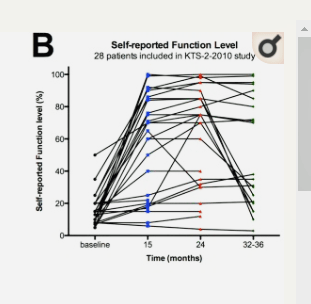
See the transition from the blue dots at baseline to the red dots at 24 months. After that – with no more maintenance doses – the patients declined.
She compared the results of the 3rd trial to the many patients in the Phase II trial who went from severely impaired to recovered, or close to recovered, 10-15 months later.
Scheibenbogen proposed that the reduction of the maintenance dose in the Phase III trial, though, may have doomed the trial. The Phase II trial used maintenance infusions at the rate of 500 mg/m2 (maximum 1,000 mg) at 3, 6, 10, and 15 months follow-up. The Phase III trial used a fixed dose of 500 mg at 3, 6, 9, and 12 months. Scheibenbogen reported the fixed-dose used was caused by financial problems.
Scheibenbogen called the maintenance dose “critical” and questioned whether it was too small in the Phase III study to knock the B-cells down. She also questioned whether the criteria for success – > 4.5 fatigue score for 8 weeks – was too brief and noted that the Phase I trial found it took 3 months for a response to show up. She also noted that patients in the placebo group received “standard” care, which may have improved their symptoms.
It should be noted that 26% of the patients in the Rituximab group had “serious adverse events” – Rituximab is a heavy-duty drug – but also that 19% of the people receiving the placebo had serious adverse events as well; serious events are not uncommon in ME/CFS.
Other B-cell Mediated Drug Trials in ME/CFS
Cyclophosphamide
The Rituximab saga is over – there will be no further Rituximab trials, but the recent open-label, Phase II, 2020 cyclophosphamide trial had good results, with over 50% of the patients responding. The long-term trial – 18 months – extended in some cases up to 4 years – found about a 50% increase in activity to over 6,000 steps per day. (While substantially less than recommended, 6,000 steps per day is above average). Four years later, 68% were still “in remission”.
Fatigue was down and functionality was up in the responders. 77% of the responders achieved a fatigue score increase of at least 4.5. (Because a fatigue score increase of 4.0 was deemed a slight improvement, not everybody had dramatic reductions in fatigue). Functionality scores, though, appeared to improve dramatically as the SF-36-PF increased from 35.0 at baseline to 69.5 at 18 months (p <0.001). Only two of the participants were working part-time when the trial started. By the end of the study, 9 of the 22 responders were able to return to part or full-time work or study.
Cyclophosphamide did not appear to be a magic bullet, but a subset of patients clearly experienced significant improvement. It’s an older and more toxic drug and the authors called the treatment period “demanding” (nausea and constipation were common). Eleven patients were hospitalized at one point, but the authors assessed the toxicity as “acceptable” given the high symptom burden and low quality of life of many people with ME/FS.
Particularly after the Rituximab results, the authors urged caution regarding interpreting the cyclophosphamide results. A placebo-controlled, double-blinded study is clearly needed.
Subcutaneous IgG

Peolple with high lactate dehydrogenase (LDH) levels did better on IgG. Their LDH levels dropped while on the treatment.
An IgG trial, “Tolerability and Efficacy of s.c. IgG Self-Treatment in ME/CFS Patients with IgG/IgG Subclass Deficiency: A Proof-of-Concept Study – PubMed (nih.gov)“, provided another indication of B-cell mediated immune dysfunction. Immunoadsorption removes IgG antibodies from the patients, including the autoantibodies Scheibenbogen’s been tracking.
The Gist
- Dr. Scheibenbogen has transformed Germany from a non-entity in ME/CFS research to a major player. Since 2010 she and her now large multidisciplinary team have co-authored 30 papers on ME/CFS.
- While autoimmunity is not proven, the infectious onset, gender prevalence, and family histories, as well as some research studies and clinical trials, suggest autoimmunity may be present in a large subset of people with ME/CFS.
- Dr. Scheibenbogen has been focused on naturally occurring autoantibodies that have positive effects but have also been shown to contribute to disease in some diseases. Several are responsible for vasodilating or enlarging the blood vessels enough to allow sufficient amounts of blood to get through to the muscles, brain, etc. A recent study associated these autoantibodies with more symptoms in ME/CFS.
- The large Rituximab trial failed but Dr. Scheibenbogen – after demonstrating the excellent results from the phase II trial – proposed that financial considerations which limited some of the maintenance dose levels in the large Phase III may have doomed the trial.
