


Amantadine is the go-to drug for fatigue in multiple sclerosis.
It seems a bit odd that we haven’t heard much about amantadine (Gocovri, Symadine, and Symmetrel) in chronic fatigue syndrome (ME/CFS) or long COVID. They are, after all, the two big fatiguing diseases on the planet, and amantadine is the go-to drug for fatigue in another highly fatiguing disease – multiple sclerosis (MS).
Fatigue is often the most limiting symptom found in MS. Given the generous funding that MS receives, it’s perhaps not surprising that the fatigue in MS has received many more treatment trials than the fatigue in “chronic fatigue syndrome” has. A recent systematic survey of clinical trials focused on fatigue in MS came up with a rather incredible 91 reviews and systematic reviews, 78 randomized controlled trials, no meta-analyses, and 107 clinical trials.
That review found that “all trials that compared amantadine (200 mg) with placebo showed a significant effect of amantadine on fatigue”. While the review pointed out the small nature and short duration of many of the trials, it also noted that amantadine is the only treatment currently recommended by the National Institute for Health and Care Excellence (NICE) for fatigue in MS.
The Long-COVID Amantadine Study
The 66-person, randomized, open-label, pre-vaccination, Iranian long-COVID trial, “A randomized open-label clinical trial on the effect of Amantadine on post Covid 19 fatigue“, was not placebo-controlled; i.e. everyone knew if they were getting the drug or not. The Fatigue Severity Scale (FSS) and Visual Analogue Fatigue Scale (VAFS) were used to assess the drug’s effectiveness. One hundred mg of amantadine was given once in the morning and once at night for two weeks.
The high p values (p<.001) indicated that the results were not due to chance; i.e. they were real. The VAFS score in those taking Amantadine decreased from 7.90 to 3.37 and in the control group from 7.34 to 5.97. The FSS (Fatigue Severity Scale) in those taking Amantadine decreased from 53.1 to 28.4, and in the control group from 50.38 to 42.59.
Put in plainer language, in those given the Amantadine, their VAFS score decreased from having “a high degree of fatigue that interferes with daily living” to “a low to moderate degree of fatigue” that does not affect daily living.

The results were promising. A placebo effect could have added to them, however.
Those not given the drug, however, also reported less fatigue. Their VAFS score dropped from having “a high degree of fatigue that interferes with daily living” to “a moderate level of fatigue, which may affect your daily functioning and quality of life to some extent.”
Similarly, those taking the drug dropped from being “easily fatigued” and possibly being disabled by fatigue to having “a low degree of fatigue” on the Fatigue Severity Scale. Those not taking the drug were still categorized as being “easily fatigued”.
The authors reported that the side effects were transient and tolerable for patients, except for one who stopped taking the drug due to severe nausea and abdominal pain. They stated that the drug is usually well tolerated, and characterized it as having a “mild side-effect profile’. Amantadine is well-known for its ability to produce hallucinations but those appear to be mostly a problem at higher doses. Care should be taken when it is used with additional CNS stimulants or anticholinergic drugs.
The trial suggested then that over a short period of time, Amantadine may be able to substantially help with fatigue in some people with long COVID. The big fly in the ointment with this study was the lack of a placebo control group meant that a placebo effect – people anticipating that they might get better and therefore improving – could have contributed to the results.
We know that Amantadine can help with fatigue in MS and this study suggests it can help with fatigue in long COVID – so what is this drug?
THE GIST
- It seems a bit odd that we haven’t heard much about Amantadine (Gocovri, Symadine, and Symmetrel) in chronic fatigue syndrome (ME/CFS) or long COVID. Amantadine is the go-to drug for fatigue in another highly fatiguing disease – multiple sclerosis (MS).
- With hundreds of clinical trials, fatigue has been well-studied in MS. A recent review reported that all Amantadine trials found that it significantly reduced fatigue.
- A 66-person, randomized, open-label, 2-week long-COVID trial found that Amantadine significantly helped with fatigue. One symptom assessment found that those given the Amantadine went from “a high degree of fatigue that interferes with daily living” to “a low to moderate degree of fatigue that does not affect daily living.
- Another symptom assessment found that people given fatigue went from being “easily fatigued” and possibly being disabled by fatigue to having “a low degree of fatigue”. People not given the drug improved their fatigue as well but not nearly to the same degree.
- Note, though, that the trial was not placebo-controlled, indicating that the placebo effect could be responsible for some of the positive effects.
- The authors reported the drug is usually well tolerated, and characterized it as having a “mild side-effect profile’. Few side effects were reported in the trial. Care should be taken when it is used with additional CNS stimulants or anticholinergic drugs.
- The drug appeared to be effective and safe in the long-COVID trial but half the participants dropped out in an 8-week 1997 ME/CFS trial. Perhaps the drug is better used for short periods. The Clinician’s Coalition for ME/CFS reports the drug can be helpful for mild to moderate fatigue.
- Amantadine tones down the excitatory neurons in the brain that may cause neuroinflammation, and increases dopamine and norepinephrine levels.
- A recent review asserted that both Amantadine and a similar drug, memantine, “improve vigilance, lack of attention and concentration, (and) fatigue syndromes… in patients with chronic neurodegenerative processes”. Highlighting Amantadine’s help with fatigue or chronic exhaustion and memantine’s effect on cognition, they proposed that both be tried in long COVID.
- Applying the Arseneau test of whether or not to try something: The evidence is not solid, but the fact that the drug might possibly help with fatigue, its low cost, and probably low risk suggests a short-term trial might be worth a try (???).
Amantadine
Amantadine has been around for a long time. Developed as an antiviral in the 1950s, it’s now used in central nervous system diseases. (It came to be used in Parkinson’s Disease after a person with Parkinson’s felt better after using it for the flu).
It’s believed to inhibit the overactivated excitatory NMDA glutamate receptors that may be causing neuroinflammation and burning out neurons in these diseases. It also increases the release of the feel-good neurotransmitter dopamine as well as norepinephrine in the brain. Like memantine – which may be helpful in fibromyalgia – amantadine also appears to have anticholinergic effects.
Amantadine also appears to be helpful in traumatic brain injury which can mimic the symptoms found in long COVID and ME/CFS.
Amantadine and ME/CFS
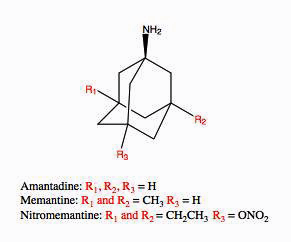
Amantadine is closely related to memantine. Both were recently recommended to be trialed to reduce fatigue and improve cognition in long COVID.
