


What treatment options to try is a question that has bedeviled ME/CFS/FM patients.
Medication and Treatment Decisions – Navigating Online (mis)Information & Evaluating the Evidence
What to do when robust evidence; i.e. evidence-based medicine – medicine that has been thoroughly assessed by scientific studies – just isn’t present? That’s the dilemma that both ME/CFS/FM and long-COVID doctors and patients have faced for decades, yet this is the first time that I remember anyone explicitly addressing it. (Thanks to Karen for letting me know about it :))
If anyone knows how to tackle this knotty issue, though, it would probably be Dr. Ric Arseneau, an ME/CFS/FM specialist who once taught medical residents how to interpret and use evidence-based medicine.
Dr. Arseneau ((MD FRCPC MA(Ed) MBA FACP CGP) has an unusual history. He started off as a veterinarian, became an MD, received an MA and a PhD in Adult and Higher Education, garnered numerous teaching awards, then got an MBA in Business Administration, and about ten years ago trained in Group Psychodynamic Psychotherapy.
In 2021, he was the Physician Lead on the Provincial ECHO Education Program for Long COVID and was the Director of Program Planning for Complex Chronic Diseases Clinic at the BC Women’s Hospital from 2013 to 2019.
Before we get to the treatment section, check out a British Columbia long COVID update from Dr. Arseneau.
A British Columbia Long-COVID Clinic Update
Lamenting the closing of the province’s Post-COVID Recovery Clinics, Dr. Arseneau reported that “very few” B.C. physicians he knows of are taking on the long-COVID patients despite the fact that:
“in the majority of (long COVID) patients that… (he sees), it’s lifelong. About three-quarters of the patients that (he sees) are unable to work.”
“This is real. We need to have an organized approach to taking care of these patients. The haphazard (approach), wherever you happen to fall, is just not going to cut it. The idea of closing these long COVID clinics — it’s like I tell my patients, we may be over COVID but COVID is not over us.”
For its part, citing a reduction in monthly long-COVID referrals, from a high of about 755 in 2021 to 80 per month, British Columbia reported that it consolidated its five post-COVID clinics into a virtual, online service.
Arseneau reported, though, that the complex, chronic diseases clinic at the B.C. Women’s Hospital has a 3,000-person waitlist.
How to Decide What Medications/Treatments to Try When Large Evidence-Based Medicine Studies or Protocols Aren’t Available
Dr. Arseneau posted this video on his ME TV YouTube channel.
Note that I added some tidbits to the talk – many of which are in parentheses – which may not conform to Dr. Arseneau’s thoughts.
Neither ME/CFS nor fibromyalgia have much in the way of evidence-based medicine – at least not in the way it’s usually thought. These diseases don’t have the 10,000-person randomized, placebo-controlled trials to go on that cardiologists, say, do. We’re really happy when we get a 100-person trial!
Dr. Arseneau noted that if he could only make treatment prescriptions based on meta-analyses of large clinical trials that provided practice guidelines, he literally would have nothing to offer his patients.
That said, evidence-based medicine has evolved and now incorporates what the patient is willing – and can – pay, what type of risk the patient is willing to take on, what kinds of treatments they prefer, who they trust, etc. Plus, deciding what treatments to try has taken on extra meaning in the era of Dr. Google, blogs, Twitter accounts, etc.
Four Factors to Keep Aware of
- Beware of vested interests – cigarettes were once touted as cures for asthma
- The credibility of the source – is it a blog (and if it is, is it a good blog?), a tweet, a patient report, doctor’s report, etc.
- Quality of the evidence – is it a rigorous study with large numbers of participants, or something else?
- The benefit, the cost, and the risk – Arseneau’s key question – what is the potential benefit of the treatment compared to its cost and possible risk. In general, the higher the cost or risk – the better quality of evidence is required.
Stay Away From the Top of the Funnel of Knowledge
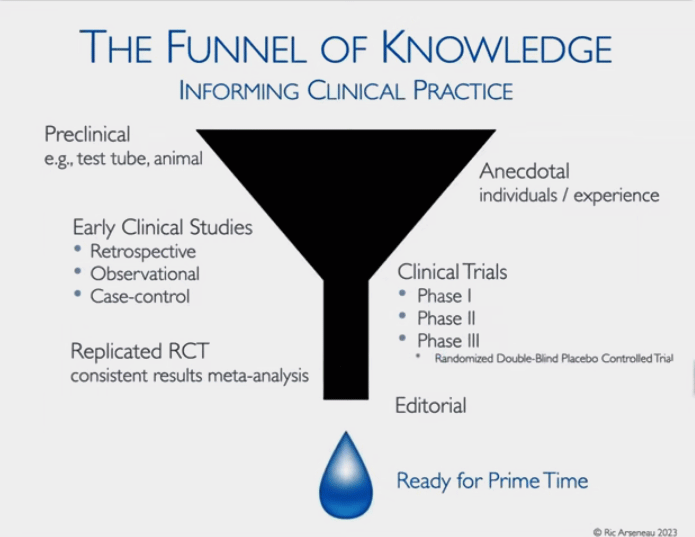
The Funnel of Knowledge – big at the top and small at the bottom – where it really counts.
The funnel of knowledge is wide at the top; i.e. there are lots of things that look good in preclinical studies, the majority of which will probably never pan out. These include lab and animal studies that may take decades if ever, to come to fruition – yet media outlets or anecdotal reports treat them as if they are real possibilities.
Beware the “monetization of early research findings” by supplement companies. It’s not uncommon at all to see supplement companies jump on the bandwagon based on a small study on rats which is unlikely to pan out.
Moving down the Funnel of Knowledge, we get the more primitive clinical studies. Going from less to more trustworthy, we have:
- Anecdotal – non-statistical subjective assessments
- Retrospective trials – historical assessment of efficacy and safety using doctor records; no placebo controls, no randomization – prone to bias and confounding
- Observational – no placebo controls – mainly used in an exploratory context
- Phase I – small trials to assess safety, treatment response to various doses, no placebo-controls, randomization
- Phase II (several hundred patients) and phase III (double-blinded and randomized trials that can contain thousands of patients) – are required for FDA approval of a drug…and need to be validated
- Meta-Analysis of Past Studies – indicates that the treatment really is… ready for prime time.
(The only meta-analysis of treatments for ME/CFS that I can remember have been for cognitive behavioral therapy and graded exercise therapy – two treatments the UK and the Netherlands governments poured enormous amounts of money into. Neither fared particularly well.)
The Most Important Thing
The most important thing is pacing and energy conservation, but we tend to go for the goodies, the quick fixes which he called the icing, and the colorful sprinkles on the cake – many of which are quite expensive.
When you have a “well-developed” practice of energy conservation and pacing – and your symptoms are stable – that’s when you go for the goodies – which is what this video is about.
(Arseneau’s focus on pacing and energy conservation brings to mind Dr. Martin Lerner’s admonition to his long-term herpesvirus antiviral treatment patients – that it would not work unless the patient practiced pacing.)
Two examples of going for the colorful sprinkles on the cake are stem cell therapy – one clinic offers it for $50,000 (with nary a single human or animal study) to recommend it and blood washing ($60,000).
He had one patient who spent a quarter of a million dollars traveling around the world and learned the hard way about unproven claims.
Source credibility – he likes Health Rising and the COVID Weekly newsletter, as well as Canada’s Drugsearch.ca which shows which drugs are covered by which plans and what the cost is.
The Three Main Questions
The three main questions in his algorithm regarding possible treatments are:
- How good is the source?
- What’s the cost?
- What’s the risk?
The higher the cost and/or the higher the risk – the better quality evidence is needed to move forward.
Next, Dr. Arseneau used this formula to assess a wide variety of potential past and present treatments. A big part of the formula depends on the patient. Is cost a factor? Are they comfortable with the risk involved? Is it the kind of treatment they want to try?
Rituximab – An Unfortunate NO
The Rituximab trials are a case in point how a seemingly good thing can go bad when it’s fully and rigorously tested. The Rituximab saga started off well with a placebo-controlled, double-blinded trial that was successful, but note how small it was – just 28 patients. Still, the results were good with 64% of patients achieving good results – some of whom had REALLY good results.
That was enough to convince some people to pay several thousand dollars to try out this powerful drug. (While most people probably weren’t harmed, Rituximab made Whitney Dafoe much worse – which brings up another factor doctors surely assess: if a bad reaction occurs, how well will the patient be able to tolerate it? One pilot, in vitro (lab) study suggested that Rituximab may impede natural killer cell functioning.)
