

Several studies suggest that butyrate-producing bacteria are reduced in people with chronic fatigue syndrome (ME/CFS) and possibly fibromyalgia (FM) and long COVID. The fact that three different techniques have found reduced levels of butyrate-reducing bacteria in ME/CFS may make the finding one of the most solid in the ME/CFS research literature.
As butyrate is the primary energy source for the endothelial cells lining the gut, the leaky gut found in ME/CFS could be a function of low butyrate production. Lower butyrate levels are also associated with increased gut transit times and impaired gut motility. Besides the fact that getting the waste out of our body as quickly as possible is a good idea, slowed transit times also affect the composition of our gut flora – producing a more inflammatory gut flora. Butyrate’s support of regulatory T-cells and its anti-inflammatory activity also helps keep the immune system in check.
A reduction of butyrate-producing bacteria could be contributing to a host of symptoms in ME/CFS.
Low butyrate levels may cause sleep to suffer as well. Gut microbial diversity, in particular the diversity of the phyla (Bacteroidetes, Firmicutes) associated with single-chain fatty acids (SFCA’s) like butyrate, has been positively associated with total sleep time and sleep efficiency. A rodent study, in fact, found that butyrate supplementation caused an almost 50% increase in non-rapid-eye movement sleep (NREMS) and reduced body temperatures (enabling entry into NREMS sleep.)
Low butyrate levels may even impact energy production by decreasing the conversion of pyruvate to acetyl-CoA – the main substrate for aerobic energy production in the Krebs cycle.
Besides ME/CFS, low levels of butyrate-producing bacteria have been found in a variety of intestinal diseases (inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), diabetes I and II, celiac disease, colorectal cancer, obesity), as well as in multiple sclerosis, Parkinson’s Disease and Gulf War Illness.
Some researchers, in fact, question whether gut dysbiosis (altered gut flora) triggered inflammation may be sparking Parkinson’s disease (PD). Parkinson’s disease is interesting for ME/CFS because both gut dysbiosis and basal ganglia dysfunction (the key feature in Parkinson’s) may also occur in ME/CFS. Outside of the obvious motor symptoms, GI issues are the most common symptoms found in Parkinson’s and often precede the disease by over a decade.
The same process envisioned in ME/CFS – a breakdown of the gut barrier that lets loose inflammatory species which make their way to the brain – is being mulled over in PD and other central nervous system diseases. (Some parts of the basal ganglia appear to be particularly sensitive to inflammation.) Several proposed treatments for Parkinson’s (ketogenic diets, NAC, glutathione, niacin, and butyrate) are being considered in ME/CFS
The type II diabetes (T2D) connection is intriguing given the interest in metabolism in ME/CFS. The low butyrate connection in T2D – particularly the reduction in Faecalibacterium prausnitzii (the same gut species primarily reduced in ME/CFS) – has been well established.
Low butyrate levels, then, are being studied in a variety of diseases.
The Short-Chain Fatty Acid (SFCA) Issue
A reduction in short-chain fatty acids (SFCA) like acetate, propionate, and butyrate appears to play a large role in the gut dysbiosis in ME/CFS. At first glance, SFCAs seem like the last substance to help one’s guts out. Derived from the fermentation of indigestible carbohydrates which pass through the intestines untouched, the SFCA’s only get produced when the bacteria in the colon begin to gnaw on them.
Once produced, though, the SFCA’s are largely responsible for maintaining the integrity of the gut lining, in producing mucus, and play important role in metabolic health, energy expenditure, glucose homeostasis, and controlling inflammation.
Given their indigestibility, it’s perhaps not surprising that only a select few bacteria (mostly Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium hallii and Ruminococcus bromii,) are able to break them down and produce butyrate. One of them, Faecalibacterium prausnitzii, is the bacteria studies have find most reduced in ME/CFS.
Faecalibacterium prausnitzii
F. prausnitizii has been found reduced in at least one fibromyalgia and two ME/CFS studies. F. prausnitizi produces butyrate and other short-chain fatty acids, as well as an important anti-inflammatory product. Called “a potential biosensor of human health” and the “sentinel of the gut”, the bacteria clearly plays an important role in gut health.
ME/CFS is not alone in its low F. prausnitzii levels. The fact that low fecal F. prausnitzii levels have been found in a variety of diseases, including inflammatory bowel disease (IBD), IBS, celiac disease, colorectal cancer, obesity, type II diabetes, dementia, and most recently in long COVID, indicates quite a bit of interest had developed in finding ways to increase its abundance.
F. prausnitzii seems like a leading candidate to become the “next-generation probiotic“, but its abhorrence of oxygen and its pickiness about its growing conditions has made it difficult to produce in the large quantities needed for commercial production.
Recent studies indicate, as well, that F. prausnitzii strains can differ widely, and teasing out the most effective ones is necessary (and has already begun happening). Still, picking an F. prausnitzii probiotic from the local health food store is not likely to happen for some time. It’s going to be necessary to increase F. prausnitzii’s levels in other, more indirect ways for now.
Enhancing Faecalibacterium prausnitzii and/or Butyrate Levels in ME/CFS
How effective your gut is at producing butyrate is dependent on a wide variety of factors including the types of non-digestible carbohydrates you eat, the different types of acetate and lactate-producing bacteria your gut contains, and the levels and types of butyrate-producing bacteria present in your colon.
Diet
By supplying the prebiotic precursors F. prausnitzii needs to produce butyrate, red wine, soluble corn fiber, wheat, and oat bran, soy, almonds, apples, and fermented foods may help increase butyrate Levels of these prebiotic factors (FOS, XOS oligosaccharides, etc.) are low in foods, however, making supplementation a better way to provide them.
High fat, high protein, and low complex carbohydrate diets may deplete butyrate levels while low fat/high fiber diets may enhance them. People on ketogenic diets might want to make sure that their diets include ample amounts of fiber.
Fermented Foods

Fermented foods may increase bacterial diversity and butyrate production.
Surprisingly, little research has assessed how effective fermented foods are at increasing butyrate levels. Still, several studies suggest eating fermented like kimchi, some yogurts, kefir, probiotic-enhanced hemp seed drinks, and fermented buckwheat milk may increase butyrate levels and/or protect the gut lining.
The most impactful fermented food study recently occurred at Stanford. The study found that while fiber can be helpful in improving gut flora diversity, it suggested that increasing fiber intake may not be enough for many people.
It suggested that people with a low diversity of gut bacteria (as has been found in ME/CFS) may need to increase their consumption of healthy gut bacteria for the fiber to work. This is presumably because they lack the bacteria needed to break down the fiber and release its many helpful components.