Once again, we see long COVID triggering treatment trials that could’ve, would’ve, and should’ve have been done in ME/CFS. A small early trial in ME/CFS, though, may have turned the field off to the drug. A 30-person, 8-week 1997 trial found that half the patients dropped out and no discernible effects on fatigue or other symptoms were found.
Pointing to their quite positive findings from the long COVID trial and the lack of significant side effects, the authors suggested that 8 weeks may have been too much. In any case, amantadine is used from time to time in ME/CFS and the Clinician’s Coalition for ME/CFS states that amantadine “may help mild to moderate fatigue. May interact with psychiatric medications but does not warn about side effects in its usage section.
Another glutamate inhibitor – memantine – presents a possibility. A review of memantine’s effects on neuropathic pain stated that memantine has the “safest side-effect profile” and that the “excellent benefit/risk ratio” the drug presents made it a good target for larger studies. A recent placebo-controlled fibromyalgia study found that memantine moderately reduced pain levels. Another study indicated it was able to increase cerebral metabolism.
A recent review asserted that both amantadine and memantine “improve vigilance, lack of attention and concentration, (and) fatigue syndromes… in patients with chronic neurodegenerative processes”. Highlighting Amantadine’s help with fatigue or chronic exhaustion, and memantine’s effect on cognition, they proposed that both be tried in long COVID.
Applying The Arseneau “Should I Try a Treatment or Not” Test
The Arseneau test assesses the factors below to help decide whether or not to try a treatment. Note that different people will get different results. For instance, people with more resources may feel more comfortable trying more expensive and unproven treatments. Likewise, people who’ve had bad reactions to treatments in the past may be less likely to try things that don’t have a strong evidence base. In other words, the final results are person-dependent.
- The credibility of the source – a journal publication, plus many studies in MS – the credibility of the source is good.
- Quality of the evidence – lacking. A small study and good results but no blinding means a placebo effect could be present. Plus, we have an old negative ME/CFS study. Countering that, the drug is on the list of the Clinician Coalition’s drugs for possible fatigue reduction in ME/CFS and has been well-studied in MS.
- The benefit, the cost, and the risk–benefit analysis – the benefit is probably moderate, and the cost is low (@$25/month). The risk is a bit iffy as a longer-term trial of the drug had a high dropout in ME/CFS but the drug has been tolerated well in MS and was in this long COVID trial.






amantadine didn’t work at all for me , but provigil (modafinil) helped a lot for fatigue.
In long covid?
Comment *Gracias por enviarnos estos rayitos de luz y esperanza. Yo tengo ambas enfermedades, SC/EM (2007) y LONG COVID (2020) y lejos de ir mejorando, mi sistema inmune no deja de inventarse nuevas enfermedades.
Thank you for posting this Cort! I just wanted to clarify some clinical trial terminology. You stated that the trial was “not placebo controlled; i.e. everyone knew if they were getting the drug or not.” However, the trial was in fact “controlled,” it just wasn’t “blinded.”
A controlled trial refers to any trial that compares a new treatment to a “control” treatment, which can be a placebo or the current standard treatment. In this trial, patients were put into two groups: the “treatment group” received amantadine and the “control group” received a placebo.
Blinding refers to whether the participants and/or the researchers know which group each participant is assigned to. In a double blind trial, neither the researchers nor the participants know which group each participant is in. In a single blind trial, the researchers know which group each participant is in, but the participants do not.
Double blind, randomized controlled clinical trials are the gold standard. The lack of blinding in this study introduces a lot of potential bias and limits the validity of these results.
Thanks for clarifying that. It’s placebo-controlled but not blinded 🙂
Does blinding add a lot of complexity or cost?
Thanks, I appreciate the explanation.
Interesting that the study referred to side effects of Amantadine as mild. Two women in my caregiver support group said their husbands had severe reactions….both men had hallucinations, one of them also had severe swelling in his feet. Who funded the study? Big Pharma?
Read the link from CJD MD below. Use in the elderly can cause hallucinations. (Study done in Iran).
Hallucinations can occur with Amantadine but typically at higher doses than used in multiple sclerosis. Again, the systematic review of amantadine studies in MS concluded that it was well tolerated.
What were they given the drug for? At higher doses the drug is well known to produce hallucinations and produces them quite frequently but they appear to be rare at the doses given in multiple sclerosis or ME/CFS (100-200 mg/day)
A systematic review of Amantadine studies in multiple sclerosis also states the drug is well tolerated and the ME/CFS Clinicians Coalition reports the drug can be used for mild to moderate fatigue and in its Comments on usage simply states “may interact with psychiatric medications”
https://mecfscliniciancoalition.org/resources/
How can you speak of fatigue instead of PESE / PEM? Fatigue was the feeling of hiking all day with a backpack. Stop hiking and the feeling fades; after a night of sleep, I was ready to hike again.
With M.E., I wake up feeling unrested, and exertion extracts a price in symptoms in cognition, sore throat, altered digestion, and long term muscle loss. The word fatigue does not fit the situation at all, in my view.
I know little about MS. If it helps people with MS, fine.
If amantadine lessens the feelings that keep a person with M.E. resting, will that person exert too much and still have PESE?
Long ago I was in at trial of amantadine and a supplement, alternated. L-carnitine was the supplement. Neither had a beneficial effect on me. The researchers published the results, in the 1990s.
I agree with this, I don’t feel fatigued either, but do feel poisoned, beaten up, etc. I usually am not stopped by fatigue at all, but by my legs not working, my muscles stiffening up, my heart racing, pain and headache. Have we all accepted this description of our illness as if we were fatigued, even if that’s not at all accurate? Or do we have different diseases? If some of us are fatigued, maybe we’re just having different diseases? I really don’t feel like fatigue describes my illness at all.
This issue of fatigue vs PEM is puzzling, and at least within long Covid there’s the idea that patients can be divided according to their response to exertion. There’s the patients who rapidly suffer extreme fatigue/exhaustion and the other group who have the more delayed PEM like reaction of ME. It makes it difficult to describe the illness when it seems to have these subtypes. My feeling is that these responses may be closely related or on a spectrum, I’ve heard of people whose fatigue became more PEM like after a sudden worsening or change in their condition.
Agree!!!!
I’m tired of reading and reading about all these studies that have been going on and the teachings on Long Covid when I already know all about it ..”why”? Because I’m living in the “LONG COVID” Nightmare, it had taken 4 years of my life, it has left me isolated, it has left my family without a mother, a daughter, a grandmother, a contributor to society advocating as a nurse, it has left me for no where to turn for medical treatment- when will there be some answers with treatment for the “Bottom line” to the problem which all of these researchers by now seem to be aware of to the reason for ongoing & long term sickness from the entry of the COVID virus into human cells and caused ….,
“Immune Responses “
“Chronic Inflammation “
“Cytokine Storm “ “
Isn’t this The Leader to all of our symptoms and to the onset of new illnesses/diseases/multi-organ & systemic destruction….where are these treatments and remedies to ending this? ??? Why aren’t doctors being educated on this subject?? So patients like me have some where to turn?