Three years later, though, the large follow-up trial failed. Despite the early reports of patients recovering, more patients getting the placebo did better than those on the drug.
(While it seemed clear from earlier studies that some patients did recover on the drug (or was it the placebo effect?), the second trial also made it clear that that progress didn’t apply to the ME/CFS population as a whole; i.e. the risk-benefit ratio was out of whack – it’s simply not worth spending thousands of dollars on a powerful drug that the study indicated probably will not work.
(One last note on the Ritxumab saga. Dr. Scheibenbogen believes that cost-cutting measures which included a reduction of the maintenance dose in the Phase III trial could have affected its results.)
Low Dose Naltrexone (LDN) – A Clear YES
Low-dose naltrexone (LDN) presents a different kind of possibility. LDN is a relatively inexpensive drug that usually produces minimal side effects (if dosed up slowly). The drug increases levels of the feel-good chemicals called endorphins that are likely lacking in these diseases, and can reduce neuroinflammation as well. (Dr. Arseneau joked it puts the glee back in glial cells :))
The quality of the evidence is not great – a couple of small, randomized, fibromyalgia studies (none in ME/CFS) – show about a 20% improvement in symptoms.
If about 70% of ME/CFS patients have fibromyalgia, and pain and fatigue are common in both diseases, though, and there’s evidence that LDN is helpful with chronic pain – you can infer that LDN is worth a try in ME/CFS.
If it was expensive or posed a real risk, Dr. Arseneau would give it a pass based on the evidence – but the fact that it’s relatively affordable, safe and there is something of an evidence base for it makes it a no-brainer for a knowledgeable, ME/CFS/FM expert. (Another reason to question the RECOVER Initiative passing on trying it out in long COVID).
Dr. Arseneau noted that a large, randomized, placebo-controlled LDN FM trial and a long-COVID trial are underway – and an ME/CFS clinical trial – one he will surely be glad to see is about to be announced.) The 99-person Danish LDN FM trial has finished up and a 120-person FM Spanish trial and a small FM+migraine trial is underway in Philadelphia.
That long-COVID trial, by the way, is a nice-sized trial (n=160) that’s taking place at BC Women’s Hospital. According to clinicaltrials.com, it hasn’t started recruiting yet. Contact Travis Boulter, 236-990-9519; LDNtrial@phsa.ca for more information.
The cost is the fly in the ointment for some LDN – which is usually compounded. It’s not expensive so far as drugs go but is not covered by insurance and with 2/3rds of his patients not working and the majority of his patients on disability, the cost can be a problem.
A new study, though, which indicated that naltrexone is stable in liquid makes it possible, though, to put a 50 mg tablet into 50 ml liquid and use a 1 or 5 mg syringe to titrate it (starting at 0.5 – 1 – 1.5, etc. all the way up potentially to 4.5 mg). Theoretically, you get to very low doses – like .1 mg or even lower using this approach. Three naltrexone tablets cost $30 making it affordable for many but the real punch line comes in the fact that while low-dose naltrexone is not covered by insurance – naltrexone is.
Use the handouts on his website “Naltrexone – How to Make it Yourself” and “Naltrexone” to bring to your doctor and get the prescription. With its low risk and zero cost when using the titration option, it was easy for Dr. Arseneau to recommend LDN.
Abilify (aripiprazole) – safe, cheap and possibly effective – a definite YES
In low doses Abilify (< 2mg) increases dopamine levels (another feel-good chemical) in the brain. A retrospective study – not very high-quality evidence – found that about 75% of patients responded.
The study results were almost too good to be true: about a 50% reduction in fatigue and brain fog and 20% of patients reported that their post-exertional malaise had disappeared. With no healthy controls, the quality of the evidence was not great, but the drug’s mechanism of action – reducing neuroinflammation in the brainstem – made sense.
Abilify clearly fits in the LDN category. While the evidence base is not great, the drug makes sense, is cheap and the risk of side-effects is low. It’s a clear YES in Dr. Arseneau’s book.
Oxaloacetate – Safe and Possibly Effective But Very Expensive – a Clear NO for Most (but Not All) Patients
Oxaloacetate is another intriguing candidate. It’s mechanism of action – it generates NAD+, helps with glucose metabolism, amino and fatty acid synthesis, and energy production – makes sense and the risk of side effects is low. Since it’s a supplement, it’s readily available. Like LDN and Abilify, it has something of an evidence base but not a strong one.
The 76-person, 6-week study found that more was better (1,000 mg 2x/day was better than 500 mg 2x/day) with the higher dose resulting in a 60% reduction in fatigue (with the proviso that the fatigue reduction did not show up in all the fatigue scores). Overall, 80% of the patients showed improvements, with some patients showing really dramatic improvements. (Indeed, Dr. Kaufman began the trial because he was surprised at the improvements some of his patients reported.) The study was not, however, randomized.
Nevertheless, so far so good – it’s a safe treatment, has a good mechanism of action and while the quality of the evidence is not great, it does have an evidence base. The minimum cost (500 milligrams 2x a day) is about $500/month and the more effective dose (1000 mg 2x/day) at $1,000/month is double that.
In this case, the evidence base is not robust enough for Dr. Arseneau to recommend oxaloacetate given its high cost. Of course, for people for whom a couple of thousand dollars a month is a drop in the bucket – it’s another story. He has some patients who have told him it works but doesn’t have enough of those patients, though, to be able to get a handle on how effective oxaloacetate is.
A long-COVID oxaloacetate study is underway…
Repetitive transcranial magnetic stimulation (rTMS) – a qualified YES
rTMS is in an entirely different animal. Instead of existing on the fringes of medicine like LDN and oxalacetate, rTMS is FDA-approved for depression, obsessive-compulsive disorder, migraine and smoking cessation, has obviously been well studied, is being used in fibromyalgia, and is being assessed in many diseases including ME/CFS, cognitive issues, neuropathic pain, Alzheimer’s, Parkinson’s Disease, stroke, ADHD.
It’s even received two small studies in ME/CFS (one a case-report series, the other a “open-label” study) – both of which concluded that it may help with fatigue. Plus, it’s also painless and safe!
What’s not to like? While the quality of the evidence base (case report series, open-label trial) in ME/CFS is not great, the evidence suggests it can work in FM and the mechanism – boosting the prefrontal cortex (a known problem in ME/CFS) and taming the limbic system and the fight/flight response – makes sense.
In this case, if a patient can find someone to pay for it, or if an insurer will, Dr. Arseneau usually recommends giving it a try.
An intriguing Solve M.E.-funded UCLA ME/CFS study is underway that’s targeting two areas of the brain in an attempt to tame the amygdala (the screaming almond of terror!) and the limbic system and calm down the fight/flight response and reduce pain.
Of his patients who’ve tried it, some have improved and some have not.
Amygdala Retraining Program – YES
Ashok Gupta’s Amygdala and Insular Retraining program (AIR) is the first to give neuroplasticity a test in ME/CFS/FM and long COVID. A randomized trial in fibromyalgia which used relaxation therapy as a control found that the AIR program produced significantly greater reductions in functional impairment, anxiety, and depression and a higher improvement in mindfulness and self-compassion. No changes in cytokines were found, but significant decreases in a pain factor called BDNF were found.
A US long-COVID study that used a control group that participated in an online program that involved dietary management, stress reduction, etc. also achieved good results. The study found a significant decrease in fatigue after 3 months and double the increase in fatigue compared to the control group in the AIR group. The authors proposed that the program had helped calm overactive and hypervigilant nervous and immune systems.
If a patient is open to neuroplasticity, trying Gupta’s AIR program given its low costs was a clear YES for Dr. Arseneau.
CT38 – Promising Mechanism – Not Ready for Prime Time – NO
Cortene’s CT38 drug presents yet another intriguing scenario. Arseneau called the hypothesis behind the drug – a quick reset of the limbic system and the HPA axis “interesting”, and noted that it fits the current thinking about an always-on stress response in ME/CFS/FM. The drug attempts to turn off a receptor called CRF2 – and return the system to its normal state (homeostasis). He linked the drug’s action to the amygdala (that screaming almond of terror).
The mechanism was good, but the very small trial – which primarily assessed safety – had moderate results. Since the drug would be very expensive to get, CT38 is not ready for prime time without further study. (Cortene is trying to raise the money for a larger study).
Metformin – Gold Standard Studies Abound – YES (if you get a COVID infection)
Next, a new entry to the ME/CFS/FM field – a commonly used drug with a long history behind it – metformin. The huge, long, multi-site, randomized, blinded, placebo-controlled COVID study clicked all the boxes – the first to do so in Arseneau’s review. While the study was a preprint- and had not been peer-reviewed – it looked to be a strong study – indeed, Dr. Arseneau called it a “gold standard” study.