In the end, people on high-fiber and fermented food diets improved the most in the study. The levels of butyrate-producing bacteria increased, markers of inflammation decreased, and bacterial diversity increased.
Preparing the Way
By the time carbohydrates make it to the large intestine, all that’s generally left are insoluble fragments of plant fibers (plant cell-wall polysaccharides, starch particles, oligo, and polysaccharides).
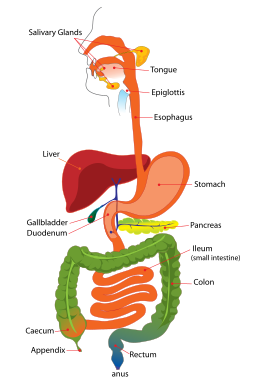
Most digestion occurs in the small intestine. Butyrate production occurs in the large intestine (green). (Image from Wikimedia Commons).
Only a few gut bacteria are able to degrade these tough fragments. Those that do, however, are able to release a wide range of products that other bacteria feed on. The process of breaking down these fragments is akin to what happens to trees in the forest. As they decay, they release products that are available to other decomposers. In the gut, this is called “cross-feeding”.
Because most butyrate-producing bacteria cannot break down these plant fibers, it’s essential that bacteria which can break them down be present and produce the acetate and lactate butyrate-producing bacteria need to function. (F. prausnitzii is a partial exception to this rule: it’s one of the few butyrate-producing bacteria able to directly degrade non-digestible carbohydrates such as inulin and pectin.)
Prebiotics
Prebiotics are tough plant fibers that do a surprising amount of good things for our gut including providing the raw materials butyrate-producing bacteria need. Prebiotics come in several forms.
Fructooligosaccharides (FOS)
FOS are found in plants like onion, garlic, bananas, asparagus root, and tubers of Jerusalem artichoke but can be produced commercially, believe it or not, from sucrose.
Inulin – Inulin is a soluble long-chain fructoligosaccharide thats digested in the lower gut to form short-chain fatty acids. Inulin, a fermentable fiber, is often found in the roots of various plants (whole wheat, onions, garlic, and artichokes, chicory roots).
Several studies, including a 2021 one indicate that F. prausnitzii’s fermentation of inulin provides a good energy source for the intestinal epithelial cells that protect the gut wall. Inulin is commonly found in prebiotic preparations.
1-Kestose – may be the most effective FOS prebiotic of all. This very short-chain FOS proved to be much more effective at enhancing butyrate production than inulin in several studies. 1-Kestose was able to increase muscle mass in the “super-elderly”. If it’s available in commercial mixtures, though, I haven’t been able to find it.
Xylo-oligosaccharides (XOS) and arabinoxylanoligosaccharides (AXOS).
Xylo-oligosaccharides (XOS), and arabinoxylanoligosaccharides (AXOS) are heteropolysaccharides and -oligosaccharides produced from the residues of crop materials like sugar cane, corn cobs, and rice straw. They appear to be particularly effective at increasing a wide variety of butyrate producers. They can be found in some prebiotic mixes.
The XOS prebiotic really shined in a study comparing FOS with XOS and antioxidants, as it consistently increased the levels of important bifidobacteria spp. and butyrate levels in different areas of the colon and over both the short and long term. (Bifidobacterial colonization, it might be noted, of the infant begins during natural childbirth.)
Galactooligosaccharides
Galactooligosaccharides are non-digestible plant sugars found in beans, root vegetables, and many others. They are readily fermented by some bacteria (lactobacilli and bifidobacteria spp, in particular) and can enhance the abundance of butyrate-producing bacteria. They may also be able to allow lactose intolerant individuals to tolerate dairy products again. They are available in some prebiotic mixes.
Others
Other prebiotics such as pectin, guar gum, alginate, arabinoxylan, psyllium fiber, red wine, and soluble corn fiber have all increased F. prausnitzii levels in human studies.
Resistant Starches
Resistant starches (RS) are another type of indigestable plant fiber that make it to the colon practically untouched where they undergo fermentation and produce the same small-chain fatty acids (acetate, propionate, butyrate) seen with other plant fibers. (Digestion – a different process than fermentation – occurs in the small intestine.) As with prebiotics and probiotics, depending on their bacterial makeup, different individuals may benefit from different kinds of RS.
Different categories of RS exist. According to Dr. Sarah Ballantyne RS1 occurs in grains, legumes, and seeds; RS2 in roots/tubers as well as green bananas, green plantains, and raw potatoes; RS3 or “retrograded starch” is found in rich in cooked roots/tubers such as potatoes as well as rice and other grains. RS4 occurs in chemically modified starches. Recently a new form of RS – RS5 -may be the highest butyric acid producer of them all.
Type 2 (RS2) (green bananas, green plantains, and raw potatoes) appears to be the best-studied of the RS’s and are most commonly used in supplement form. RS2 plus arabinoxylan (a dietary fiber) was found to increase bafidobacteria spp., acetate, and butyrate in one study. An overview of RS2 human and animal studies concluded that RS2 supplementation increased short-chain fatty acids levels, and enhanced Ruminococcus bromii, Bifidobacterium adolescentis, and other healthy gut taxa. Perhaps because of the focus on one type of starch, bacterial diversity, however, tends to decline.
Dr. Ballantyne prefers adding RS via whole foods rather than supplementing them as focusing on one type of RS may imbalance the gut flora. She does note, though, that this is likely a non-issue for people eating vegetable and fruit-rich diets.As with other plant fibers, resistant starches should be gradually added into the diet.
The FODMAPS Conundrum
A lack of healthy bacteria could be causing people with IBS to be unable to tolerate the foods that are eliminated in the FODMAPS diet.
The ingestion of prebiotics comes with an odd catch – they may exacerbate IBS symptoms. In fact, despite the fact that people with IBS are low in butyrate, a common and often effective IBS diet called FODMAPS (Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols) explicitly deletes butyrate and other short-chain fatty acid-producing foods (apples, beans, cruciferous vegetables (broccoli, cauliflower, cabbage), caffeine, chocolate, nuts, fats, wheat, garlic, onions, dairy products) from the diet.
A recent study showed that a low FODMAPS diet that reduced F. prausnitzii, short-chain fatty acids, and n-butyric acid levels – all factors associated with good gut health – substantially relieved IBS symptoms.
Other studies suggest, though, that while the FODMAPS diet can be helpful in the short run, they may produce nutritional problems, gut problems, and gut dysbiosis in the long run. While low FODMAPS diets may relieve IBS symptoms, they have also been shown, for instance, to “rapidly and negatively change the gut microbial community, abundance and diversity”.