I feel poisoned AND severely fatigued almost constantly, except when asleep! Plus awhole bunch of other symptoms!!
When you talk about fatigue in a medical sense it is definitely something else than the tirednes or maybe exhaution from trailing/hiking. And fatigue is also a big part of ME, even if you remove PEM.
I understand what you are saying, but again there has to be studies done IF it may work in some aspect. Like B12 helps for the brain fog, this might help in other aspects, and the answers for ME might be a combination of several treatments?
Good points, Kajsa.
Sarah, I had the same thought. My key symptom is PEM. I wouldn’t call it fatigue.
I have extreme fatigue at times if my day goes beyond what I can handle. When “normal” people say they are fatigued, I always think that they may not know what fatigue truly feels like. I cannot function — literally, physically cannot move, and mentally I am forced to shut down. Go to bed (or be helped) get into position, see ya’. It it the most uncomfortable feeling; I don’t believe I can describe it but to try, it’s a bit like having restless legs syndrome all over your body with pain that has turned to numbness, and shaking all over. That’s extreme fatigue to me. A lesser version of that is more prevalent. I mainly have to keep tabs on what I do each day or I’ll know what’s coming. It’s far worse than PEM in my case.
Hmmm – an interesting article – I will follow ongoing updates….. Research is one thing, but clinical use is another. While in academia, I used to occasionally prescribe amantadine, and teach how to use it only when/if needed and appropriate. It takes a skilled and informed clinician to know with whom to use this, and how to follow up and titrate it, given its many potential, sometimes severe side effects and drug interactions. Here’s one site to read thoroughly if considering its use – not just this part about “before using”. Use with care and caution!!
https://www.mayoclinic.org/drugs-supplements/amantadine-oral-route/before-using/drg-20061695
Cort I appreciate the ideas you bring to the table for consideration. If I am stating this correctly, Ron Davis recently discusses his findings of a subset of ME/CFS patients ( test tube blood test) had findings identical to MS. So he believes one virus can cause two illnesses, ME/CFS and MS. So for some Amantadine May very well be beneficial.
My experience: I took Amantadine for about a year towards the beginning of my ME/CFS, POTS, MCAS, etc. It was no help at all to me, same as Sarah L. has posted.
My physician meant for it to reduce viral infections but instead, it increased adrenaline, worsening my hyperadrenegic POTS. At that time, there was no such term as POTS or hyperadrenegic POTS, so I had no diagnosis for my orthostatic hypotension alternating with severe hypertension.
Minnesota Mayo and Dr. Low had no idea how to treat me, nor did the Syncope Dept. at the Cleveland Clinic. And Dr. Cheney or Dr. Lapp never really help my ME/CFS in the 80’s/90’s. Just travel making me worse and very broke.
Thank you, Cort, for keeping us up to date. I’m glad people today have infinitely more info, data and treatment options than I did back then.
Is it possible that the POTS is a condition, where blood can go out from the head fast enough than it gets in, that’s was we feel relief when we seat or even better lie down?
I used to prescribe this years ago, and teach how to use it when/if needed and appropriate. It takes a skilled and informed clinician to know with whom to use this, and how to closely follow up and titrate it, given its many potentially serious side effects and drug interactions.
Here’s one site to read thoroughly if considering it’s use – not just this part about “before using”. Use with care and caution,
https://www.mayoclinic.org/drugs-supplements/amantadine-oral-route/before-using/drg-20061695
Why isn’t fibromyalgia listed in the top two. There are more people with fibromyalgia than MS. Yet fibromyalgia is not even mentioned. It is disheartening to have the major symptom of CF/MES but with the additional burden of widespread pain and a host of other comorbid diseases and then to rarely see it mentioned in articles about debilitating diseases. I would gladly lend my body to people for a day so that they can experience what it is like to live with fibro. I bet more attention would be given to it then.
there are plenty of articles here about fibro, it just doesn’t happen to be the focus of this study 🙂
alot of people with cfs also have widespread severe pain and/or comorbid fibromyalgia, so believe me, we know where you’re coming from, don’t worry. a bunch of chronic illnesses are severely under rescourced and it’s honestly criminal, makes me so angry tbh, but idk how much it helps to point out how fibro is understudied on an article partially about how cfs is understudied
I know there are lots of articles on fibro. My point was that you said “chronic fatigue syndrome (ME/CFS) or long COVID. They are, after all, the two big fatiguing diseases on the planet”. Leaving fibromyalgia out of the top two made your statement incorrect and also made fibro and everyone who has it invisible.
Oh I figured out where the misunderstanding happened, I think Cort may have meant “top two most fatiguing” instead of “the main two fatiguing” (e.g. in terms of average fatigue severity scale studies CFS and MS come out on top)
Now I understand what you meant, that makes sense! Perhaps Cort could clarify?
To SW-I agree, I like ur last 2 sentences.
Did anyone out there try it and have a good effect?
I used to prescribe this, and teach how to use it when/if needed and appropriate. It takes a skilled and informed clinician to know with whom to use this, and how to closely follow up and titrate it, given its many potentially serious side effects and drug interactions. Here’s one site to read thoroughly if considering its use – not just this part about “before using”. Use with care and caution,
https://www.mayoclinic.org/drugs-supplements/amantadine-oral-route/before-using/drg-20061695
I was prescribed amantadine in the early nineties by my -then- neurologist, who knew about ME, and it did nothing for me. Just nothing. So don’t expect too much from it.
Hi Bettine – You are an N-1, and we know that ME/CFS is a heterogenous disease and people respond differently to all sorts of treatments. We can’t really use your experience to predict how others will do (i.e. “don’t expect too much from it”). Thanks for sharing your experience, though.
you are right ofcourse, Cort. Though I had to think really hard what an N-1 was! 😅 I hope it works out for other people with ME.
🙂 Thanks Bettine for your understanding!
I honestly think we’re all different & what works for some, may not work for others. Articles like these brings it to our awareness that there are things to try that MAY work (getting our hopes up).
Once we start reading the comment section and find it doesn’t work for some dashes our hopes and may prevent us from even trying to find out if it’s a good “fit” for us. I say we stay open to try it and pray for good results.
Agreed! I think our brains – that is our brains as humans – pay more attention to negative results than positive ones. We should always remember to try to stay open…Thanks!