It found metformin produced a 42% reduction in the risk of coming down with long COVID over time. The drug is cheap, is “relatively well tolerated” (diarrhea can be a problem), and is relatively “low risk” if you’re trying it for two weeks. Plus, it’s cheap.
Arseneau noted that while he’s traveling, he brings it, Paxlovid and COVID tests so he can take it if he gets ill. Trying metformin for ME/CFS is fine given its cost and risk profile, but he’s not had patients respond to it.
The same study found that Ivermectin – (a hot internet item at one time that so many were sure – absolutely sure – was helpful)- failed miserably.
Guanfacine – YES (if you can afford it).
A very small study (n=12) of guanfacine (1-2 mcg) plus NAC (600 mg) found that 2/3rds improved. One-third stopped it because of dizziness – adding salt may help with that). The drug’s typical use – ADHD – was promising given how much ADHD is found in ME/CFS/FM.
Plus unlike other ADHD drugs, it’s not a stimulant and can calm the “screaming almond of terror” – the amygdala – and thus the fight/flight response. Stimulant drugs like Vyvanse, Ritalin, and methylphenidate, can be helpful in ME/CFS/FM but can increase anxiety and dysautonomia and provide a false sense of energy which can send patients crashing.
The vast majority of his patients who stay on Guanfacine have reported a reduction in their fight/flight response and some found an improvement in their heart rate variability – a physiological measure of the fight/flight response.
The drug is not cheap in Canada (@$100-$165/month) (but is much cheaper in the US). He starts patients at 400 mcg/3xs a day or 600 mcg/2xs a day. After two months, double the dose to see if it helps.
Micronized PEA (palmitoylethanomide) – A clear YES
Micronized PEA shows how wide the world of treatment options is as PEA is in a category all to itself. A “food product”, it’s been well assessed in Europe and indeed has been studied enough for a meta-analysis to have been done which concluded that it can be effective in treating chronic pain (but has rarely, until recently, been mentioned much in the U.S. and Canada.) Dr. Arseneau noted that 5 years ago it had to be imported from the Netherlands at about $200/month but can now be purchased for about $30-60/month.
PEA has been so well-studied that Dr. Arseneau called the evidence base “the best we ever get for these conditions at virtually no risk”. It’s a clear yes for those who can afford it.
THE GIST
- It’s a great question – given limited resources of energy, money, and time how to choose which treatments to try? Dr. Ric Arseneau, who was the Director of Program Planning for Complex Chronic Diseases Clinic at the BC Women’s Hospital, has some ideas.
- Noting that diseases like ME/CFS and FM don’t get the 10,000-person randomized, placebo-controlled trials that major diseases rely on for answers Arseneau asserted there are ways to filter the wheat from the chaff.
- He suggested assessing 4 factors. First, beware of vested interests – companies willing to sell anything to make a buck. Ask if the information is from a credible source (he likes Health Rising and the Long COVID watch), what kind of evidence backs up the claim (full-fledged, large, randomized, and placebo-controlled, trial?), and finally, the benefit, the cost, and the risk.
- The three main factors are: “How good is the source?”, “What’s the .ost?” and “What’s the risk?”
- Whether or not someone decides to try something depends on their tolerance for risk, their financial situation, and the kinds of treatments they feel comfortable with.
- Dr. Arseneau then worked his analysis on a dozen well-known ME/CFS treatments including Abilify, LDN, rTMS, Guanfacine, and Metformin. Check out what he said on those in the blog.
DAO – A Mast Cell Stabilizer
A small 2019 open-label study found that 0.3 mg of DAO taken before meals 3xs a day in patients with histamine intolerance “improved all symptoms” and when the supplementation stopped – the symptoms came back. The evidence base was not great, (small open-label study, no placebo control/randomization) but with the risk (side-effects) being virtually zero, it all comes down to cost – which at $65-$90 Canadian is not low.
Dr. Arseneau then presented a most interesting slide – a common medication list he might give to a patient.
Q & A
What about hyperbaric oxygen treatment (HBOT)? It’s definitely useful – the problem is the cost. He’s interested in nicotine patches. There is a risk of addiction and nicotine is a stimulant that could make things worse but he is going to look more into it. He’s never seen any evidence that nutritional IV’s are helpful.
Resources
- Great Resource! Dr. Arseneau has handouts that describe benefits, side effects, dosing, etc. for over 40 treatments on his website. You can either download them to your computer or to Dropbox.
- Check out Dr. Arseneau’s past video’s and signup for his ME TV YouTube channel here.
I hope that Dr. Arseneau does another session like this and covers things like stellate ganglion block, blood clotting medications, vagus nerve stimulation, nicotine patch, Rapamycin, craniocervical instability surgery (apparently not available in Canada).






Arseneau pushes his ‘think yourself better’ course – that’s all patients need to know.
Thank you. That’s very helpful to me. Also, having looked at the Health Rising posts on the studies linked here, I see that some of the things he says “yes” to help patients for a few months and then have no effect, according to all the comments.
I don’t think I learned anything here, except for two things: take metformin for 2 weeks (along with Paxlovid) during a Covid infection, to lower chances of long Covid; and look into PEA, which has zero side effects and helps all kinds of things because it’s a potent anti-inflammatory.
Well, it looks like you’re up on a lot of stuff Agatha! I hope the PEA helps. I have yet to try it but plan to.
Hopefully you won’t need the metformin but if you should – it’s a nice option. Dr. Arseneau isn’t seeing much help from it in his ME/CFS patients, but we had a few people report in the Metformin blog that it was really helping so it may help some.
Cort, I got covid for the first time in March of 2023. Very concerned about possibly getting long COVID on top of my CFS, I contacted my doctor ME doctor. After discussing I was put on metformin 500 mg. I started taking it on the third day of my COVID infection. It took me several weeks to get back to my baseline but then something very interesting happened. I continued to improve and went 15% above my baseline prior to taking metformin. I still continue to take 500 mg a day and even my primary thought it was perfectly fine. I’m not diabetic though my levels get close to the borderline. It’s just taken a little bit of the struggle of the up and down cycle we all go through and has put me in a better place to wait it out until we get real medical relief down the road.
Interesting. Thanks for sharing that Roger – a few people have said it helped. Good to hear.
I had a similar experience, Roger. I got covid in October 2023, and started on Metformin on the second or third day. I tried 1000 mg twice a day, but lowered it to 500 mg twice a day due to nausea, and then tolerated it well. I continued taking it after the acute infection cleared. After a few days I noticed I was doing better than prior to my covid infection. It wasn’t a huge improvement – but it was noticable and helpful.
About 2 weeks later I got a flu shot, which I’ve had trouble recovering from. So I’m not yet sure if the benefits of Metformin are going to last. Only time will tell.
I had no signs of diabetes. My fasting blood glucose and A1c are both very good.
My last bottle of Metformin cost about $2, which was quite refreshing given the outrageous cost of the prescriptions and supplements we all rely on.
Brian, I’m glad it also helped you. Keep in mind that it took me several weeks to recover from covid. I got back to normal in 7 weeks and then kept improving for another 4 more weeks. So you have to be patient. Keep in mind it takes time for the body to fully clear the lingering COVID virus. I just got the most recent COVID vaccine yesterday. I will wait 4 weeks to fully acclimate before getting the flu vaccine. I feel we have to be patient with our bodies to reset and recover.
PEA is a laughing joke. Took it at high doses for two straight years and it was a complete waste. No change whatsoever in symptoms or severity.
I’m curious about PEA less because I think it would impact my ME/CFS than because it seems to have overall anti-inflammatory effects, and that matters to me for reasons like lipid profiles and osteoarthritis…things I’m having to think about as I age.
I hadn’t heard of PEA before, but reading up on it now, it seems to be widely promising…though not for ME/CFS. After two decades of illness, I have become skeptical that any ingested substance (aside from extra hydration) will help with PEM at all. I don’t expect it any more. And I won’t get hopeful about that kind of thing until there are solid studies to make me hopeful. No more roller coaster of hopes for me! (I stay hopeful about other stuff in life….just not this.)
PEA works to stop pain, etc for me. If you can afford it, at least start with Mirica because it works better than some other brands that will take twice the stated dosage to see the same results.
If ME/CFS was a homogeneous condition (I dont’ think any condition is probably homogeneous actually) but if it was we could go by your experience, Shea – but since ME/CFS is clearly VERY heterogeneous we can’t go by any one patient’s experience.