One study attempting to have it both ways is adding a fiber mix called Fibre-fix to the FODMAPS diet in hopes of improving the gut flora, sleep quality, quality of life, and inflammation as well as IBS symptoms. Some commercially available fiber mixes specifically state they do not produce symptoms in people on FODMAPS diets.
Another study found that diets, as we know, are personal. Depending on their gut composition, some people did well on butyrate-depleting FODMAPS diets while others did better when given butyrate and other compounds.
People producing high levels of colonic methane and high short-chain fatty acid production did well on the FODMAPS diet, while everyone else did better when taking butyrate, propionate, and probiotics which supported SCFA production.
The solution may lie in increasing the bacterial diversity of IBS patients, thus giving them the ability to break down the prebiotics that are essential for good health.
Conclusions – Prebiotics
Since prebiotics are essential for good gut health, they can be as important as probiotics. Since different bacteria feed on different kinds of prebiotics, it may be best to try different prebiotics to see which ones work. Since fermented foods can help enhance the digestion of prebiotics (via cross-feeding) it may be good idea to use them as well.
Increasing gut flora diversity may be able to reduce IBS symptoms, increase the effectiveness of prebiotics, enhance butyrate production, and help heal the gut and possibly provide other benefits (sleep, cognition, energy production).
With regard to prebiotics, several studies show that prebiotics can increase F. prausnitzii levels, but prebiotic products which contain shorter-chain fructoligosaccharides and/or Xylo-oligosaccharides (XOS) and arabinoxylanoligosaccharides (AXOS) or 1-Kestose may work better.
A wide range of plant fibers, however (pectin, guar gum, alginate, arabinoxylan, psyllium fiber, red wine, and soluble corn fiber) have all been shown to increase F. prausnitzii levels in human studies.
Resistant starches, particularly RS2 and RS3 starches, have also been shown to increase small-chain fatty acids and gut promoting bacteria including, at times F. prausnitzii,

Bifidobacterium are readily available in probiotic mixes and may indirectly support the production of butyrate. (From Wikimedia Commons)
Because they provide a variety of substrates and hence opportunities for digestion indigestible plant fibers might be best be taken in combination in the midst of a diverse and vegetable/fruit-rich diet.
Probiotics
Ensuring that enough upstream bacteria are present that can supply the raw materials like acetate and lactate that butyrate-producing bacteria need is essential. There are some provisos, though.
The bacterial mix in your gut, for instance, may respond differently to probiotics than another person’s gut does. Plus, different strains of the same bacteria may behave differently. Because many probiotics do not indicate strains you could get a strain of a bacterial species (say Bifidobacterium bifidum) which isn’t as potent as a different strain of the same species.
The Gist
- Butyrate-producing bacteria are a big deal. They protect the gut lining (which is almost certainly impaired in ME/CFS), reduce inflammation, and may even impact neuroinflammation and sleep.
- Studies pretty definitively indicate, though, that these bacteria are reduced in chronic fatigue syndrome (ME/CFS) and may be reduced in fibromyalgia and long COVID as well. Reductions in these bacteria have also been found in gut disorders, multiple sclerosis, Parkinson’s, and other diseases.
- Butyrate and acetate are two small-chain fatty acids (SFCA’s) that butyrate-producing bacteria need. These small-chain fatty acids occur when difficult to digest carbohydrates and starches – known as prebiotics – get fermented in the colon. Prebiotics are used, then, to provide the raw materials for the production of butyrate.
- Without the right mix of bacteria, though, prebiotics will not get broken down properly and produce butyrate. FODMAPS diets specifically avoid foods containing the tough, indigestible carbohydrates and starches which produce butyrate. While they may be helpful in the short term in diseases like IBS, in the longer term, they run the risk of impairing gut functioning. Increasing gut bacterial diversity by using fermented foods or probiotics or using butyrate supplements to supply butyrate directly to the gut may help those who cannot tolerate prebiotics.
- Prebiotics occur in two general forms: indigestible carbohydrates (FOS (inulin and others), XOS, etc.) and resistant starches (RS2 (green banana powder, raw potato starch) RS3). Both have been shown to improve butyrate production and both are available commercially. They should be taken slowly and gradually increased over time and used in concert with a diet right in vegetables/fruits.
- The bacteria that directly produce butyrate are not commercially available but other bacteria such as bifidobacterium, in particular Bifidobacterium animalis ssp. lactis 420 (B420), enterococcus bacteria and Akkermansia muciniphila (if you’re taking indigestable carbs), and Bifidobacterium adolescentis (if you’re taking resistant starches) may be able to enhance butyrate production as well.
- Microencapsulated butyrate has been shown in several studies to increase butyrate levels and improve gut health. Butyrate supplements are readily available. (I’m don’t know if they are microencapsulated or not.)
- Several drugs (metformin, Rifaximin) have been shown to increase butyrate production. Studies suggest that taking Andrographilide, Sparstolonin B (SsnB), antioxidants, melatonin and B-vitamins may be helpful as well.
- A supercharged way to increase butyrate might be to slowly add an array of indigestible carbohydrates (with XOS perhaps preferred) or resistant starches (green banana starch), supplement them with the appropriate probiotics (including Akkermansia muciniphila if possible) add Andrographilide, take a broad-spectrum antioxidant and B-vitamin as well as melatonin and slowly increase fermented vegetables and see what happens.
- If the prebiotics cause stomach problems concentrate on increasing microbial diversity by slowly increasing fermented foods and adding probiotics. Alternately, or in addition, you might want to try butyrate supplementation.
- Note that the current bacterial composition of your gut may influence which treatments will work and how effective will be. Ultimately personalized gut treatments.
- Butyrate enhancement appears to be a growth field and quite a few studies are underway.
Bifidobacteria
That said, some types of bacteria such as the Bifidobacterium spp. have been used for many years to improve health. (Bifidobacteria are the first bacterial species transferred from the mother to the infant during natural childbirth.)
Because the fermentation of carbohydrates and resistant starches by bifidobacteria yields large quantities of acetate and lactate, supplementing bifidobacteria – which are often found in probiotics – may be helpful. B. breve, B. bifidum, B. longum ssp. Longum all appear to improve intestinal health including, in some cases, helping to prevent leaky gut.
One study found that culturing F. prausnitzii and Bifidobacterium catenulatum, and adding fructooligosaccharides as an energy source, resulted in large increases in butyrate production.
Enterococcus spp.
The enterococcus strains found in some probiotics and fermented foods may be helpful. Enterococcus strains such as E. faecalis have been used to treat a variety of gut conditions including
diarrhea and IBS as well as asthma and bronchitis. E. faecalis, E. lactis, E. hirae, E. durans, and E. faecium have all been used in probiotics. Enterococcus durans EP1 and M4-5 are two high butyrate-producing strains if you can find them.