I also was prescribed Amantadine in 2003 when I was first diagnosed with ME/CFS. It never helped and did nothing for me. I remember my CFS Dr. saying that it was best taken right at the onset of CFS as an anti viral medication. Unfortunately it did not help and made me very dizzy. 22 years later and still struggling…
Well, I guess a good thing about long COVID is that, in addition to more of useless antivirals, a few promising ones, including LDN, are now getting trialed. I’m all for it as long as it is safe, especially if it’s got to do with dopamine.
According to medplus.gov, “amantadine is used to treat the symptoms of Parkinson’s disease… It is thought to work to control movement problems by increasing the amount of dopamine in certain parts of the body.”
Low risk? Per CJD MD’s link, I don’t think so! Considering how many herbs and supplements I take to try and keep multiple symptoms under control, I would not try this without better research. As per all else I suspect genetics has a role to play here as well. I’ll definitely think twice about trusting any research coming out of Iran in the future even though I trust many foreign countries research over that from the US sponsored by Big pharma.
That was the conclusion of the study and this is the conclusion of a 2020 systematic review of the dozens of the studies that have been done in MS
“Amantadine is the most studied drug that has shown improvement of MS-related fatigue, with mild side effects and good tolerability.”
https://pubmed.ncbi.nlm.nih.gov/33012266/
Over the years since my diagnosis of ME/CFS and long covid, I’ve found that a combo of low dose naltrexone, micronized PEA and a one year neuroplasticity zoom group, with an additional focus on pacing, have been the most helpful, amongst other treatments. I’m more able to enjoy life and have lessened my symptoms.
Once again, it takes an informed and skilled clinician to know when, with whom and how to use and titrate drug therapy. I was one of what I was told is 7% of patients who reacted with side effects to LDN, but responded to titrating, using “ultra-low dose” naltrexone. It was carefully titrated down to a place where the side effects left.
I also needed a higher than normal dose of the micronized PEA. We are all unique, and often require individualizations of dosage to achieve maximum benefit. This also applies to Amantadine.
This is very interesting. I have had what I assume to be MECFS for about 4 years now, and then LC starting with a booster 2 years ago, and greatly exacerbated by 6 covid reinfections over the last year.
But I’ve also just been diagnosed with ADHD (I’m 53), so the dopamine connection in this article and discussion is very interesting. And PEA, which I’ve seen supplementation suggested for people with low PEA and ADHD.
I’ve had success with aspirin + nattokinase, antihistamines + a low histamine diet, (cleared up a most of my brain fog, the peripheral neuropathy and the fasciculations) ,
brain training/neuroplasticity, daily yoga nidra, meditation and LDN.
However, every time I get reinfected, it sets my baseline way back, to square -1. Then it takes a couple of months to start getting better again, but I always get reinfected before I can even get back to where I was before I was infected. I’m in a much better place mentally than I was a year ago, when I thought my life was over, but physically, the POTS and the exertion limitation is much worse.
Now I’m masking at home around my family, even while I sleep, hoping to avoid another reinfection.
I’m hoping to do a neurotransmitter test soon, and find out if there’s any more supplementation I can do.
CJD MD
I agree with you with respect to LDN and PEA. I take both every day. The most important thing with LDN is starting at an ultra-low dose and increasing the dose very gradually. I find my best effects at 6mg every bedtime. I had to start at .10mg and it took me eight months to get to 6mg.
I take PEA at every meal, but micronized PEA is not good enough. You need ultra-micronized PEA for absorption. I buy it here:
https://www.elevationterpenes.com/collections/best-sellers/products/ultra-micronized-palmitoylethanolamide-powder-99-pure-lab-tested-pea?variant=32705087733807
and I put it into capsules myself because it’s cheaper that way.
Thx for the info and suggestions . I did restart low at .1 mgm, after starting originally at 1 mgm and not tolerating. I also very gradually built up over several months, but could never tolerate more than .6 mgm . I tried again after a year, but my intolerance remains. A colleague who attended a conference on LDN informed me of the 7% who need and benefit from ongoing ultra low dose.
I’ve just written away to see if the ultra micronized PEA can be delivered to Canada – they don’t say where they are located? Sometimes Canada doesn’t allow some less well known supplements across the border, and at times, even common supplements have been restricted.
They are in California, and I am in British Columbia. I buy this stuff by the kilo!
Thx – I’m also in BC , so will follow up – useful info!
What does the PEA do for you both?
PEA is a great pain reliever, better than Tylenol. I take 400mg at every meal. Without this, I would not be able to walk. When I take this, I even have to be careful not to touch hot things because I can burn my fingers and not feel severe pain, just mild pain.
Palmitoylethanolamide also binds to the Ace 2 receptor where Covid 19 would latch onto the cell and therefore prevents the virus from attaching there. I have not had Covid.
I have extreme fatigue at times if my day goes beyond what I can handle. When “normal” people say they are fatigued, I always think that they may not know what fatigue truly feels like. I cannot function — literally, physically cannot move, and mentally I am forced to shut down. Go to bed (or be helped) get into position, see ya’. It it the most uncomfortable feeling; I don’t believe I can describe it but to try, it’s a bit like having restless legs syndrome all over your body with pain that has turned to numbness, and shaking all over. That’s extreme fatigue to me. A lesser version of that is more prevalent. I mainly have to keep tabs on what I do each day or I’ll know what’s coming. It’s far worse than PEM in my case.
I take 4 caps of 400 mgm a day to help reduce my fibromyalgia pain and back pain. The kind I take and what it says is :
“AOR Palmitoylethanolamide (PEA) is a long chain fatty acid naturally produced by the body but also found in foods such as egg yolk, soy and sunflower oils. This molecule, researched for its unique and varied health benefits for eighty years, is now receiving attention because of its crucial role in the endocannabinoid system (ECS). The ECS is a lipid communication network with critical physiological functions. It is a signalingnetwork that communicates with other systems throughout the body, helping to regulate them. PEA is an endocannabinoid produced locally by the cells, and it accumulates in tissues following any injury, physical stress or pain.
By inhibitingthe activation of mast cells that cause additional inflammation (such as the release of histamine), PEA acts as a potent anti-inflammatory agent. This unique molecule has other direct and indirect mechanisms of action; it even enhances the action of other endocannabinoids through its ‚Äúentourage effect‚ . The PEA molecule has been clinically demonstrated to relieve pain in osteoarthritis of the knee as well as to reduce symptoms of low back pain. This mighty little molecule is well toleratedfor athletes, children and seniors.
Benefits:
Scientifically shown to help reduce chronic pain and inflammation
A naturally occurring long-chain fatty acid amide generally produced in response to various triggers including inflammation
Provides a multi-pronged approach to activating and sustaining the endocannabinoid system
Studied for 80 years and by more than 300+ medical studies”
Is there a less expensive way to get this? I haven’t even the money close to this.