Since Arseneau has found that it works with some of his patients I don’t know that we can put it in the “laughing joke” category. 🙂
Works very well for me!
Same here. Tried several times and no effect.
Did you try Mirica or one of the other newer brands? Mirica works for me. I’ve tried other cheaper brands and they ending up costing more because it takes 2 capsules to the 1 Mirica. Could be some of the failures people report are from not taking a high enough dosage.
It definitely doesn’t work for everyone, it didn’t work for me either unfortunately. But I know many who it has helped greatly. We all respond differently I suppose!
PE works great for me. I’m in remission. Everyone is different.
But Scott, Arseneau just give his thoughts on 11 supplements/drugs – and if you look at the resource docs on treatments he provides – he uses a lot more than that.
Cort, my statement is not based on your article, it is based on attending a couple of his zoom webinars and hearing his pitch for his course. At one of them he said that peptic ulcers were caused by stress, no mention of H pylori — that was one of many huge red flags.
I can understand that some people will dismiss any doctor who uses neuroplasticity in his approach. I just want to point out that’s only one part of his treatment approach. Along with the neuroplasticity video’s he also has long extensive video’s on mast cell activation, POTS, myofascial issues and physiotherapy on his site.
SImply labeling him as a “think yourself well” guy – which you didn’t do – doesn’t do justice to all the other parts of his practice that he brings to bear.
Cort, I do not think neuroplasticity is a think yourself well approach. I have seen many people get quite well with Neuroplasticity work. What are your thoughts? Could we have limbic loops that are driving inflammation and immune responses? I also see baseline training along with diet, moderate excercise according to pushong baseline slowly with some rewiring of the nervous system working really well for some. Like the folks at CFS Health in australlia Thoughts?
Absolutely, the parts of the brain that regulate the stress response have been found dysregulated in ME/CFS. For me, my guess is that these practices are tamping down a highly overactive stress response. Makes sense to me. 🙂
Agree with you, Scott. Not hopeful about this article that offers zero value and other recent ones like taking a Prozac to increase serotonin. Every ME/CFS patient has tried every obvious treatment already. Let’s focus on more forward thinking therapies like the recent study published about hydrogen gas.
Ach!!! Zero value – well maybe for you Shea. Maybe you missed it but the serotonin blog was more about the study finding that low serotonin than the treatment option that particular group was doing – and it included another treatment approach that was much more comprehensive.
Hydrogen gas, by the way, is no more “forward-thinking” that oxaloacete, guanfacine or Abilify all of which showed up in the past year or so. We did a blog on using hydrogen gas embedded in water three years ago!
Hopefully you’ll find the nicotine blog coming up more stimulating 🙂
Shea, you are grossly mistaken about every patient having already tried every obvious treatment for ME/CFS. I live in Michigan and, there may be knowledgeable medical practitioners here, somewhere, but I have not found one. All I have is my PCP, who knows almost nothing about the disease. I did have a rheumatologist for a while who was very caring, believed his ME/CFS patients were indeed suffering but, I knew infinitely more than him, just by reading Cort’s reporting. I did finally get an appointment at Stanford University but it’s a year out. I am a member of Solve ME, the advocacy group and have met with Congress People for three years in a row but still haven’t learned much about treatments. Perhaps you could tell me where you have received treatment, but if you have not been helped, maybe I wouldn’t either. I am so grateful for Cort’s reporting. Without that I would have completely lost hope a long time ago.
How does Dr. Gupta’s course differ from DNRS?
Hi Diane
As a long term yoga and mindfulness meditation practitioner I recommend people with health problems who want to give meditation a shot to try to find a well researched medicalized / clinically pschologized, secular meditation programme like Mindfulness Based Stress Reduction (MBSR) or Mindful Self-Compassion course. Most importantly, you want to find a teacher who is a committed long-term practitioner who has herself studied with leading Western meditation teachers or even with meditation masters in Asia.
In my opinion there are too many medical meditation programmes popping up now developed and tought by medical professionals who promise to cure x,y, z, who are neither experienced practitioners nor teachers. The problem with unexperienced practitioners teachint mindfulness is that because of our all hard-wired greediness for a quick fix the rist with such teachers is very high that they unwillingly turn their mindfulness training in a short-term cure or a therapy. When just the opposite is true. Mindfulness works when you learn to be with everything that shows up in your life in a welcoming and accepting manner in order to gain that moment where you then can decide from a calm and peaceful place what you want to chose to support yourself in a difficult situation.
A couple of weeks ago I read a wonderful summary about what to expect from starting a mindfulness practice in a health crisis, understand its principles, and avoid frustration on the blog of Inner Space. A center for mindfulness and psychotherapy in Mumbai that was founded by Sadia Saeed, a very experienced clinical psychologist, psychotherapist and meditation practitioner/teacher.
https://innerspacetherapy.in/mindfulness-during-stressful-situations/
Another great resource for people who have already started a meditation practice is the course Dancing in the Dark Field by Zenshin Florence Caplow that she is doing with the San Francisco Zen Center. She is a long term Zen practitioner who suffered from something very similar to ME/CFS and rheumatoid arthritis for many years and the course is about learning to take the very best care of yourself with an illness that may hang around longer than you’d wished.
https://www.sfzc.org/calendar/events/online/dancing-chronic-illness-and-pain-ongoing-group-online-1112-47
Dancing in the Dark Field sound great – thanks for mentioning it 🙂
I am a trained MBSR teacher and have 20 years of lived fibro. Unfortunately, research has shown that the drop out rate for fibromyalgia patients in MBSR and similar programs is high because the course requirements are too much for many. I’m in the process of developing a mindfulness for fibro short course in an effort to share the teachings in a more adaptable way.
DNRS is very focused on the idea of doing brain retraining rounds (these include steps and visualizations) and stopping POPs (pathways of the past). Also important is the idea of getting into parasympathetic as much as possible, and avoiding sympathetic activation as much as possible during your 6 month commitment. A militant program…
Gupta also focuses on brain retraining rounds (similar to DNRS) and changing thoughts. There is also focus on a breathing exercise, meditation, and calmness (bringing a “relaxed attitude” to things). A gentle program…
Cort, could we see that slide about common meds Dr Arceneau gives his patients? Or have a summary? I’m always up in the air about all of those recommended supplements & this doctor offers a very closely evaluated list, I’m sure. Thanks!
You might find this helpful! 🙂 It’s directly from his website and many of the documents are also found on the CCDP website.
https://www.dropbox.com/sh/r0ggay2szphqfgq/AAATpXbA-Q50N9LPiQG1wkGta?dl=0
https://drricarseneau.ca/
Controversial but from my experience: SSRI. Not for everyone, but in my case helps. Iam not depressed or anxious. It lifted brainfog, improved sleep.
For me its a difference of being able to work as a legal prof at a university or not being able to work.
Happy for you! Which SSRI please?
Lexapro
There might be something there. With the serotonin findings previously and now this exploratory study on DSRIs https://www.nature.com/articles/s41598-023-45072-9
SSRIs
Great blog & resource! Shame that Guanfacine (Intuniv) is rather expensive in Germany too (currently it seems 113 EUR/28 x 1mg) – I’d love to try it! If we’re lucky it might get cheaper in about 3 years once the exclusivity of the original producer expires.
Just not sure it can be taken by patients with low blood pressure as it may lower it – did he say something about that?
Also may I ask if “3xs” means 3 times? (I’m not familiar with US medical abbreviations) Thanks!
Good blog! I have tried many of the things listed and can say that almost all did not improve my fatigue to any significant degree. LDN was useless and Abilify, as I was part of the study, had…umm…issues with data collection and the intense desire for the doctor to put a feather in his research cap.
I have been offered a stellate ganglion block but because it is such an invasive procedure, I’m inclined to wait for more results.
Refined drugs that have effects most often also have side effects and that’s why I favor trying supplements (after assessing risks, dosage, possible cross reactions with other meds etc) however the usual reaction I get from doctors is discouragement.The reasons are usually 1) it hasn’t been studied (of course it hasn’t because drug companies cannot make a profit on a substance that can’t be patented. Or 2) supplements are not very well regulated so the strength could be incorrect or the substance contaminated or even non-existent.
I believe that the body has its own wisdom and if one can figure out how to balance the body systems, there is a good chance of improvement. In that light, for myself, P.T. (mostly core exercises), when I can actually manage to do them consistently, has offered more improvement than most drugs. Usually however, I fail to do them. Hyperbaric oxygen is often used in France with good results, but is not supported by insurance in the U.S. I cannot afford it so it remains on my wish list.