- Small clinical trials – all involving agents that affect the B-cells (prime drivers of autoimmunity) have been helpful in a subset of patients.
- While cyclophosphamide treatments are “demanding” some participants in that trial were able to return to work. The small immunoadsorption trial produced rapid benefits and long-term gains in some participants. Some people weren’t able to tolerate IgG but others were able to and functionality increased as well as blood vessel function and blood flows.
- These trials are far too small to hang your hat on but the results are generally pointing in the right direction.
- While Rituximab is off the table for ME/CFS Dr. Scheibenbogen pointed to the more effective monoclonal antibody drugs being developed as well as other drug approaches to autoimmune diseases. She hoped that with the advent of long COVID drug manufacturers will take another look at ME/CFS and said she was in discussion with one company.
Biomarkers – markers that can tease out which patients are likely to benefit – are particularly helpful in expensive treatments like IgG – and they may have found one in this trial. All the patients who improved on subcutaneous IgG had high lactate dehydrogenase (LDH) levels at baseline, which subsequently dropped during the treatment. Lactate dehydrogenase converts lactate, which at high levels becomes toxic, to pyruvate and back again. When oxygen is in low supply, as has been conjectured in ME/CFS, it converts pyruvate, the last product of the glycolytic cycle – to lactate. Lactate dehydrogenase (LDH), then, plays a key role in energy production. The fact that IgG appeared to help reduce LDH suggests that the immune system is involved in the energy production problems found in ME/CFS. They hope to do a randomized, placebo-controlled trial.
Immunoadsorption
The small 5-day immunoadsorption trial found rapid symptom improvement in 7/10 patients and sustained improvement in three of those patients after 2 years, as well as dramatic autoantibody declines. A follow-up study in 5 responders two years later found sustained improvement. Interestingly, the number of infections went down in the patients who responded to the treatment.
A B-cell Mediated Autoimmune Disease?
These different treatment approaches all have one thing in common: they target B-cells – one of the most important cells in autoimmunity – in different ways. Rituximab and cyclophosphamide (Endoxan) wiped out the memory B-cells that were telling the B-cells to go on the attack. (Endoxan has a broader effect and also affects T-cells). Immunadsorption removes the autoantibodies from the blood. IgG flooded the patients’ blood with helpful antibodies.
While the Rituximab failure was a bitter pill to swallow, Scheibenbogen noted that better, next-generation, monoclonal antibody drugs are being developed and coming on the market. New approaches to autoimmune disease such as targeting Fc receptors will also be helpful Scheibenbogen pointed to a 2019 paper Next-generation Fc receptor-targeting biologics for autoimmune diseases – PubMed (nih.gov) which listed alternative approaches to IgG that were under development. She believes that the advent of long COVID will make companies more receptive to giving these drugs a try in ME/CFS and has had discussions with one.
Scheibenbogen suggested that clinical trials of autoimmune therapies only include patients with infectious onset, that the Canadian Consensus Criteria be used to filter patients, and that activity measures be used to assess responses.
During the Q&A, Scheibenbogen reported that she knows Andreas Goebel, the leader of the fascinating fibromyalgia study, which found that transferring IgG antibodies from FM patients into healthy mice turned the healthy mice into fibromyalgia mice. (The IgG from healthy people had no effect). It appears that Goebel is interested in doing a similar study in ME/CFS and they are discussing how that might happen.
Conclusion
To sum up, both studies and small trials suggest that a subset of people with ME/CFS have autoimmune problems. The field is being thwarted from producing results we can count on, though, by its lack of financial resources. Scheibenbogen noted that problem with financial resources even extended to the Rituximab trial and its need to fix the maintenance dose at a certain level (instead of tailoring it to patient size). Given the very strong response seen in the Phase II trial, which did not have a fixed maintenance dose, Scheibenbogen believes the reduced maintenance dose in the big trial may have had a big effect.
Scheibenbogen noted that every attempt to impact B-cells (Rituximab, cyclophosphamide, immunoadsorption, IgG) has had some success. The fact that lactate dehydrogenase levels dropped with IgG treatment suggested that B-cell mediated issues may play a role in the energy production problems in ME/CFS. Except for the Rituximab trial, though, the trials have been small, not blinded, etc. – a situation she called “frustrating”. Still, the small trial evidence is generally pointing in the right direction – not for all patients, for sure – but for a significant subset of them.