Akkermansia muciniphila
A. muciniphila is a different beast entirely. One of the most abundant bacterial species in our gut, A. muciniphila , produces acetate, which was low in ME/CFS, and is used by Faecalibacterium prausnitzii as fuel. A. muciniphila also specializes in producing the mucus that protects the gut lining.
Decreases in A. muciniphila have been associated with type 2 diabetes, metabolic syndrome, inflammatory bowel diseases, and a score of other diseases, and the gut bacteria has been effective in protecting the gut lining in mouse studies. It’s not easy to find though. I found one brand that (at a high price) purported to be the only supplement to contain A. muciniphila.
Bifidobacterium animalis ssp. lactis 420 (B420) increased both short-chain fatty acid and A. muciniphila levels in one study. This probiotic contains it and others may as well. Note that you can more often find B. lactis in probiotic concentrations but it is not the same.
Supplementation with pomegranate extract, resveratrol, sodium butyrate, and inulin may also be able to increase A. muciniphila levels. Cranberries, pomegranate, green tea, and even red wine contain A. muciniphila as well.
One person actually significantly increased his A. muciniphila levels by eating a lot of pomegranate (!).
Resistant Starch Fermenting Bacteria
Ruminococcus bromii and Bifidobacterium adolescentis are the two bacteria known to break down resistant starches. As Bifidobacterium adolescentis is available in supplement form, taking it in combination with resistant starches might be a good idea.
Other Probiotics
Many of the bacteria species mentioned (F. prausnitzii, Bacteroides thetaiotaomicron, Clostridium spp.) may not be present in commercial probiotics. They serve to note though that having a diverse gut flora is best.
Most probiotic mixtures do feature Lactobacillus and bifidobacterium species (breve, bifidum, longum). Two studies have found that two bacterial species commonly found in probiotic preparations (Lactobacillus paracasei and/or Bifidobacterium bifidum) increased fecal butyrate levels.
Another study that combined butyrate-producing species (E. hallii, Clostridium beijerinckii, and C. butyricum), with other gut bacteria (A. muciniphila and Bifidobacterium infantis) and inulin modestly increased butyrate levels.
One study found that coculturing F. prausnitzii with Bacteroides thetaiotaomicron – a bacteria able to metabolize apple pectin (an “indigestible carbohydrate”) – caused F. prausnitzii to produce more butyrate.
Conclusion – Probiotics
The effects of probiotics can vary from person to person, and even beneficial probiotics can have negative effects in some people. The most effective butyrate-producing bacteria also do not appear to be available commercially. Still, adding probiotics to a prebiotic mix may provide the best bang for one’s buck.
Probiotics containing bifidobacteria, enterococcus strains, and/or A. muciniphila may be helpful. Because even the strains of the same bacteria species can differ, buying a specific strain that has been identified and tested may work best.
Fecal Transplantation
Because fecal transplantation should be able to provide a wide diversity of bacterial strains that can enhance cross-feeding, theoretically it should be able to help increase butyrate levels. In general, fecal transplants in IBS have resulted in increased levels of short-chain fatty acids such as butyrate. Results of fecal transplants may vary though and more study is needed.
Drugs
Metformin – It’s interesting that metformin – a drug that one study suggested may be helpful in fibromyalgia – has been found several times to enrich butyrate-enhancing bacteria including Akkermansia muciniphila. In fact, the use of germ-free mice suggested that metformin’s effects in T2D diabetes may be entirely due to its effects on the microbiome. One study found that both metformin and type 3 resistant starch improved microbiome diversity, increased small-chain fatty acids, etc.
Rifaximin – an antibiotic used by some to clear out bad bacteria in IBS – has been shown in some studies to increase levels of F. prausnitzii as well.
Supplements
Microencapsulated Butyrate
Some websites pooh-pooh the use of butyrate as a supplement, stating that it gets absorbed before it reaches the colon but microencapsulated butyrate has been helpful in ulcerative colitis, inflammatory bowel disease, and IBS and is being tested in several clinical trials. Microencapsulated butyrate may also enable some people to bypass the symptoms produced during the fermentation of prebiotics.
Microencapsulation in its simplest form simply means coating something to protect it from being degraded. One study stated that a “new butyrate oral formulation (ButyroseR Lsc Microcaps‐BLM) has been developed” where butyrate is “contained in a lipophilic microcapsule that provides extensive capacity for intestinal diffusion and facilitates slow release of the active ingredient.”
Unfortunately, I’m not sure what constitutes microencapsulated butyrate, but this product which contains the butyrate triglyceride found in butter appears to feature it. The Remission Biome project is using a product called Tributyrin-X to increase their butyrate levels. Check out how they are trying to reset their gut microbiome and return to health.
Vitamins and Supplements
A recent study suggested that using antioxidants (riboflavin, ascorbic acid, vitamin E, and β-carotene) in combination with fructo-oligosaccharides (FOS) and xylo-oligosaccharides (XOS) further enhanced butyrate levels. The antioxidants appeared to clear out bad bacteria – possibly opening the way for increases in F. prausnitzii. The use of antioxidants made sense given a recent finding that redox imbalance helps to perpetuate the leaky gut found in Gulf War Illness (GWI).
A Melatonin Connection?
Oddly enough, several studies suggest that melatonin may be able to enhance butyrate production (and that butyrate production may support the effects of melatonin as well.) In fact, one study stated that “The benefits of melatonin mainly depend on the ability of modulating gut microbiota and regulating butyrate production“.
A multiple sclerosis study linked melatonin, orexin, ceramide, and butyrate production in the gut with the circadian dysregulation of the mitochondria. (Butyrate is a mitochondrial enhancer.) It specifically states that “the effects of melatonin may be enhanced by the adjunctive use of sodium butyrate”.
(On the gut bacteria/sleep subject, one study suggests that fermenting milk with a high GABA producing strain of Lactobacillus brevis (L. brevis DL1-11) may help with insomnia.)
Sufficient vitamin-B stores – produced either from the diet or from gut bacteria – also appear necessary for butyrate production.
Gulf War Illness researchers have uncovered several unusual products that might be able to help.
- Andrographilide – improved the gut microflora in mice, and tightened up the crucial gut lining junctions by increasing the abundance of Bifidobacterium and other bacterial species
- Sparstolonin B (SsnB) – a compound derived from a Chinese herb, it increased the abundance of butyrate-producing bacteria and helped tighten up the crucial junctions lining the gut, while also reducing inflammation and neuroinflammation in GWI mice.