I’m constantly scouring research papers for clues. As you wisely recommended, always ask yourself how the person who offers these solutions is likely to benefit. Or if it is a doctor, will they get in trouble for giving their blessing to a dubious treatment.
I look forward to using AI for more individualized treatment suggestions. Do hope you see my recent post on your Kaufman/Ruhoy blog regarding the connection between hyper mobility and ME/CFS–somewhat overlooked research.
I know many ME patiënts who used LDN it didn’t help. So, i wouldn’t put my money on this drug. De Meirleir used it to his patiënts if i am correct. It is old school. I don’t expect much from this trial. Also mestinon will not do the trick. But a negative outcom in science is also a result.
What do you mean ? Hyper mobility of joints In ME/CFDS?
@Elaine, I’m not sure what you mean either. I don’t think I wrote ‘ME/CFDS’…Not even sure what that is.
Opps, apologies for my mistake. I meant ME/CFS. I’d never heard of hyper mobility related to this-seems like it is related to the joints or mobility of the spine. Thought that was interesting-I’ll look into that. Thanks.
I’m a patient of Dr. A’s and tell everyone i know that he has been a gift from the universe for me after suffering alone with ME for 8 years before finding him. He definitely DOES NOT use a think yourself out of it approach. He’s extremely science based but aknowledges that things like meditation and other brain calming techniques can be useful to help the anxiety that is a consequence of a disease like ME or long covid. It would take two seconds of listening to him to figure this out. To anyone who can’t be a patient of his because they don’t live in BC, I highly recommend his website and YouTube channel for education.
Am I the only one who gets depressed of these results (ill since “94), verry severe and this is all we get, what happens? what has happened? I am way to severe and getting to old to have any hope anymore if for excample there is not given worldwide huge amounts of monet to research ME/cfs…
OMF wants to do a double blind crosover placebo controled trial on 40 persons-40 persons-40 on mestinon and LDN (takes 2 years) and call it statistical significant!
Help!
I like the Funnel of Knowledge and welcome this kind of detailed guidance from a physician who has treated many ME patients. Otherwise, there are innumerable options & it’s confusing without a guide.
Actually there was a research study done on LDN for people with ME/CFS in 2021, https://www.frontiersin.org/articles/10.3389/fimmu.2019.02545/full
Thank you for this summary, Cort. Wondering if you or anyone on here has experience actually finding where to get rTMS treatment for fibromyalgia (or anything else) in the US? Trying to search for it doesn’t bring up a lot of results…
Seems crazy to me that there still isn’t a 5mg Naltrexone tablet.
From what I gather there have been hordes of ME/CFS people compounding Naltrexone for many years now. Isn’t this on it own evidence of how totally dysfunctional the medical world is surrounding ME/CFS?
I asked the same. It’s such a cheap drug there’d be no ‘profit’ to gain. The pharmacies definitely make money compounding it. Get the 25mg tablets, usually covered by insurance, and crush, make your own.
Hi Cort, I have been suffering from CFS for 33 years now, since the middle of my bachelor’s degree in mechanical engineering at university, pretty much like you. It took me 20 years to get ‘diagnosed’. Indeed, none of the many doctors I have seen never ever mentioned or suggested to me that it could be CFS. All the tests I have done at the beginning or later were ‘normal’ although I was half dead all the time. After 20 years, I found by accident in a library a book talking about chronic fatigue syndrome and I knew instantly that this was what I had. I then searched for and found an open-minded doctor knowing about CFS and, after having discussed with him during one hour during which he asked about how it started and all the symptoms (mostly heavy brain fog and heavy PEM), he immediately validated my auto-diagnosis.
Before that time, not knowing what I had I was constantly forcing myself and crashing deeper and deeper. From that moment, I learned about pacing. This is only then that I started to learn how to ‘manage’ CFS and be able to feel good again for very small period of times which began to lengthen very slowly. Since then, I have tried many supplements like antidepressants (SSRI and others) and I felt ‘very weird’ but absolutely nothing good, mitochondrial enhancers (D-Ribose, CoQ10 and others) and either they did not work at all or I was awfully cranked-up (like in the movie with Jason Statham) even with extremely small doses, Omega-3 and I got sick like a dead cow and it stared a period of 10 years of very intense food intolerances and allergies with IBS and finally many other supplements that never improved anything. ONLY pacing is doing something good for me. Time goes by and I am more and more reluctant to try new supplements or medications that have not been fully scientifically validated because this destabilizes my ‘equilibrium’ based on pacing and so far never improved anything, just causing a period of instability until I stop the test and go back to my natural ‘equilibrium’. Far from being perfect but still an equilibrium which is stable without crashes. All this to say that Dr. Arseneau is perfectly right to mention that the first thing to do is get an equilibrium based on pacing and then try something else slowly and one at a time with a lot of care.
By the way, I have been reading your blog for many years now and when I discovered it, it was the first time that I felt like I was not the only Martian cast-away on planet Earth. Thank you very much!!!
Amazingly useful, thank you so much for all of this!!!
Quick question on the guanfacine- looks like the doses are off? Usually it’s like 1mg or 2mg a day, but here it says 400 mg/3xs a day, 600 mg/2xs a day, etc.
Thank you again!
You’re right, the dose for guanfacine is 1-2mg. The dose listed here is actually meant to be for PEA (below). I’m taking both 🙂
My mistake – fixed.
Yep – should be micrograms – fixed.
Has anyone tried a random-over-time trial on themselves with help of a pharmacy? Each week you’d either get a drug or a placebo, and at the end of say 20 weeks you evaluate if a drug helped. I wonder if pharmacies and GPs are open to the idea.
We’ll likely see only moderate effects of many of these drugs and it’s difficult to test if they work by just trying them.
Hi Cort, I have been suffering from CFS for 33 years now, since the middle of my bachelor’s degree in mechanical engineering studies at university, pretty much like you. I was a very althletic guy being an alpine ski instructor and training hard for road bike racing. And suddenly THAT. It took me 20 years to get ‘diagnosed’. Indeed, none of the many doctors I have seen never ever mentioned or suggested to me that it could be CFS. All the tests I have done at the beginning or later were ‘normal’ although I was half dead all the time. After 20 years, I found by accident in a library a book talking about chronic fatigue syndrome and I knew instantly that this was what I had. I then searched for and found an open-minded doctor knowing about CFS and, after having discussed with him during one hour during which he asked about how it started and all the symptoms (mostly heavy sleep problems, brain fog and PEM), he immediately validated my auto-diagnosis.
Before that time, not knowing what I had, I was constantly pushing myself and crashing deeper and deeper. From that moment, I learned about pacing. This is only then that I started to learn how to ‘manage’ CFS and be able to feel good again for very small period of times which began to lengthen very slowly. Since then, I have tried many supplements like antidepressants (SSRI and others) and I felt ‘very weird’ but absolutely nothing good, mitochondrial enhancers (D-Ribose, CoQ10 and others) and either they did not work at all or I was awfully cranked up (like in the movie with Jason Statham) even with extremely small doses, Omega-3 and I got sick like a dead cow (this also stared a period of about 10 years of very intense food intolerances and allergies with IBS) and finally a list of many other various supplements or medications that never improved anything. Only pacing is doing something good for me. Time goes by and I am more and more reluctant to try new supplements or medications that have not been fully scientifically validated because this destabilizes my ‘equilibrium’ based on pacing and so far never improved anything, just causing a period of instability until I stop the trial and go back to my natural ‘equilibrium’. This is really far from being perfect but still it is an equilibrium which is stable without crashes. All this to say that Dr. Arseneau is perfectly right to mention that the first thing to do is get an equilibrium based on pacing because this is the absolute basement that you can build on anything else. Only after that can you maybe try something else slowly and one thing at a time with a lot of care.
By the way, I have been reading your blog for many years now and when I discovered it, it was the first time that I felt like I was not the only Martian cast away on planet Earth. Thank you very much!!!
I agree with you on whyupset your pacing results with yet another trial. I got ME suddenly in 2002, but was misdiagnosed. I always felt I had a low level infection and had several health, physical injuries and infections prior.
I finally got a proper diagnosis, not psychiatric, 2016! I knew I had CFS but could not get the diagnosis.
I’ve been a guinea pig, poked, drugged, prodded, and discounted. Yet my job was a high level IT programmer/database expert for large corporations and could be trusted there! Yet treated like an idiot when it came to my own health, and the fatigue, etc. makes it difficult to self advocate.
I now added Neuro Lyme to the list so it’s worse than ever and no one is really investigating CLD, and if they did well, as you said, pacing and keeping low stress has helped more than anything. A bout of antibiotics can help, but no one is mentioning that type of treatment.