Ultra-micronized PEA (palmitoylethanolamide) is cheapest when you fill the capsules yourself. I buy it here:
https://www.elevationterpenes.com/collections/best-sellers/products/ultra-micronized-palmitoylethanolamide-powder-99-pure-lab-tested-pea?variant=32705087733807
and you can buy less than 1kg if you want by choosing from the drop down box. One kilogram lasts me a year.
Can you give the full spelling of PEA? There are two supplements with that acronym. Also wondering what dose ended up working for you.
Here are two Health Rising blogs on PEA
https://www.healthrising.org/blog/2022/12/13/pea-luts-long-covid-chronic-fatigue-syndrome-fibromyalgia/
https://www.healthrising.org/blog/2014/09/19/palmitoylethanolamide-pea-medical-food-fibromyalgia-chronic-fatigue-syndrome-mecfs/
CJD MD. LDN works for me but last year, after being on it for a couple months, I noted serious side effects. Hair loss and pain in my tendons so I went off it. It however did cure me of a flare I was in. I am back in quite a state. Post viral exhaustion and post exertion malaise etc. LDN is the only thing I know to work. So I’ve asked my doc to go back on it.
I’m going to start at 1mg but I’m curious about how to ask for ultra low dose LDN. Where do you get it? In what form? My doc is great and will prescribe for me.
A compounding pharmacy will make it for you with a prescription. The dose itself will specify to the pharmacist that it is ultra lo dose. I actually started at .1 mg in capsule form at bedtime, and every week increased by .1 mg up to .6 mg. When I increased beyond this, I experienced a major sleep disturbance with nightmares. I tried twice to increase the dose a few months and then a year later, but once again experienced the nightmares, so have remained on.6 for the past 3 years or so. My body is sensitive, and told me what it needed dose wise. I did work with a functional medicine practitioner, who suggested how to do this.
CJD MD
Thanks this is helpful. I have taken it in the morning because of the sleep disturbance. I really like the idea of taking a very very low dose. Do you feel it helps even at that low dose? Is there a reason that you wanted to escalate up?
It has really helped me in the past. I went from feeling horrible post COVID to feeling so much better. But I learned on this forum of the muscle wasting and hair loss side effects – which I experienced myself. (Both symptoms stopped when I went off the LDN. I was shocked that LDN was the culprit.) I was on 4.5 mg capsule at the time. I’m in the middle of a flare and I really need some help.
I have found at this dose that it decreases my fibromyalgia pain, and when stopped, the pain increases. I was told years ago that 7% of those who take LDN need ultra lo dose. Titrating to maximum tolerable (no side effects) but effective dose is the way I find works best – trial and error work for me!! I also feel it helps my immune system at the best titrated dose for me – aside from one covid episode in early 2020, I have had no respiratory illness, even in flu season. We are all unique and individual, so I am grateful for the helpful advice from my functional medicine practitioner, who knows my complete medical history and can advise me best by being fully informed. I wish you well in recovering soon from your flare.
Thank you to all who have shared the ultra LDN info. I have a prescription, and it definitely helps in some ways, but it seems to build up and create unacceptable side effects at 1.25 mg. I’ll talk to my pcp about an even lower dose.
So Cort, in your opinion, would this be something to consider for short periods of time when we know we’ll be under considerably more stress than we’ve built into our normal routine? (Such as navigating a major house update that will take a few weeks.) I’ve constantly adjusted my lifestyle over the last 23 years but sometimes it’s impossible not to overdo it. Or would that be the worst time to try it since we wouldn’t have a normal baseline anyway?
Has anyone been tested for Bornavirus? .
The only treatment is Amantadine.
Bornaviruses are RNA viruses that infect blood and brain cells. Most infected patients suffer from reduced intellectual capacity, mood disorders and problems concentrating.
It is estimated that in Europe around 30% of the population is infected by the virus which can remain in a dormant phase for a long time. 15% are at risk of developing mental disorders often triggered by chronic stress which weakens the immune system and, as a result, leads not only to activation of the Bornavirus, but to other chronic infections such as, for example, borreliosis (lyme) and its co-infections.
I am weakly positive for Bornavirus. I tried Amantadine, but quickly stopped. I’m too afraid of the side effects.
Hi Claudine, did you have side effects so far? P.S. incidentally, I posted some of the controversial Borna story below.
I remember a comment on a Cort article in 2020 or 2021 where a person mentioned having taken amantadine injections in Germany. She described feeling ‘cured’, but that the effects did not last long. She also pointed out that intravenous amantadine was quite dangerous, which is why she did not continue. this treatment.Medicinally, it didn’t work.
What is fatigue? It is a feeling that can be different for everyone. In my opinion there are different types of fatigue. There is no medicine against fatigue. You need to know the underlying cause and mechanism and what type of fatigue before you can try a medication. When someone says I’m tired, people think they understand that, but that’s not the case.
Agreed, Gijs. I’ve identified at least 5 kinds (like flavors) of fatigue in my own experience of CFS. There is when my brain is tired and I can’t think or function; when I wake and am a zombie for the first couple of hours; the wired-and-tired fatigue of stress; the fatigue of coming down with a bug; the fatigue of being unable to motivate… some of these I experience physically in my body, some in my head/brain, some just expressed in lack of capacity… I’d love to hear from others how you might describe your fatigue(s).
Apologies, this is a bit off topic, but relates to ME/CFS and long covid treatment.
Wondering if anyone has tried CBD oil, and if so how effective or otherwise it was?
My working theory for a long time is that our illnesses are primarily caused by neuroinflammation. Unfortunately, many substances struggle to cross the Blood Brain Barrier, but apparently CBD oil can. And it has anti-inflammatory actions.
So interested to hear from anyone who has tried it. Thanks
I’m not surprised that CBD oil can get into the nervous system. I find it very helpful with sleep at times.
How about fatigue Cort?
Matthias, I take CBD daily, and it helps a lot, but CBDA is even better. Cannabidiolic Acid is the precursor to CBD in the raw cannabis plant. It is not psychoactive, but it is a powerful anti-inflammatory. I take it daily and it helps me with neuroinflammation.
Thanks Ann. Does it help with fatigue, or other symptoms?
No, just with neuroinflammation. So it helps with focus, mood and brain fog. But it wouldn’t work if I didn’t also take LDN and methylene blue. I find so many of my supplements only work if they are taken together as a group. If I try to eliminate any one of them, the whole thing falls apart.
“” I find so many of my supplements only work if they are taken together as a group. If I try to eliminate any one of them, the whole thing falls apart. “”
Thank you for saying that !!!!!!!!!!!!!!
While I may be a pain in the butt of your brain as I try to put you all through the ringer of theories and explanations, this is because, to me, the best path is becoming clearer and clearer, and is becoming simple.