Next-generation monoclonal antibody drugs, as well as new drugs being developed to fight autoimmunity, will likely be more effective than drugs like Rituximab and cyclophosphamide. She hopes the attention to long COVID will help bring needed attention to ME/CFS, and more and better trials.







Wow. I have hereditary hyperhomocystienemia. I have 2 gene abnormalities that cause my body not to be able absorb B vitamins. I got sick with what I believe is ME/CFS after flu/bronchitis, (post viral), in winter 2017.
This is so spot on. I believe more people should be tested for hyperhomocystienemia. It is one blood test. There could be a link.
Is there any treatment for it mary
Was the placebo in Rituximab trial a saline infusion? If so, those with POTS would have improved
This idea has come up again and again and again. If you’ve ever taken saline you know the results only last a day. The Rituximab trials, on the other hand, lasted for a year or longer. To put it another way getting fIve shots of saline IV over a year are going to produce five days or so of feeling better – and that’s it.
There’s no way that saline is going to produce lasting results. People have been consistently taking saline for long periods of time. As soon as they stop the saline the party’s over.
Yes, B vitamins, given topically, injectable, or IV, if oral supplements aren’t effective.
I tired SC IgG for a years without any effect. Very expensive and weak in effect. For me the following substances had a noticeable effect:
GcMAF: full symptom resolution for 2 years. Disadvantage: treatment may take 12 months with lots of side effects (women experienced balding…)
Nexavir: 60% improvement but had an effect only for 2 years. The body adapts.
Finally, BCG revaccination every 10-14 days: very stable effect. Not full health but a miracle for me.
BCG revaccination? I’ve never heard of it. Could you elaborate please?
BCG (bacille Calmette-Guerin) treatments have been around for a long time. It is used to treat TB patients in many countries. It is also injected directly into the bladder via a catheter and is used to fight bladder cancer with varying degrees of success.
There are some caveats to adopting this treatment.
BCG vaccination should not be given to persons who are immunosuppressed (e.g., persons who are HIV infected) or who are likely to become immunocompromised (e.g., persons who are candidates for organ transplant).
BCG vaccination should not be given during pregnancy. Even though no harmful effects of BCG vaccination on the fetus have been observed, further studies are needed to prove its safety.
I hope you find this information helpful.
Dear Deb, I do not know how much experience Joe has with BCG. This vaccine has been introduced 100 years ago and it is part of mandatory vaccination programs throughout Europe. It’s safety profile has been excellent (much better than that of COVID vaccines). Discuss it with your doctor, you have nothing to loose. It saved my life.
That’s really something about the BCG vaccine. We did a blog on it:
https://www.healthrising.org/blog/2019/12/26/epigenetics-fibromyalgia-fma-diagnostics-vaccine-treatment-chronic-fatigue/
My general internist tells me patients with ME/CFS ARE immune-suppressed and that’s why my immune system can’t keep EBV and HSV1 from flaring occasionally. So I thought I could not take a live vaccine. My internist knows little about ME/CFS though, so I’d appreciate an answer to that from someone more knowledgeable.
BCG vaccine can be in some way similar to the effect of Staphypan vaccine , even different in general.
My mother was given BCG therapy for a year to combat bladder cancer (carcinoma in situ). It worked wonderfully and she’s now 19 years cancer free. I had no idea it was considered for ME/CFS. Interesting article.
It is effective not only for fibro. I administered it to my parents too before the introduction of Pfizer vaccine. It normalizes immune reactions too. None of them caught COVID, I did. I developed long COVID again and recovered with BCG revaccination. I got 20 shots and still get it on biweekly basis.
News about booster shots. An independent advisory committee of the American drug watchdog FDA is overwhelmingly against the administration of an additional corona vaccine to Americans who have already been fully vaccinated with the vaccine from Pfizer/BioNTech. They are against the so-called booster shots for people aged 16 and over.
The experts had to advise the FDA on whether such a ‘booster shot’ has sufficient added value. Many expressed concern about the risk of heart problems from a third dose in younger men.
A third booster shot for those over 65 years old is being considered.
Thank you for sharing the details of the experimental treatment you have received and the corresponding response for each. What symptomatic improvement did the BCG lead to for you?