So nice to hear people with similar stories, as I have felt I was an anomaly as my peers ski and mountain climb. I found some brain relief with crochet!
Still considering a trial of Abilify but have failed with other similar type drugs.
Very unfortunately for me though, the rather open-minded doctor I found also believed that the solution was CBT and more sports based on the infamous UK PACE study. I stopped seeing him rapidly and from this point, I have been on my own with pretty good results in the circumstances of course.
The second most important thing I discovered after PACING is SLEEP from Dr. Jacob Teitelbaum. The production of all hormones and body repair mechanisms occur during night.
The funnel concept from Dr. Arseneau is also very important. No matter how sick a person is, who would walk in the forest and taste everything he encounters? Any medicine, even ‘natural products’ can be dangerous and harmful. Many of them are totally uncontrolled and real crap. It is extremely interesting to have a list of potential medicine suggested by a real certified doctor with arguments based on these four factors. Many others, like Dr. Teitelbaum, also do proceed so. Although, based on my own experience, the doses suggested are almost always far too high and the side effects can still sometimes be very strong.
I did not give up totally trying new potential remedies and I will NEVER either but I am extremely careful now weighting the ratio risks/benefits before making my decision.
I do believe that ME/CFS, FM, Lyme and even the very old sickness named ‘neurastenia’ dating back as far as the end of 19th century (if I am not mistaken) are pretty much the same kind of disease as long Covid and I strongly believe that with so many data available now from long Covid, AI will significantly help discover sooner than later the Saint Graal for us like insuline was for diabetes.
Keep hoping, it is not over yet !
I definitely wouldn’t touch Guanfacine or brain training, both of which have a high chance of harm and many patients have reported lasting consequences. Guanfacine seems can be quite damaging in some patients and leave permanent damage and Brain training is all about telling you that you don’t have fatigue and push through and we all know crashes can lead to permanent baseline reductions.
I don’t know why you think this. Guanfacine from what I know is an old blood pressure drug and has been used for ages.
If you’ve ever done any brain retraining (I’ve tried two of them – Amygdala and DNRS) and neither asked me to push beyond my fatigue. It was actually quite the oppoite with Amygdala – which addresses working too hard and actually suggested that I should work less.
Both Amygdala and Dan Neuffer of ANS Rewired had ME/CFS and both well know the consequences of pushing through fatigue. Dan explicitly said his program does not ask people to push through fatigue.
I would love to chat with you Cort. Are you ever open to conversation?
Pushing through fatigue can lead to permenant baseline reductions? Is there not anything one can do to raise that baseline? Brain retraining programs do not tell you to push through baselines at all. Not in my experience.
I’d suggest trying a compression garment, up to the abdomen if you can manage it, or thigh high or knee high as tolerated.
https://pubmed.ncbi.nlm.nih.gov/35056360/
https://www.sciencedirect.com/science/article/pii/S0735109720379079?via%3Dihub
But then I always bang on about orthostatic intolerance 🙂
I believe there is a typo in this very interesting report: the usual dose of guanfacine is 1 to 2 milligrams, NOT 400 to 600 mg as stated in the article. Perhaps the author meant 400 to 600 micrograms?
Wouldn’t expect to get that past you, Dr. Lapp! Ironically, I’m trying Guanfacine and its a VERY small tablet. Thanks -fixed.
I would like to share some things that I inadvertently found that helped my CFS. I was diagnosed 8 years ago with FM/CFS. I now believe I had the FM portion approximately 4 years prior to the virus and subsequent CFS appearance which, I understand is common. Apparently I do have issues with mast cells because I found that, if I am having severe, debilitating flu-like symptoms, if I take 2 Benedryl tablets, I feel so much better within one-half hour. It works every time. I can be so sick that I only want to go to bed but, if I take the two Benedryl, I can actually go about my day and am able to accomplish quite a bit. Benedryl doesn’t make me sleepy anymore if I take it during the day, which might be a problem for many.
Also, I discovered that if I am very sick with flu symptoms and am sleeping 18-hours a day, if I take a full dose of Nyquil, I feel so much better the next day. It’s not because I have slept better because, fortunately, I rarely have the common sleep problems that most patients have. It doesn’t matter what formula of Nyquil I use so, I expect that it is the Guafanesin ingredient that is helping.
Another discovery I have had is that, if I get my hands n the clay and work on my ceramics projects, I feel better. I am guessing that it is the mindfulness aspect of the clay work that helps. We know that mindfulness is the goal and that, meditation is just ONE way of reaching a state of mindfulness.
Gardening results in the same benefit for me. While just vacuuming two rooms can send me to bed, in the summer when Michigan has sun and warmth, I can work outside for a continuous stretch of 4-6 hours, some of which is quite strenuous. I will be tired when I stop for the day but the next morning, I feel great. This is the ONLY physically-challenging activity that doesn’t make me sick if too much is done. I suspect that part of it, again, is the total mindfulness of the work. There probably is an additional benefit of doing it in the sun. We know that the pill form of vitamin D doesn’t even resemble what the vitamin D our own body makes from being in the sun. Perhaps that somehow relates to the recent study of serotonin puzzle piece. Any thoughts or similar experiences?
Hi Kat,
Have you explored histamine intolerance and/or mast cell activation disorder? The Benedryl helping your symptoms may suggest this could be an issue for you.
When I discovered these issues I cried with relief to have figured out what was wrong with me (part of my FMS/CFS complex), but also frustration, as I had been suffering for so long and no doctor had ever mentioned these health conditions. Once I went down the path of a low histamine diet and lifestyle I started to feel a lot better. I’m glad you have found some things that work for you.
Kim
Kim, I really appreciate your support and knowledge. I have not been helped by my medical professionals because, again, I know much more about the disease than they do. How do you find out this information and treatment?
Kim, where and with whom do you get your medical treatment?
Maybe it is minerals in the clay, or silica that makes you feel better also. No doubt a focus thing there though..Recently I found the vit D in chlorella is helpful.. I do better when I garden also. Sometimes the sun and bigger movements, like shoveling or trimming stuff can be helpful..maybe makes me feel more alive. Or I may actually hurt more, but eases my mind to be in nature.
Elaine, yes, I suspect you are right. Scientists are learning how important it is to be outside, whether you have our illnesses or not. I will have to look into the Chlorella.
But it is important to get a toxicity anylasis on herbs, powders, chlorella -for heavy metals content (or not). Supposedly the best chlorella comes out of Korea, and is grown in the sun (and sun dried), not in a lab under lights. I also am doing breath work..am excited it can knock me out (ugh, don’t get too excited.) My first doctor thinks I was having problems as a kid. And I remember at the age of 4 playing in a sand box and felt greater peace. Either it was the creative focus or something I absorbed by the sand..maybe both. That’s why I replied to the clay thing.
I don’t understand why this Dr has publicly stated to other medical professionals that “We have to talk about the elephant in the room which is that most of the patients you’re going to see with these conditions have Borderline Personality Disorder”:
https://www.youtube.com/watch?v=E7d6ZrqWOWM&t=1035s
Everyone makes mistakes along their learning path, but to my knowledge this statement has never been recanted. It would be so much easier to take the above information on (mostly) bio-physical treatments seriously if it had been, and/or if this video had been taken down. But here it is still propagating dangerous medical misinformation.
As pwME we have to take whatever help we can get, and it’s possible that the same Dr who is harmful in one way could be helpful in another. But statements like the one above are disheartening for those of us who have suffered medical gaslighting and don’t exactly inspire confidence.
Ouch – I would be the first to agree that this illness changed me emotionally – in particular I have much more “emotional lability”, much greater mood swings, and am less settled and more uptight generally but borderline personality disorder? I don’t know.
I don’t think I’m an adult who acts like they are in the “terrible two’s” or a “belligerent teenage” – altho I’ve been there at times – but not much!
Borderline personality disorder
https://www.mayoclinic.org/diseases-conditions/borderline-personality-disorder/symptoms-causes/syc-20370237
That seems a bit much. Arsenau included – at least in 2017 when he made that video – migraine, fibromyalgia, tension-type headaches, TMD, Interstial cystitis, IBS – in this category – he does not mention ME/CFS (but I can’t imagine that its not in there) – and believes its due to arrested development and traumatic events that happened in the past – which we never got over. A lot of research indicates that these events or a stress ridden childhood harm us – except in my case, it doesn’t apply.
He believes this problem affects 20% of people who see doctors. That’s a lot of people to give that label to.
I thought that Arseneau’s discussion of somatic disorders – which he had at one time – was good, as well as his discussion of the biological origins of the chronic pain was very good.