- Immunomodulatory Glycan LNFPIII – Lacto-N-fucopentaose III (LNFPIII), a glycan found in human milk increased butyrate producers (e.g., Butyricoccus, Ruminococcous) and reduced neuroinflammation in GWI mice.
Clinical Trials
How big a deal butyrate enhancement has become can be seen in the many clinical trials spread across different diseases that are attempting to achieve just that. These trials should help us come up with better ways to increase butyrate production and may provide some ideas for those attempting to do that. These trials include:
- Targeted diets to increase both Prevotella bacteria and the short-chain fatty acids (e.g.) butyrate.
- Adding encapsulated butyrate and probiotics ( L. rhamnosus, L. acidophilu, B. longum, B. bifidum, and B. lactis) together to reduce symptom in IBS.
- Using Dibuzin (calcium butyrate, zinc, vitamin D) to improve colon health.
- Adding hydroxycobalamin to butyrate to help with ulcerative colitis.
- Using Butyrate to Improve Insulin Sensitivity and Lower Triglycerides in Type 1 diabetes subjects.
- Using Butyrate Ultra (butyrate-producing Butyricicoccus pullicaecorum 25-3T, vitamin K2, and fenugreek) and Metabolic Rheostat (Ginseng extract, Ginseng root extract, berberine chloride, Livaux (gold kiwi powder), MegaSporeBiotic, and MenaquinGold (vitamin K2-7) powder) together in pre-diabetes patients.
- Trialing microencapsulated butyrate in inflammatory bowel disease.
- Studying a drug Beta-hydroxy-butyrate in heart failure and type II diabetes.
- Using a “colon-targeted” dietary supplement (BKR-017) in Type II diabetes.
- Daily oral supplementation with 237 ml of a polymeric nutritional formula added with 1.5 g of HMB (Ensure Advance®) in older adults.
- Adding inulin to increase butyrate production in vegans and non-vegans.
- Using the Omni-Biotic Stress Repair probiotic to increase butyrate-producing bacteria
- Using special forms (acetylated and butyrylated) of high amylose maize starch (HAMS-AB) that’s been effective in increasing short-chain fatty acid production type II diabetes.
- Using OptiMized REsistaNt Starch to increase butyrate production in Inflammatory Bowel Disease: The MEND Trial.
Conclusions
It’s not clear why, but studies clearly indicate that reductions in butyrate-producing bacteria occur in ME/CFS. Butyrate is a short-chain fatty acid that provides numerous health benefits. Reductions in butyrate-producing bacteria in the colon may produce a variety of impacts that begin with a leaky gut and could extend to neuroinflammation, problems with sleep, and even energy production.
Much remains to be learned about how to enhance butyrate-producing bacteria, but many studies are underway. While the most common butyrate-producing bacteria appear to have been identified other bacteria could play key roles. It should also be noted that most of the studies done in this area are animal studies.
Because the butyrate-producing bacteria need acetate and other materials, it may be necessary to: a) provide the raw materials needed to produce them by supplementing prebiotics (indigestible carbohydrates and/or resistant starches); and b) by enhancing the activity of the bacteria that break those raw materials down via fermented foods and/or probiotics. Prebiotics should be added gradually into the diet.
Prebiotics and resistant starches can, however, upset the guts of some people with irritable bowel syndrome who lack the bacteria needed to break them down. Fermented foods, probiotics, and/or the use of microencapsulated butyrate supplements may help avoid that. Antioxidants, vitamin B supplements, and melatonin used in combination with prebiotics may enhance butyrate production as well.
Andrographilide and Sparstolonin B (SsnB) are two herbal supplements that have shown promise in Gulf War Illness mouse models. Metformin, immunomodulatory glycan LNFPIII, and Rifaximin are three drugs that improved butyrate levels in some studies.
While specific butyrate-producing bacteria such as F. prausnitzii are not currently available other bacterial species including the bifidobacteria, enterococcus, A. muciniphila, and others may help provide the raw materials F. prausnitzii needs to produce butyrate. Fecal transplants show promise as well.
A supercharged way to increase butyrate might be to slowly add an array of indigestible carbohydrates (with XOS perhaps preferred) or resistant starches (green banana starch), supplement them with the appropriate probiotics (including Akkermansia muciniphila if possible) add Andrographilide, take a broad-spectrum antioxidant and B-vitamin as well as melatonin and slowly increase fermented vegetables and see what happens.
If the prebiotics cause stomach problems perhaps concentrate on increasing microbial diversity by slowly increasing fermented foods and adding probiotics. Alternately, or in addition, you might want to try butyrate supplementation.
A personalized approach that supplements the bacterial strains in one’s gut with the needed bacteria strains via diet, prebiotics, probiotics, and/or fecal transplants will probably work best in the long run.
(Ken Lassesen’s work on possible F. prausnitzii enhancers was very helpful in preparing this blog.)







Thanks for a very interesting article, Cort! I was expecting more info about resistant starch (RS), which afaik is a very good precursor for butyrate. You do mention the OptiMized stuff briefly at the end, but I would have liked more discussion of the different generic types and sources of RS, especially what their various effects/benefits are.
RS is mentioned in the following passage:
“SFCAs seem like the last substance to help one’s guts out. Derived from the fermentation of indigestible carbohydrates (also known as resistant starches) which pass through the intestines untouched, the SFCA’s only get produced when the bacteria in the colon begin to gnaw on them.”
But I think this in inaccurate and needs correcting. RS and “indigestible carbohydrates” are not identical or synonymous. Rather RS is just 1 type of “indigestible carbohydrate”/dietary fibre. I imagine “indigestible carbohydrate” would be a larger set than “dietary fibre,” though practically identical.Maybe I have misunderstood something though.
A source: https://en.wikipedia.org/wiki/Resistant_starch
Thanks Nick, I poured through articles on enhancing butyrate and while I certainly remember reading about resistant starch I don’t remember reading about the different types and didn’t actually know they differed from the other things mentioned.
Now that you mention RS’s I find a rather large literature on them! Don’t know how I missed them but thanks.
Glad I could help! I’ve found RS a pretty confusing subject and there are a lot of (I think obvious) questions it is hard to find good info about. The stuff you added is nice! I think there is an inaccuracy where you talk about RS3 – retrograded RS – “found in rich in cooked roots/tubers such as potatoes as well as rice and other grains”. That should be “cooked AND COOLED”. AFAIK that retrograding occurs in all dietary starches.
The prebiotic livaux, from golden kiwifruit, has helped me.