(Not easy, nor without finances or efffort, but elegant…)
Attack all deficiencies at once, after being counselled by a doctor, or naturopath, if that is your preference, to be sure that no treatment will overtax you.
Justification: sometimes what one system in the body is failing to do relies on or is impeded by what a different system in the body is failing to do.
Example:
You may be anemic. So take iron, right? Maybe not. Maybe you already consume enough iron in your diet, but your gut is not absorbing it well enough. We know that vitamin C and Iron mutually aid the absorption of the other.
Therefore, think of how the symptoms in your *personal* body systems may be relying on BOTH of them getting better at the same time.
WHY?
Well… if one stressor can cause a myriad of symptoms (toxicity is a great example) then you might need a myriad of (or not-so-many) simultaneous therapies.
But please remember: My entire thrust is always: Nutrition. Our food can give us all that we need, organic is preferred, supplements can subsitute,
and some herbs and foods naturally help us to detox.
One of the GREATEST factors in all autoimmune diseases is
ELECTROLYTE balance.
Again:
1. One stress on the body, in an already weak state
(disease, toxicity, what we *call* stress)
can be a trigger for SEVERAL symptoms/conditions
and I would say ALSO… for the usual suspect autoimmune diseases
(the ones we can all name, mostly affecting women)
2. SEVERAL COMBINED STRESSORS , listed above, can result in seemingly only ONE condition (and i use the term loosely, to mean symptom, syndrome, disease with a name, mystery with no name)
3. I have endeavoured to synthesize:
-the blogs by Cort Johnson
-the doctor and researcher observations brought to my attention by Cort
-other research (mostly on NIH websites)
-basic or more complex nutritional/vitamin/mineral/herb/supplement studies.
For me… this seems simplified. To me, it is.
Now I keep on repeating.
Where do you get the CBDA? Thanks for your info.
Sarah: Just google “buy CBDA” + the name of your country. I can get it in Canada, but it’s cheaper in the U.S.
its never just fatigue! Many symptoms are interwoven. To me, treating the multi symptoms of both long covid and/or ME/CFS requires that a practitioner take an extensive medical history, and has current info re multimodal, individualized treatments. I believe that we all need our individual health care team, medications, awareness of healthy lifestyles, supplements and diets, for prevention, support and treatment – it’s multi modal.
While I have a history of autoimmune issues, and am soon to be 84, I believe I have developed lots of natural immunity over the years. I have not had a viral illness since I had covid 4 years ago. I personally do not get flu vaccines and have not had the covid vaccine. I also don’t use masks except at places where they are mandatory – I’ve read, and keep reading all the up to date research on specific vaccines and masks – pro and con, and have made my personal informed choices.
I use individualized doses of Vit C, D, magnesium, zinc, probiotics etc. and have had micronutrient testing to help both choice and dose for any needed macronutrients.
I have had holistic nutritionist consultations with testing to find the best diet for my various health conditions, and in general cook all my own food – an organic, gluten and dairy free, non-processed, individualized diet. I pace my cooking to times when I have the energy, and freeze batches for when I’m feeling too fatigued to cook.
I go for daily walks, and my social life with friends and family is fulfilling, and they all understand and respect my need for pacing. I’ve been retired for 10 years, but I keep active with medical webinars and research, as well as reading the 20 eclectic books a month my home library delivers. This regime has taken time, energy and commitment, is now routine, and has paid off in spades. We each find what works best for us. I still have some symptoms, and they vary with stress and weather, but on the whole, I feel lucky I can focus more on gratitude than on symptoms.
For those who wish to experiment with Amantadine, I encourage you to find a practitioner who is well informed in this area, knows your full history and treatment regime, and understands how to titrate doses should they feel you might benefit from it.
A fun fact from Germany: There used to be 2 German doctors/scientists (amongst them Liv Bode) who, a couple of years back, developed an Amantadine protocol to treat a ME/CFS-like syndrome that they attributed to Borna virus infection.
Bode also acted as a kind of whistle blower warning against contamination of blood donations with Borna virus. They got discredited at the time, the official opinion of the most renowned virology institute in Germany then being that Borna (a zoonotic i.e. animal-born virus) could not infect humans, or if it does in very rare cases, it almost always causes a severe often deadly encephalitis in humans.
Bode was since then
I tend to believe her about Borna virus being able to cause ME/CFS (at least as one of the known range of disease triggers), because if so many other virus infections are known to initially trigger ME/CFS, why should Borna be any different?
Moreover, Borna is a neurotropic virus (i.e. attacking the nervous system), and is known to also cause chronic infectious disease https://www.sciencedirect.com/topics/medicine-and-dentistry/borna-disease-virus .
Bode’s warning was somewhat vindicated when recently (since 2016) a few cases became known where people developed severe Borna related encephalitis (brain inflammation) after receiving transplants contaminated by Borna virus, and some of them died.
Still as far as I know, the official opinion remains that cases where Borna jumps from animals to humans are rare and will mostly present as a severe, often deadly encephalities. To me, this reminds of the early-pandemic “0-1” opinion that assumed you either die from Covid or are completely fine afterwards.
I would not be surprised at all if there were also more unspecific or asymptomatic cases of Borna virus infection that cause ME/CFS like chronic disease (similar to Long Covid).
Also, a question then (which still remains) was that if the Amantadine protocol causes patients to improve, is this really because of antiviral effects against Borna virus, or rather because of other effects of Amantadine on the brain.
In a recent publication https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8953669/ , Bode et al. comment on versatile antiviral properties of amantadine, including inhibition of Borna virus infection in vitro (in the lab), as well as antidepressive properties of amantadine.
…or, I don’t known, if maybe Amantadine might have influenced another kind of virus in those patients taking part in the Borna amantadine protocol?
As far as I remember, there was also criticism questioning the reliability of results of an independent lab test used by fatigue patients to show Borna infection.
Anyways, knowing what we know about post-infectious disease today, I am not sufficiently convinced to exclude Borna as one possible trigger of post-infectious disease.
And here’s a study the Bode team did on Amantadine effect on depression in conjuction with presence of Borna virus https://bmcpharmacoltoxicol.biomedcentral.com/articles/10.1186/s40360-020-0391-x .
By the way, a 2020 email from Institute of Tropenmedizin said the exact opposite (that amantadine was proven in lab experiments to NOT affect bornavirus), and a 2020 article said that based on sequencing, results from earlier borna virus tests were likely to be lab contamination.
Still, knowing what we know e.g. from current herpes virus research like Bhupesh Prusty’s, how viruses hide in specific tissues, can interact with cells without replicating and may not show up in the blood, I would not be surprised if Borna virus prevalence would be higher than shown by current borna antibody tests.