I don’t think he’s made borderline personality disorder a big deal since then (?) but I wonder if, six years later, he still stands by that statement?
BPD!!! Wow. I am a trained psychotherapist whom specialized in BPD for many years. I’d say no to the BPD, although could a small percentage of folks have it? Sure. I could see how someone not trained in all the nuances of that diagnosis could consider this and see it as applicable in other cases, when it is not. I believe it is an over diagnosed and often poorly understood condition, especially since the days of internet. I have had a quite a few clients think they have BPD when they did not meet the criteria. All of us (as humans) can have some to the traits, some of the time, in certain contexts, but it does not mean we have BPD. There may be some brain similarities in some folks, but it takes a lot to meet the criteria for that diagnosis.
Thanks I appreciate your professional opinion on this Jamie. Even from my lay perspective I understood that the criteria for BPD was quite specific, and not an appropriate explanation for chronic pain etc…
I wonder if the more relevant traumatic experience came from the doctor’s office – not a childhood experience. I don’t know if I’ve experienced anything as devastating as watching me go from a strapping young man to someone who could hardly walk and having a doctor totally dismiss me.
I was probably lucky – it only happened twice to me – and the last one was decades ago but
I still remember the feeling isolation and loneliness I was left when I came to the conclusion – at my lowest point ever – that I was basically on my own and that the medical profession not only didn’t have anything more for me but actually acted with disdain towards me.
That’s a pretty stressful place to come from when seeing a doctor – let alone an emergency room doctor. I wonder if Dr. Arseneau – who was trying to help emergency room doctors deal with chronic pain patients – and did with the exception of that statement quite well I thought – thought of the stress most of us carry inside from dealing with the medical profession
Thanks and yes ‘ouch’ is right. The idea that much or any of this can be explained away as originating with BPD seems very reductionist and unhelpful to me. Especially in light of the pandemic we now know that specific infections can cause these conditions to develop in some people. No one is claiming, for instance, that BPD caused long covid. As for whether he would still stand by the statement, because it’s a dangerous view shared with other medical professionals I think it would be especially important to recant it. Or at the very least there could be a request made to the Chanel on which the video appears, for it to be taken down. None of us are perfect, but when mistakes of this magnitude are made, which affect people’s medical care, they should be owned up to. Then we can all move forward with renewed confidence and be open to the information presented in articles like this one, without wondering what unmentioned misperceptions about the illness in question might underlie it.
Did you know that emotional lability has been linked to low cerebral blood flow in at least one study of POTS – and I see no reason it couldn’t apply to folks with low cerebral blood flow in other disorders.
https://pubmed.ncbi.nlm.nih.gov/32298990/
I have OCHOS, and, when I am medicated, my emotional lability reduces.
Cort, this person’s suggestion that patients suffering from ME/CFS have BPD is a travesty. It is almost as insulting as the idea that our illness is, “all in our heads”. Yes, the incidence of BPD in patients suffering from ME/CFS would be the same as the general population. BPD is an awful mental disorder, with not much successful treatment. In days past, many psychiatrists would not even accept patients with BPD and family and friends were unable to tolerate the person with the disorder. While we do suffer from anxiety and depression; in my case, only during a flare, I cannot accept that our illness causes BPD.
I can’t accept it either!
I agree that considering ACE’s and trauma (both shock and complex) should be part of any chronic condition work up, but extrapolating that to Borderline seems a bit extreme in my view. Childhood trauma can develop into a variety of mental complexities and all should be treated with care, respect and competent, effective therapies and treatments.
I did years of psychotherapy, somatic experiencing and trauma work to re-wire my nervous system and integrate difficult experiences from the past. It certainly helped and I felt better, but all my fibromyalgia and CFS symptoms persisted despite having a very well regulated nervous system and well integrated psyche.
I finally discovered my core driving factors (food allergies, herpes) and most symptoms went away (after 20 years). I was still having periodic flares but 4.5mg of LDN has stopped those thankfully. I’ve now been symptom free for about 12 months.
Hi Scott, thanks for this article. Im a patient of Dr A, and take PEA and Guanfacine. Unless he’s changed his dosing recommendations: PEA 400mg x3/day or 600mg x2/day (so a total of 1.2mg/day) After one month double the dose (increases to 2,4mg daily total) and see if there’s any incremental difference. Guanfacine start at 1mg/day for a month, then increase to 2mg/day.
Dr. Arseneau is the first doctor I’ve ever gone to who knew what he was talking about. I’d been sick for about 20 years at that point, and had to endure applying for disability coverage without having a diagnosis yet.
Going to the Complex Chronic Fatigue Program was a big breath of fresh air. This was about in 2015 or so. Every person on staff was informed and knowledgeable in regards to ME/CFS. It took me 2 years to get into the CCDP, and I see by the 3,000 person waiting list that more resources should still be allocated.
Dr. Arseneau had a guest doctor sitting in that day, and they spent 2 hours with me. Going in, I realized there was no magic bullet and my expectations were guarded. He made several suggestions, but to do them all was and is beyond my pension budget.
The biggest thing I got out of the visit (other than validation) were 3 recommendations, 2 that helped. He put me on modofinil, to clear brain fog. Big improvement for me. Next, he sent me to a trigger point doctor for the worst of the knotted muscles. I had endured an egg on the back of my neck for about 10 years, and couldn’t afford massage therapy. The results were shocking. The trigger point injections were simply lidocaine shot right into the knot, and the egg was completely gone after 2 treatments.
Modafinil and TPI were the 2 best things to come out of my visit to the CCPD program. Another side note was, my visit was just prior to the Institute of Medicine Report, and he listened to me as to how I thought my illness worked. When I finished, he said, “I’m writing a paper on that at the moment.” Wow.
The third recommendation I tried was Low Dose Naltrexone, and I found it didn’t help me at all. As an aside, he also recommended CoQ10 for heart health. But for me, pacing and avoiding adrenaline still remain as the best two options for things I CAN consciously control.
Dr. Arseneau helped me realize that you can’t think yourself better, but could be aware what what was happening in the background. Becoming aware of what the subconscious was spinning on helped me calm it. It didn’t think myself better, but I could help things from deteriorating.
I read about and started taking PEA a while back. I found that it was helpful with brain fog (nothing else) and so I’ve kept taking it.
I believe that Dr. Ric Arseneau’s approach is possibly the best way forward in complex, chronic illesses like ME/CFS and Long Covid etc. I think most people will have their own combination of factors, contributing to their ongoing issues and these need to be very carefully worked out. Especially for the severely and very severely ill, who have no capacity for dangerous errors.
I was disappointed though, to hear him dismissing *blood washing* by which I presume he means HELP Apheresis. Some people have reported that they found it gave them the break they needed, to begin improving their health and that was crucial to them.
I would love to have a doctor like Dr. Arseneau and have the opportunity to discuss my health issues, with someone who seems genuinely interested. He reminds me of Dr. Kaufman and Dr. Ruhoy. 🙂
I wonder why there’s nothing about Mestinon in this review of treatments. Previous Health Rising articles and other sources have made it sound worth trying. Is there something I’ve missed about Mestinon no longer being a promising treatment option for ME?
Just wanted to comment, for the people with histamine issues, DAO does work and it’s not as expensive anymore. There is a brand called NaturDAO that is plant based, made in Spain from legumes (no allergy issues for me),which now has a distributor in the US. It is 1,000,000 so I only need to take a 1/4 before each meal unlike the expensive versions that are only 10,000HDU per cap. Right now it’s .67/ tab on Amazon. FYI Do not take a whole tab except on special occasions because it will crash your dopamine or serotonin. I forgot which but you’ll get depressed after awhile.
This is so informative and useful. Thank you so much!
It’s interesting that some people have recovered by doing Erik Johnson-style mold avoidance via Lisa Petrison’s writings. Of course there is no research profile associated with that but it is medically low-risk (but very high-challenge, because it involves moving out of your normal accommodation).
I’m one of the people who achieved partial recovery this way, from unable to work to able to teach a class for four hours, three days a week plus commute. So in that sense the facebook groups and totally anecdotal information have been the most important information I have ever accessed in my life.
This is not to challenge the analysis above, which is really important because there’s a lot that gets talked about on social media that is potentially much more risky. But still interesting — until research actually happens, some of the anecdotes will still help people. At their own risk though!
Thanks for this hugely helpful article — I learnt so much.