Btw, I have been a moderate red wine drinker for quite a long time. Not sure if its coincidence or not, but often when I have had a break from drinking my health has deteriorated.
Just shows how we are all different. Can’t tolerate red wine or any alcohol
I have had CFS for 25 years. I struggled with tolerating alcohol in the first few years, but like my food allergies that pretty much disappeared after 6-7 years.
I too had alcohol intolerance for a long time and then started drinking again in the last a few years. The change seemed to have coincided with the improvement of the CFS condition from severe/moderate to moderate/mild side of spectrum.
I’ve noticed that if my stomach is having a bad day, and my friends and I gather for a drink, a brandy always makes me better!
Matthias, where did you find the Livaux?
TK, me too! I have CFS at the milder end of the spectrum. Did you also have food allergies early on which cleared?
A major study a few years ago showed that our immune systems are overhyped earlier in the illness, before calming down and going even slightly underactive. I can only theorize that’s why I hard allergies earlier in the illness which largely disappeared by year 7 or 8.
Hi Ann, it’s ‘Kiwi Bio-Boost’ by Lifestream.
Thanks, Mattias.
Food supplements with Microencapsulated Butyrate Triglyceride (Tributyrin) -> http://www.Butycaps.com
Have you tried fresh golden kiwifruit, Matthias? If so, have you found Livaux more effective than the whole fruit? I have always just eaten the whole fruit since Livaux is hella expensive and it seems like the 300mg or so dose would give you much less of active component than a 150g fruit. I have not found the whole fruit eaten even 1 per day had any noticeable effect. Now I just eat it now and then. But maybe I should try the Livaux.
It’s crazy to take a supplement when the fruit is available. The fruit gives you fibre when a capsule can’t and is a natural prebiotic.
Cort, thank you very much for an outstanding and thorough article. I imagine that even more effort than usual must have gone into this.
Thanks – this blog took longer than in memory. It just kept growing and growing which I guess is a good thing.
Thanks Cort. I stick to mainly a form of keto but have lactose free dairy. This is not by choice though as I have Sibo. I’m aware of the necessity of prebiotics and occasionally sneak in a tiny amounts of onion garlic and asparagus just because I love them but my gut does not like it. I am okay with a small amount of nuts and ground linseed which give me some fiber in my diet. I still think a balanced whole grain diet is still best if you don’t have digestion or Sibo issues to contend with. It’s all about finding what works for you.
Research has been done on gut bacteria in ME/CFS patients for a long time. With many varying results. There is a reduced diversity and an imbalance of good and bad bacteria. An excess or deficiency of some specific bacteria also jump out. The question remains cause or effect. What can cause this? And what is the connection with a post-viral illness such as ME often arises. Are our gut bacteria causing a loop that we can’t get out of?
Many ME/CFS patients often had some intestinal problems before they became ill. So is this the common denominator? Can it also run a loop due to other infections or stress?
can no more research be done on intestinal bacteria that end up in the blood in ME CFS patients. Is that being researched?
Bacteria and endotoxins with a deficiency of vert acid and probably other important (nutrient) substances can fully explain ME/CFS
Clostridium butyricum is a butyrate-producing probiotic.
https://cfsremission.com/2015/10/19/miyarisan-clostridium-butyricum-revisited/
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8078720/
Thanks George – do you know if it’s available in supplement form?
You can get miyarisan from Japan via Ebay
Another great post.
Cort,are you still planning to review the Gut Health Protocol book?
I agree with Dr Ballantyne that we should be eating whole Green Bananas rather than its powdered form. Doctors that argue the opposite such as Mark Hyman are wrong on this point.
On Dr Ballantynes website… Paleo Mum you can find articles and recipes on Green Bananas etc
Cort you mention a low fat/high fibre diet I prefer(asothers here do) a resistant starch/high fibre diet.
Personally i have benefitted from intermittant fasting.
Dr Ballantyne points out that a typical western diet incluldes roughly only a tenth of the amount of fibre and resistant starch that our ancestors consumed!
Thanks. I am definitely going to do a blog on that gut health protocol. I really enjoyed Dr. Balantyne’s website. I believe I read that intermittent fasting can increase butyrate levels.
Thanks for the great article Cort. About a year and a half ago I went on a whole-foods vegetarian diet and used the company Thryve to analyze what was missing in my microbiome and supplement it. That pretty much solved my gut/IBS issues reducing misery, and I lost some weight, fixing my diabetes issues. I suppose the whole foods provided more prebiotics/ fiber and less of foods that aggravate things. I still have celiac disease and can’t eat wheat, and I REALLY have to stay away from free glutamates (like soy sauce) that wreak havoc with my gut, POTS, and brain. I’ve slacked off a little on the whole foods, but my gut seems ok now. Of all the things I’ve tried that help (Abilify, Oxaloacetate, Spironolactone, Acetyl-l-carnitine, big riboflavin, whole foods diet, low glutamate diet, Thryve probiotics), I still get relapses with too much exertion. They aren’t as horribly painful, but they still last with brain fog and weakness and POTS at least a week. My life is a lot better with a clearer mind and minimal pain, but I’m still stuck at home, up a little bit but in my recliner most of the day. I can’t get stronger because I can’t exercise. I am waiting so impatiently for them to figure out the biochemical sequence that is a post exertional malaise relapse. I guess they are working on it. I have been trying to be grateful for the significant improvements, but, AAARRRGGH as my relatives vacation in NYC and the Azores and get together for Christmas and and…. I guess I’m feeling jealous and whiny today. But I can do digital art with my newly clear mind 🙂 Yaaaayyy! Sorry, got off track there but I wanted to affirm your article and point out the glutamate issue that I think you wrote about before, and all the good things that come with improving your gut. But I’m not real optimistic that the gut things are going to resolve post-exertional malaise, from my own experience. Maybe I’m wrong. Thanks again 🙂
My personal guess is that the ME cure will come in an anti-autoimmune drug that undoes the suppression of mitochondrial activity. But I’m not ruling the gut out yet. Do you have a favorite theory?
Thanks for relating your experience Chris and congrats on getting the stomach in better shape. I like the autoimmune/mitochondria idea – that would explain so much.
I don’t really have a favorite theory but whatever happens my guess, for a long time, is that it involves the blood vessels and either getting nutrients to the muscles or the muscles/mitochondria being unable to take them up. I think its intriguing that Wirth/Scheibenbogen have now written four papers seeking to explain various parts of ME/CFS. That’s pretty impressive.
I think I’m going to send Wirth/Scheibenbogen this paper, since it may be why spironolactone is helping me and seems related. Also not a cure, but keeps me from being bed bound. https://www.ahajournals.org/doi/10.1161/HYPERTENSIONAHA.116.07911
Post exertional malaise. Yes, pacing is very tricky.