Comment *Cela fait quelques années que j’ai essayé l’Amantadine. J’ai vite arrêté car le médicament contient du soja, auquel je suis intolérante. De plus, je déteste prendre des médicaments car j’ai souvent des effets secondaires aux niveaux des yeux ( corps flottants permanents)
Le protocole du laboratoire allemand DEDIMED pour le Bornavirus est de 2-4 mg/kg. Pendant les trois ou quatre premiers jours, la dose est de 1 mg/kg pendant 3 mois, avec une amélioration au bout d’un mois. DEDIMED affirme avoir de bons résultats avec l’Amantadine pour la dépression, mais ils ne mentionnent pas la fatigue. Cependant, je ne suis pas dépressive.
Mon immunologue, spécialiste de l’EM/SFC, m’a informé qu’une étude réalisée au Japon dans les années 90 mettait en cause le BORNA dans le SFC.
Thank you, Claudine! I’ve entered your reply into Google translate:
Claudine de France: It’s been a few years since I tried Amantadine. I quickly stopped because the medicine contains soy, to which I am intolerant. In addition, I hate taking medications because I often have side effects in my eyes (permanent floaters)
The German laboratory DEDIMED’s protocol for Bornavirus is 2-4 mg/kg. During the first three or four days, the dose is 1 mg/kg for 3 months, with improvement after a month. DEDIMED claims to have good results with Amantadine for depression, but they do not mention fatigue. However, I am not depressed.
My immunologist, a specialist in ME/CFS, informed me that a study carried out in Japan in the 90s implicated BORNA in CFS.
Thanks JR, I forgot to translate my comment.
Hello All,
First: Cort: you kindly emailed me , and I don’t mind who contacts me whatsoever: at xtoferdx@hotmail.com. The problem was that your sending address wasn’t spelled out such as *cort@healthrising.com* it was
down deeper into the email server code and was like “cort@healthrising3
26587655467643467647654654” to which my replies could not reach your inbox. Please email me your correct (simple) address so that I can send YOU long messages rather than flatten the readers. Thanks. Chris– Ottawa
I return
now and again to Health Rising
to share my educated-guess interpretations of current research and meta-analyses as they pertain to long-covid and OBVIOUSLY and pertain to ME/CFS, the more recent medical ACRONYM and definition of which is supposed to be named SEID: Systemic Exertion Intolerance Disease. I don’t think CFS is very useful as a descriptor, but Myalgic Encephalomyelitis actually is. And I think that SEID is best, but I now believe that ME/SEID are a dynamic duo for awareness. With regard to M.E. , the abbreviation draws our attention to the most poignant current patient recognition that I have witnessed on Health Rising over the past 3 years: the statement that “oh… now research is showing some brain inflammation? We could have told you that 10-20 years ago. We’ve always known that.”
I feel this one point is CENTRAL in the pathology of SEID. The BRAIN, regardless of the body’s own problems, may be governing body temperature and metabolism to AVOID overheating and damaging the brain. And this choice may be based on HYPOTHALAMUS SET POINT temperature.
I will just insert a spoiler: The body can go feverish from Fahrenheit 97 to 104 and even to 108, and with immediate intervention… even survive. Fever is a FUNCTION of immunity, not a bad side-effect. But the BRAIN is only tolerant within a +/- fluctuation range of LESS THAN 1 degree Fahrenheit. Correct me if it’s actually 1 degree Celcius. I doubt it. The brain is VERY likely to “spoil everything” in the body for self-preservation of the brain.
My contribution today is
to: comment on AMANDTADINE,
to: tie together the fatigue trend in various autoimmune conditions, and
to: give an update on my personal learning and theorizing which is guided by NIH, Dr. Roger Seheult, and people such as Bob Naviaux, Rob Phair, Ravindra Nath, Nancy Klima
and many others.
I read Cort’s precis on this Amantadine trial, and then all of your individual comments. I am tempted to call myself C.J.D-not-md since the first 3 letters are my real initials, but since my interest and care for CFS long-haulers draws from care for a friend and my knowledge from academia– yet not from a formal medicine education.
Also I have overcome some Long-Covid sequelae through the use of Citrus Bioflavonoids. Lastly I will add that my niece developed schizophrenia at around 16 yrs, yes because of her phenotype to be genetically prone to it, but triggered by severe emotional trauma. I was recently educated here on the importance of “phenotype” but… let us never forget that EPIGENETICS is a 2-way street !! We can intervene. The phenotype can predispose, but epigenetic attention can REVERSE the reaction.
Cort and many of you are already familiar with the papers of
Dr. Bob Naviaux.
Correct me if I am wrong, but it seemed to me— and only through the lens of my *intermittent Health-Rising visits that there was Dr. Bob researching CFS, and then he went , kind of… broad-based and theoretical on chronic disease in general. And to his credit, he is qualified if not overqualified to be doing so.
But I like what he has done. He seems to have tackled WHY a body could be fighting a pandemic virus or a bacterial infection, or even just personal work or family burn-out and then instead of hit the HEAL stage, the person remains in a stage before that. A stage that requires destruction of the pathogen (attack) before the cell will go back to Naviaux’s newly coined term:
Salugenesis.
To explain what I mean: he has gone past the usual definition of the “pathology” of a disease to show how the body or organ was healthy before, changed its mitochondrial capacity and protocol , under orders from “head” office (HPA Axis), but conquered the pathogen and resumed normal mitochondrial function.
Naviaux, in “Mitochondrial and metabolic features of salugenesis: the healing cycle” in Mitochondrion Journal (Elsevier/ScienceDirect)
tells us:
that if the pathogen is not eradicated, the cells insulted will continue a loop in the second last phase of the cycle toward healing/recovery. It seems clear that this happens in all autoimmune intolerances of “self cells”, for whatever cause.
But also, other 2023/2024 researchers are teaching about reversing autoimmune intolerance through detox, toxin avoidance, supplemental nutrition. Consider Dr. Terry Wahls who healed herself of MS, and Dr. Amy Myers who knows she COULD have reversed her hyperthyroidism and could have avoided a thyroidectomy.
Again: “if the pathogen is not eradicated, the cells insulted will continue a loop”
I think that this well-describes CFS since CFS seems to be an autoimmune reaction to protracted infection. One of the comments in this thread on this page was that fatigue (and non-restorative sleep) are not limited to CFS patients. MS has fatigue, so does Fibromyalgia, so does Lupus, so does Myasthenia Gravis and there are more…. at least 10. They are in 8:1 thru 10:1 ratios of affecting women versus men. Why? I have thoughts on that. Cortisol levels, hormone dominance, whether the primary hormone producer: Ovaries or Testicles is supplying the hormone, or whether, either AFTER Menopause, or even BEFORE that… hyper cortisol production bumps out of the system the very necessary salt/water hormone regulator: Aldosterone.