There are some sound take aways from this article but I have issue with the Funnel of Knowledge for a few reasons. Firstly, many lifestyle, natural and non-patentable treatments will never have Phase 2 or 3 trials because they will simply never get funded. Secondly, clinical trials usually only test one treatment at a time when our complex conditions require multiple treatments. Thirdly, as you have mentioned in the comments Cort, these conditions are heterogeneous and the clinical model assumes everyone with he same diagnosis or symptoms has the same pathology. It’s clear to me after 10 years of following the research that this is a major problem when clinging to the mainstream ‘evidence based’ model.
Dr. Datis Kharrazian (with a Master of Medical Sciences degree in Clinical Investigation from Harvard Medical School) explains this here:
N of 1 clinical trials are getting a lot more support in research publications. With a clinical trial, the focus is on generalizability to the entire population and not on individuality. Also, there is a limitation to only one treatment approach. In a real clinical setting, we do multiple treatment approaches because we are trying to do many different things to make the patient feel better. We get patients to change their lifestyle, maybe try to sleep more, as well as change their diet, and take a few supplements. We are not doing these one at a time for 12 weeks and then measuring a primary outcome. There is a lot of difficulty in establishing a clinically realistic model with a standard clinical trial for complicated conditions that have a lot of diversity in their pathophysiology. For example, we really cannot design an accurate clinical trial with so much variability with each patient who suffers from autism. Each patient has a unique set of physiological parameters that makes the standard clinical trial approach an ineffective model because of the wide range of uniqueness that cannot be accounted for in a group study despite the power of randomization.
The BBC Producer in the UK Published a book on Histamine Intolerance, it hit me on the head when I saw the red wine bottle on her book page cover, I got so sick once with red wine never touched a glass again since then.
The mention of DAO in this article, she also later on found out she had Fructose Intolerance.
I am not one who believes it is Fructose Intolerance I think it is another name for misdiagnosed (HFI) Hereditary Fructose Intolerance & countless are never ever tested for this there are not many clinics that do this test in labs.
A lot of medicines today have fructose inside & even sugar.
One Woman I read about she had been diagnosed with CFS for over 48 years she had Hereditary Fructose Intolerance all along from Birth the adolase B gene.
I believe everyone should be tested for (HFI) it should be part of the criteria. One Guy all he eats is macoroni & plain cheese sauce he cannot tolerate other food at all.
Another thing that goes with Histamine Intolerance is (HATS)
Hereditary Alpha Tryptasemia Syndrome tested at here: http://www.genebygene.com *key in tryptase home page
Thank you Cort for your unending dedication and clear writing! I have tried most of these and have a few things to add that may help others. I’ve been on LDN for years and it could very slowly be helping. I tried Guanfacine with NAC after the Yale report last year but it caused me low BP, more brain fog and fatigue and NAC can increase histamine. I tried PEA but if you use any cannabis products (guilty) it can and did make me pretty spacey. I’ve also tried DOA and have so many allergies my Dr thinks it must be Mast Cell related but did not notice any improvement. I’m very curious if anyone has ideas why us ME/CFS folks often have a med work a while and then lose all effect if not give bad effects? Unfortunately I think I’ve tried about everything I can get my hands on and/or talk my Dr into and I don’t have it in me to try many more. Many thanks to this community!!!
I would love to know that too. You’re not alone in having tried everything to little effect unfortunately :(. Keep up the faith, though, things are showing up all the time…
We’ve seen the two studies in the journal, Cell Host and Microbe (Columbia Univ. and the Jackson Laboratory, Feb. 2023) HIGHLIGHTED BY THE NIH, that use very rigorous science (drilling down to individual bacterial genes) to demonstrate the strong INVERSE relationship between the abundance of butyrate-producing bacteria in the gut microbiome (F. Prausnitzii and E. Rectale) and CFS. My impression is that the researchers have likely found biomarkers/(a signature) for CFS. Now that the University of Gothenburg has overcome the oxygen sensitivity of F. Prausnitzii and added a symbiotic bacterium, D. Piger, to aid in the colonization of F. Prausnitzii in the gut (and tested this encapsulised combination for safety in humans); does anyone know of researchers/clinicians planning to use their encapsulated supplement for CFS? I realize that U. of Gothenburgs supplement was created for trials in Type 2 diabetes and inflammatory bowel disease. However, it would seem to me that the connection between this butyrate-producing bacterium (F. Prausnitzii) and CFS is actually stronger than for these other disorders.
Hi Joe, Thank you so much for this information! This is very interesting and I’ve seen nothing thus far on this subject in CFS forums.
Cort, I wonder, is this something you might be able to look into for us? I barely understand the 2 papers:
https://www.nature.com/articles/s41586-023-06378-w
https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(23)00029-X
Thanks Deb! Very glad to see they are making progress with F. prausnitzii! I think we may have covered the preprint of that other paper – that was a big one! I will check.
Hi Deb. Thanks for the kind response! Please excuse my slow reply. I’m pretty overwhelmed at the moment. Yes, that is the article/study in Nature I was referring to, research involving collaboration between the University of Gothenburg and the probiotic supplement company BioGaia AB. Here’s an additional link – to a short synopsis on the U of G website: https://www.gu.se/en/news/important-step-toward-next-generation-probiotics. As I mentioned above, when I first saw that this supplement was being created for upcoming clinical trials, I was eager to see if ME/CFS was among the trials’ targets. After all, the research in Cell Host and Microbe draws such an extremely strong association between ME/CFS and deficiencies of the bacteria responsible for producing the essential short chain fatty acid, butyrate. NOPE. No ME/CFS trials. As I was well aware, deficiencies, in the gut microbiome, of our helpful friend, F. Prausnitzii, are also associated with a NUMBER of other disorders. Osteoporosis, Cardiovascular Disease and Type 2 Diabetes come to mind. The upcoming BioGaia trials are aimed at pre-diabetes. I believe there will also be trials involving IBD (Irritable Bowel Disorder). In addition, I’m planning to keep an eye on the research teams from the two Cell Host and Microbe studies. They are invested in and continuing to pursue ME/CFS (and the connection to the gut microbiome). They’ve had funding and produced impressive results; results that the NIH drew attention to, with an NIH blog attached to their release in February and an NIH bulletin sent around to doctors. Hopefully they’ll continue to be funded. It’s been a while since I researched their studies, but my memory says they’ve moved on to mouse models, where their plan is to create ME/CFS mice (via fecal transplants?!) and attempt to successfully treat them. I assume they are well aware that there will soon be a supplement containing an oxygen-tolerant strain of F. Prausnitzii combined with the helpful symbiotic bacterium, D. Piger.
Hi Joe,
I hope I don’t send 2 identical comments, but my last comment didn’t seem to post.
Thank you for this information – it’s very interesting and I’ve seen nothing about this elsewhere. Have you learned anything further about the availability of the combination F. P and D.P.? Btw, here are the links to what I believe you’re referring to – are these the studies you are referencing?
https://www.nature.com/articles/s41586-023-06378-w
https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(23)00029-X
Seems like more silver bullet info. Always this is it, that is. This part of my system or that. Getting tired of info overload with not much but grasping at straws, and dead ends ultimately. I think Dan has had some of the best ideas here.
Hi from Finland! Is anyone familiar with NIKKI wearable? Has it helped you with fibromyalgia or CFS related symptoms? I read about it a week ago.
I might by one to myself if I manage. to save money for it.
Merry Christmas to everyone!!
Best wishes, Liisa Hänninen
Great article and summary! Dr Arseneau did have a guest speaker (an anesthesiologist) review stellate ganglion block for his patients. Hopefully she will one day give him permission to post that to his YouTube channel.
Just a note that I believe the guanfacine dose suggested is wrong in this article- those are his PEA dose recommendations. I believe he often recommends 1mg guanfacine, then 2mg if needed (up to 4mg max), though please check his medication handouts directly to confirm this.
Based on this list from Dr Arseneau I am trying PEA and I just want to say it’s not true that it is completely safe and without any side effects
It does say that in many places online including from the companies selling PEA but if you dig a little deeper you find it can cause heart palpitations and anxiety and shouldn’t be mixed with antidepressants- at least some types
Some places even say it can be dangerous
PEA had an immediate good effect on me- a real boost of energy- the best I have felt in decades- but after a couple of weeks I am having bad heart palpitations so I’m worried and I’m wondering whether to quit or try to lower the dose
I do take wellbutrin so it could be the interaction
Anyway be careful out there…
I’ve been on PEA a week and didn’t clue in that the increased anxiety and heart palpitations/chest tightness were side effects, so thanks for mentioning that. Though I haven’t had any benefits yet.
I had a group consult with Dr.A today and he said to keep up with the 3X PEA400 daily and add Pharma GABA for the anxiety.