I still find a very reduced version of some yoga exercises I have practiced for 40 years tolerable + more recently a v short walk or countryside tour(not exceeding 1/4 hr) on an electric bike, later in the day…helps to keep muscles working without stressing. But visits and excursions extremely risky and there is always a price to pay. I am coming up to 79 and can’t reasonably expect remission, just desperate to hold on to what I have.
A regular kimchi and brown rice eater here. At times I’m forced to go off my regular diet (for several months at a time) and I can’t say I have noticed much difference in my energy level due to diet change. The bowel movement and poop quality definitely changes for better though.
Oops, I meant: “..changes for better when I’m on kimchi and brown rice diet”
Everything that’s supposed to be good for my gut is legitimately terrible for me. Butyrate makes me sh*t epic amounts. So does VSL3. Anything fermented gives me drunken brain fog. So does resistant starch. Can anyone help? I do not take health suggestions from ableds but welcome them here.
Darn….Sorry to hear that. Maybe slowly try to introduce more probiotics? I wonder if your gut needs more diversity to break down these substances. Maybe try mast cell inhibitors as well? I’ll have something on fermented foods and mast cells when I do the gut health blog.
@Kiwi I too have that dreaded drunken type of brain fog especially after consuming carbs/grains, the severity being directly proportional to the amount I eat. Haven’t found any supplement till date that would help with this particular symptom except avoiding/minimizing intake of said ingredients. Any ideas to counter this are welcome.
Hey Kiwi. Sorry to hear it.
I might be out on a limb here but maybe fibre isn’t for you? Some patients on HR seem to do better on a paleo high fat, low carb diet. Others, like myself, do better on a semi-vegetarian, high fibre, low fat diet. I wonder if this stems from our genes and whether our liver works best with the lypolitic (fat) pathway for breaking down food into energy or the glycolitic (carb) pathway. Nature supplies most of its food in high fat, low carb or high carb, low fat formats and generally only modern processed foods combine fat and carbs in one meal.
If you struggle with high fibre foods, maybe healthy fat is more gentle? I agree with Cort too about considering the impact of mast cells and allergies, especially if fermented foods are a problem. It certainly takes a while to figure out what your digestive and immune system can happily cope with and what it objects to. You know your own body best. Good luck.
Might be MCAS reaction/s. Common in ME/CFS. Fermented foods are very high in histamine, & majority (not all) of ppl with MCAS have problems with histamine.
The Wikipedia article on resistant starch reveals that rural South Africans eat ten times the amount of resistant starch than the typical western diet has.
Wow…
Free on Kindle The Potato Hack by Tim Steele all about resistant starch. Just started reading it
Hi,
Wow cort….i do not have CFS but my mind is reeling with all the information you managed to collate here. I feel for those with brain fog.
As ever there are a myriad of complexities in such a subject and you should be lauded for your great effort to make it as clear and understandable as possible.
I am sure all this mental exertion takes it toll on you at the time of preparation…but i sincerely hope that over the long term it will keep your grey matter in good shape for a long time to come.
Some products that I think are great quality and fit with the article suggestions:
Also backed by studies-
https://www.turmericandhoney.co.uk/personal-care-c54/tesseract-probutyrate-300mg-120-capsules-p26348/s26348?utm_source=google&utm_medium=cpc&utm_term=tesseract-probutyrate-300mg-120-capsules-tsr-06&utm_campaign=product%2Blisting%2Bads&cid=GBP&gclid=Cj0KCQiA7oyNBhDiARIsADtGRZbrvVWrOgtOXatVBcQA8eLiKnuhGkf-iwPtRTO7q4EP9udGybcYJEMaAvKSEALw_wcB
https://microbiomelabs.com/home/products/megapre/
Hoping these may be a useful suggestion for some people. Thanks Cort. Oh also- Just Thrive probiotic is awesome and backed by studies too!
Potato Hack suggests eating raw potatoes. Just started trying it!
You ok from those raw potatoes, Wallace? Raw potatoes do contain a substance called solanine that is poisonous. It usually just causes stomach ache, though that could be excruciating if you ate enough. Solanine is destroyed by cooking. Solanine is also not present in raw potato starch (whether because it’s been cooked or removed some other way, I’m not sure). I reckon potato starch (like the Bob’s Red Mills stuff) or cooked potatoes cooled at least overnight in the fridge are your best bet for potato sourced RS.
https://en.wikipedia.org/wiki/Solanine
Regarding all that government money to study long-Covid, here is an article which reviews if vaccines actually can help with long-Covid symptoms. It also details other aspects of this research such as the RECOVERY project which was set up to survey long-Covid symptoms and possible treatments.
I’m just posting this because it is our hope that the big block of NIH money to study long-Covid might actually be filtering down to sufferers of ME/CFS. Unfortunately, not much of anything was mentioned about this in the article…
https://www.nature.com/articles/d41586-021-03495-2
Really interesting…I tried supplementing with butyrate, and with inulin (Jerusalem artichoke)…in both cases, after one capsule…I became extremely sick…with the same exact symptoms that triggered my CFS 20 years ago. Sore throat, extreme fatigue/pain, aches/flu-like feeling, mucus drainage and extreme, intense coughing, that took weeks/months to get over.
This is fascinating. Could it be that butyrate is causing an issue and the reason why butyrate is low in people with cfs is because the body has responded to this issue in order that less butyrate is produced?? A lot of cfs buomarkers are likely to be things that have developed due to, or in response to, cfs and therefore don’t hold the key to a cure. Which isn’t to say that the more people can understand about what’s going on the better of course
I’m late to this thread but just wanted to thank you, Charlie, for making an excellent point!
Also, Cort, so incredibly impressed with this article!
I’m housebound with CFS except to leave the house a few times per week for necessary reasons like doctor appointments and groceries.
In 2020 when I initially switched to an almost-vegan diet after 20 years of either Keto or very low carb dieting, I felt about 20% better energy wise and my brain fog reduced by an astonishing 80%! That lasted about a year, when I lost the increased energy but my brain fog is still better.
At first I was getting vegetable and fruit boxes delivered (i.e. ugly foods or farm rescue type boxes) and eating 5 to 12 servings of fruit and veg per day (I read Fiber Fueled). Tons of fiber and reduced trips to grocery store, and reduced decision making about what to eat were all pluses. However, I’ve since cancelled those subscriptions and started eating more convenience foods; all the chopping and standing required for healthy cooking was using up much of that net gain in energy, and there were some quality issues with my boxes, so I was still just as frustrated with my condition.