@onwardandupward:
for details on BCG see the comments by Adam Keri on: https://www.healthrising.org/blog/2021/09/07/ldn-low-dose-naltrexone-chronic-fatigue-syndrome-natural-killer-cell/
My symptomatic improvement are as follows:
-from 0 hours at work to full time employment
-from 0 sport to swimming twice per week, gym twice per week
-good sleep, no anxiety
-good digestion
What has left: I am 70 % which is a stable 70 % (from 25%). Candida infection disappeared and my IgA is normal again. I have my own life again. Of course my energy level is lower than normal. Symptoms strongly reduced.
@Adam Keri
Thank you so much for your insight into treatment with BCG. I have a practical question. Once you open a BCG ampoule and mix the powder with the diluent you are supposed to use up the vaccine within a few hours. So if you only take shots every two weeks – how do you proceed? You can´t use a new amp. every time??
Thanks for an answer.
Herbert
see my response bellow
Dear Herbert,
I give my response right here.
Your question is correct. I need lots of vials bc BCG is extremely sensitive to heat and light. It shall be stored in the fridge AND shall be discarded after opening the vial. It means that you use 0.5 dose and discard 9.5 dose.. No way to salvage it. BCG does not contain preservatives and therefore it can be contaminated after 6 hours.
If more patients could participate in the treatment, it would help.
I am using it alone. I obtained 6 packages each containing 6 vials. It is for 1 year. 3 months before I run out of it, I re-file my off-label petition and ask the wholesaler to order 6 packages on my behalf.
Any more question ?
Take care,
Adam
at the beginning, it was not easy to obtain BCG. First, you have to get an authorization to use it off label. Next, you have to find a wholesaler. Next, the wholesaler contacts the producer. The Swedish producer blocked my petition and objected to the off-label use (and requested 1000,-EUR just to initiate a formal decision). Then, I switched producer and could locate another one. Not easy but life is difficult guys. Never give up !!!!
Can you please tell us more and explain what those abbreviations stand for?
Adam, thank you for replying to my earlier question. If you don’t mind sharing, which doctor has been prescribing/administering the BCG vaccinations for you?
What kind of sleep issues did it rectify?
I have difficulty remaining asleep beyond 4 hours of sleep at night for well over a decade now so am wondering if the BCG could potentially help with that along with cognitive impairment/excessive daytime drowsiness.
Thank you again.
I had similar symptoms. You have to administer BCG at least every 10 day to be effective. At the beginning treatment should start low:
0.1 dose per week
0.2 dose per week
0.3 dose per week
0.4 dose per week and cca 0.4-0.6 dose every 10 day. There is an individual variability.
I am now able to tolerate weight training too and full time work. It is very effective. However, one vial contains 10 dose and after opening you have to discard the rest. It cannot be refrigerated or frozen. If more individual would receive the treatment, you could limit the waste…
Hello, Adam
Would there be any way to trade this product with you? I’m not in the medical profession, and that’s the only way I would have this product.
BCG is available in Mexico through Myco Medico
Hmm… I often hear about LDH being normal-high in Me/CFSers.
Mine has been borderline low for years.
I have been wondering if there are others with similar value.
It’s great to hear of another researcher taking on biomedical research in ME, but am I alone in despairing at the use of the Chalder fatigue scale to measure outcomes?
I recognise that outcome measurement is difficult in ME but I don’t think the Chalder scale questions go anywhere near capturing my inability to undertake activities. On good days I don’t feel too bad, but I’m still housebound. On bad days I feel ill more than tired.
And it’s not surprising that the scale fails to capture the ME experience since Chalder is an advocate of the learned helplessness/deconditioning hypothesis.
How to accurately measure fatigue and functionality is a huge issue. I wonder about the SF-36 as well….
Measuring metabolic rate old school: O2 consumption + CO2 production. And temperature (heat)
The video on the peas and cellular respiration here are a nice way to understand things
https://www.bbc.co.uk/bitesize/guides/zg2xxnb/revision/1
Fatty acids can inhibit PDH as well
“Glucose–fatty acid cycle. This, also called Randle cycle, refers to the significant reduction in the uptake and utilization of glucose that occurs in muscle when fatty acid oxidation is intense. This phenomenon is explained as follows: the oxidation of fatty acids yields acetyl-CoA from which citrate is generated by action of citrate synthase. High values of acetyl-CoA/CoA and NADH/NAD+ ratios stimulate PDH kinase, which phosphorylates and inactivates PDH. ATP and citrate inhibit phosphofructokinase, with accumulation of substrates from previous stages, including G-6-P, which has an inhibitory effect on hexokinase. This depresses glycolysis”
McGregor and Hansen have both said they have found lower levels than normal of lactate. So there is also something else going on: where is glucose going?