Anyhow, since my brain fog improved significantly on the lower fat, super high fiber almost-vegan diet, and has remained much improved for two years, I’m trying to stick with it but in reality have settled into a low fat, whole foods, vegetarian diet which allows two slices of vegetarian and a few eggs per week, mostly because cooking low fat And vegan is either too complicated or gets too boring. Eating lots of fresh fruit is easy enough.
I don’t miss meat and do believe it, or just too much saturated fat and protein was the main reason for my brain fog.
My blood sugar normalized after about 6 months on the plant based diet going from a fasting blood sugar of 111 to 70, even with the occasional pizza cheat, so I went off Metformin about 6 months ago thinking fewer meds is always best.
Gradually since then I’ve been feeling worse than I have since 15 years ago, onset of CFS, energy wise, even with the improved brain fog. Thanks to your article here, I am convinced that going off of Metformin may have been a big factor in this, and I’m going to start it up again.
Thank you.
Fiber Fuel Doc Will Bulsiewicz is also against taking fiber supplements. We have to eat the whole food folks! No choice.
Potato Hack p188 says we need to take 20-40 grams of RS2 which is 1 raw potato or 2 Green Bananas.
I have been on Green bananas for 2 weeks now on 1 Potato and 1 Green Banana daily.
Ive eaten worse things!
I don’t think he is. At least he developed and sells his own fiber-supplement “38TERA”.
Nice article. I was surprised you did not reference that resistant starch’s fermentation in the gut produces more butyrate (vs. other fermentable fibers). http://ajcn.nutrition.org/content/73/2/415s.short. Of course, it also depends on the source of resistant starch and the microbiota. I have been intrigued with Darrell Cockburn’s new research on the topic. His research is showing that green banana resistant starch produces more butyrate across different individuals and different microbiota compared to other sources of resistant starch. https://pubmed.ncbi.nlm.nih.gov/33995299.
You might also find http://www.ResistantStarchResearch.com a good resource.
I have all of the 285 clinical studies on resistant starch referenced and linked (except for the brand new one in Parkinson’s patients – https://www.sciencedirect.com/science/article/pii/S167202292100245X?via%3Dihub#b0095)
I have ME/CFS and Fibromyalgia more then 40 years (after a Meningitis 1977 with 3 1/2 years) , 2013 they found out I have FM and 8 years ago CFS ,it started to be very hard (more then 16 hours sleep-time a day) and so they say, ok it’s more then only FM. Before it was a very long way for the diagnosis from “you are only growing ” till “don’t play ill and go to School /Work.I have 2 wonderful adult kids with no symptoms of one of the disease and I’m very proud.
2 Years ago it be badder because i have to sleep 20 -23 hours and they say, ok after so long times “it goes in the last round”. But I learned in my life to trust me and my body and not every thing one write (without having one of this one “bad guys”). And I found out that the big problem was Carbohydrate . SO I try every one from Bread to fish, from Apples to Milk Products and I found a “diet” to stop this way down. So I eat only milk-proteins and Milk-products, flesh from Chicken and turkey , and oatmeal . So I can stop it (back to 16 – 20 hours sleep a day) and I learn that 1 half Bun send me to bed and need a lot of energy . So if I read this with the butyrate it make sense why I have less Problems with the Milk Food (Proteins only from milk ,nothing vegan).I don’t believe that this is only the Illness but a “foot” and if we understand , we have only to found the rest 😉 And with Ibuprofen (for Inflammations) Oxycodon (against the FM Pain) and Dronabinol drops (against the muscle spasms) and a lot of humor I love my life.Believe yourself and your body. Ok the last 2 Month it have to be badder (20 hours + a day in bed) but with luck it was only the winter flare. I wish us and our family’s only the best stay strong take your vaccination shots and wear a mask 😉
Bravo Rhonda W your website is a treasure!
Do you agree with Dr Bulsiewicz that we need to eat whole food rater than fiber supplements to get to 35-50 grams of RS2 daily?
Looks like a great heap of info . So sad that the vast majority of people with cfs would have no hope in taking in and take meaning out of that much information, even in the highly unlikely case they had the prerequisite knowledge to understand the microbiology speak.
I’ve been taking this: https://www.cosmopharma.com/activities/product-pipeline/zacol_nmx_nutraceutical
It’s butyrate + inulin in a tablet with a special technology that ensures the active ingredients are released in the colon and not before.
I’m not sure it’s helping yet, but I tolerate it just fine.
More info about Tributyrin (Butyrate Triglyceride) https://compoundsolutions.com/tributyrin-and-gut-health-heres-the-scoop/
Thanks Cort, lots of information to try digest (pun intended) there. One thing that puzzles me though, isn’t it generally considered that fermented foods be avoided until you have fixed any leaky gut issues? As the leaking of bacteria into your system can cause nasty effects.
I am gutted (sorry!) at having found out that I cannot order Akkermansia from the U.K. as Pendulum only ship within the USA. Whilst guessing not, is anyone aware of how I might get hold of it? Another company,
https://www.a-mansia.com/
say they will be going into production this year. So it’s fingers crossed.
Hey wetherlam, have a look here:
https://www.amritanutrition.co.uk/products/akkermansia-30-capsules
Has anyone had noticeable success with diet and pre and pro-biotic supplements?
And… how do you make fermented buckwheat milk?
I recently discovered that a probiotic developed for the long covid Phyto-V trial in the UK and which was successful at alleviating long covid symptoms describes itself as containing 5 butyrate producing bacterial strains!
I have been taking it and it definitely keeps my stomach ‘together’ and stops me getting a kind of weak feeling in the pit of my stomach I associate with CFS crashes and seems reduce fibro pain elsewhere.
https://yourgutplus.com/product/yourgutplus/
Thanks for passing that on, Alice!
Thanks Alice! Wondering if you have mast cell issues? I do, and can’t always take probiotics. If you have mast cell issues and can still take it, maybe I would have a better chance of tolerating it.
No I don’t Birdie as far as I know. It might be worth asking the manufacturer if they know. It has 400iu of vitamin D3 in it also x
I have been taking butyrate supplements for 3 years now. Positive results so far. Improved health for sure. I took the Viome stool test which identified my need. I used the brand BodyBio. Bought it through Amazon.
Given that a reduction in Butyrate-producing bacteria has also been found in HIV patients, it seems that chronic viral infection might also be responsible for the alterations we see in the gut microbiota of M.E patients
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5263163/
I have been taking Butyric Acid (Butyrate) for the past four years now. MAJOR improvement. Slow and gradual. Fecal transplantation should not even be considered here. The butyrate will restore a person slowly but surely.