I haven’t seen this addressed anywhere yet – the high/low lactate and what is the problem with fatty acid metabolism.
Nad+ also connects to tryptophan metabolism.
How about correcting this before resorting to prohibitively expensive treatments? What are their side effects?
Thinking about ME/CFS – or a subset – as an autoimmune disease also helps us consider risk factors, which include what is now considered “a new paradigm of health and disease”. This is the understanding that life experiences – including adversity and trauma – influence our biology and physiology. It’s referred to as the biological embedding of life experiences.
The contribution of adverse experiences from prenatal events, birth events, adverse childhood experiences (ACEs) and even onset triggers that involve stress, trauma, accidents, surgery etc (as well as but not limited to infections) most easily seen in other autoimmune diseases where antibodies have been identified and can be tracked (such as type 1 diabetes).
That adds more tools for us to work with for healing as well as for understanding why we can often be sensitive to regular doses of meds/ supplements etc
I have been on infliximab infusions (Remicade) (TNF blocker) for 5 yrs and it has made a tremendous difference. Still have crashes but the “good” days are more often and better. At times I can do more now than in the last 30 yrs of ME/CFS.
Could you kindly expand ? Are the good days completely symptoms free or simply “better” form “bad” ? How often did you take the infusions and in what dosage ?
Thank you !
Massimo
It’s just so hard to get access to these powerful immune drugs but Corinne’s experience – after an infection triggered severe case of ME/CFS – shows they can be quite helpful.
Dr. Klimas has wanted to use them for years. Maybe with all the money pouring into long COVID they will get another look.
to treat an autoimmune disease,
where the autoantibodies are viewed as problematic
they give the patient *more* antibodies?
I find this logic puzzling…
It is weird but they are giving autoimmune patients (and others) lots of antibodies from healthy people; i.e. they are adding healthy antibodies to the unhealthy antibodies found in those who are sick.
I don’t know that anyone knows exactly how its working.
I have been wondering about this and just spoke to my doctor about remicade. So many of us have autoimmune issues that I’m sure are contributing factors to our synptoms. I have Hashimoto’s, why is this the only autoimmune disease that doesn’t get drugs like remicade or other immunesupressors? Lupus, MS, Crohns etc…all do. I wondered myself if drugs like this would reduce synptoms in those of us with Hashimoto’s and whatever other autoimmune issues other have with me/CFS.
Hey, chronicfatigue, especially auto immune, gets nothing. You are not alone. I live everyday like this.
I learn something new:
High levels of LDH in a blood test means that higher levels of the enzyme were found.
This test doesn’t measure level of enzyme function.
So it really only reflects more of the enzyme in the blood stream, but nothing on its function. We don’t know where it is coming from or where it is going to or why. It says on standard blood test info that it is due to tissue damage.
AND
there is a test that further looks at which LDH isoenzyme is high to further clarify location, type, and severity of tissue damage.
Thanks for adding that Meirav :). They clearly took an initial stab at it. I hope they can find the funds to do more.
Thank you for another excellent post. Apart from all the treatment possibilities that are raised at the end, I appreciated the overview of Dr. Scheibenbogen’s diagnostic work at the onset. The recent (Aug 2021) study dissecting autoantibody correlation to specific symptoms, with differences based on disease onset (post-infectious vs otherwise) appears quite carefully done, from how they normalize the results by IgG to how they apply statistical corrections. It seems to me more likely these results will hold up. This could be potentially game-changing for how people think about ME/CFS.
Let’s hope 🙂
Wonder why Dr. Scheibenbogen never mentioned anything about the BC 007 drug?
IgG Has helped me significantly. I infuse subcutaneously weekly. I take autoimmune doses which are higher than doses for immune deficiency. I am able to exercise on recumbent for 50 minutes 3-4 times a week. Before that, I could not exercise more than maybe 5 minutes. Functioning scale was 20-40% now at 70-80%. I was shocked I am a responder to this therapy. I developed ME-CFS, worsening of POTs which I lived with, and MCAD after exposure to toxic black mold. I was diagnosed with hEDS as well. My daughter had CCI and tethered cord (hEDS), MCAD and POTs. She has yet to have tethered cord surgery. My son has NMH and hEDS and MCAD. Both exposed to the mold and clearly have my genetics. Moving from east coast to southwest also helped. I don’t seem to react to mold the same way or chemicals.
I feel like I am dying. I had cancer in 2000 and I have had cfs for 22 years. For awhile,NADH helped me but now I am looking at life and death. I hope it helps.