One of you described that normal exercise can lead to exhaustion, but that with rest one’s body could restore itself for the next exertion. If you ask me (and I suggest that you should not 🙂 ), CFS is very similar to Myasthenia Gravis.
EXCEPT: in MG, the patient exerts, then hits the ceiling of NO MORE exertion.
The identified cause is an autoantibody against (the exact target escapes me at the moment:)
against either Acetylcholine, or against Acetylcholine Receptors of the Nicotinic type . nAChR
What I would like to UNDERLINE from the Naviaux article mentioned above is 2 main points:
A) One insult to the system (virus/bacterial infection/ or, also, let’s call it: “burn-out”)
could result in
ANY one of a plethora of autoimmune reactions or disease results and diagnoses…. in fact could progress to two or three or several of them.
But what condition is unique among all? No condition. Did Fibro invent pain? No. Did CFS invent inflammation? No. Did poor vascular health or did diabetes, or did obesity invent increase in blood pressure? No !!
There is a limited number of body REACTIONS and STATES while sick or infected,which TELL the brain what intervention is needed.
The MAIN TWO INTERVENTIONS or controls from above are:
body temperature set point
thryoid-based trigger of Citric Acid Cycle of energy metabolism — OR —
RESTRICTION OF THIS for the purposes determined by the
Hypothalamus
I may be wrong, but
We can give a mind-boggling diagnosis such as ankylosing spondylitis , but we are too close to the subject to be OBJECTIVE as to the VARIOUS insults that could result in the same conditions… based on our genetic phenotype.
The second point is the inverse:
B) It could be a COMBINATION, or perfect storm of insults to the body system,
or specifically upon the organ in question that result in the condition that we eventually have diagnosed.
Bottom Line:
1. One pathogen attack could result in a VARIETY of dis-ease states
which currently have a HOST of names.
2. SEVERAL pathogen attacks either simultaneously, or sequentially,
could reduce our cells/tissues to ONE SPECIFIC Disease state,
with a coined medical name.
3. It may be a more direct journey to recovery to examine with one’s doctor
the TYPES of attacks or nutritional/energy deficiencies
which can lead to one’s personal experience,
and to look at one’s own: pathology, the natural process of salugenesis,
then, with doctor guidance to *snap OUT * of that deleterious cycle
Conclusion:
Look at any sick day from work:
-low energy
-pain
-tenderness
-allergic or mast-cell congestion in the nasal passages
Are any of these unique to ANY disease name?
I would state: No.
In that case, why would it make sense to
go after ONE NAME, that has been dedicated
years of research,
to cure ONE SYMPTOM?
to cure ONE NAME with MANY symptoms??
The body only has so many symptoms to
choose from.
What leads to them? How am I prone to those?
What can I now — give up, avoid, increase,
supplement, and rest more… or EXERCISE MORE…
to put Salugenesis back on track??
To Conclude, or rope it all in , as I often forget to:
1. My niece who has been diagnosed with schizophrenia, but WELL treated,
suffers, clinically from 2 simultaneous disorders:
a) her frontal brain receives too MUCH dopamine, and perhaps also norepinephrine, and this leads to hallucination of voices who voice her darkest fears.
b) her , not anterior, but the segment directly behind, which is meant to control imagination or… mind wandering…. does not receive ENOUGH dopamine to slow down the former area.
If you can guess….. why a brain, under emotional trauma would switch to resisting dopamine which would have resisted running away from the point of reality,
then maybe you can help. My guess is that the goal failure is so final and .
bad that no NORMAL amount of motivational catecholemine (dopamine) can be enough.
Then, because the survival section of the brain is so DEPRIVED of motivation to hold back,
the experimental or Imaginative section of the the executive function brain, at the front, must desperately search for a new way. However, if the mindset at the back is negative, the extra dopamine will cycle upon failure.
It seems to be a catch-22, wherein there is too LITTLE dopamine in the rear portion of the brain to carry on rational activity, while there is too MUCH
dopamine in the frontal portion which is all about creativity and imagination.
But these ideas can only grow from one’s general attitude.
TWO.
Some of you are not experiencing the shut-down of energy supply during exertion as others are. There is liable to be a SPECTRUM at the body or cellular level
since we each are in unique situations:
as to what is too much exertion
and
how each exerting cell (muscle, or endothelial blood vessel cell, or Neuron in the executive brain)
will respond to overage….. we need to study and do it personally.
I would like to conclude by saying, that after 3+ years of research since:
1- suspecting covid virus
2. meeting a CFS/FIBRO friend with also 6 other vascular conditions
3. Finding out that Long-Covid seems undifferentiated from other named conditions,
That an HOLISTIC approach to what has challenged the tissues, and how your care provider and you can re-equip your body/brain to rise to these challenges,
is the only way ,
In a name-calling, summary, dismissive medical system of “you are or you are not”
To find the right path of solution, DESPITE the resistance that you may have received.
As for Amantadine and hallucinations? Well, Amantadine increases, I guess dopamine release in the brain. Why? Because the scientists developed it to enter the brain.
Yes, there is always a too little and too much titration with any drug because NO ONE could possibly guess what processes are active in your brain, nor why they are . Chris
Hypothalamus is rich in Ace 2 receptors…
Get outta town !
I may have read that too.
Thank you.
Now…
that helps long-covid research, right? But how does it help CFS?
I can suggest a fashion:
All the pandemics of 1900s had (because I needed to know, and then I researched, and can document)
a long-haul cohort. Spanish Flu, Polio, the usual suspects.
BUt:
their target was at first — not ACE2 receptors… at least, not that they were aware at the time,
but
Sialic acid receptors.
Interestingly, they can be bound by lectins. Much talk is occurring right now on the net about lectins in fruit/vegs
that can not be broken down by us, and which trigger inflammation.
So sialic acid receptor a-2,3 was the most easily accessible cell membrane receptor for Influenzas that were of Avian origin.
At some point mid-century-ish, viruses had successfully mutated to take advantage of both a-2,6 receptors.
The latter were more prevalent in swine, a major food source, but maybe more importantly, ANOTHER live source of virus in addition to bird sources of food, and their farmers, perhaps most importantly.
We began with a-2,3 friendly [pandemic flus, but then added flus friendly with both a-2,3 receptors and a-2,6 receptors, which increased the likelihood of human contraction.
Those types of earlier avian flus, plus the swine flu, dominated, but they actually still do.
It is just that ACE-2 receptor friendly Coronaviruses
have been MUCH MORE SUCCESSFUL or lucky,
in such a shorter timespan.
They may seem to outnumber sialic acid friendly flus,
but let us be real: the reach of covid has been MUCH MORE populous.
Finally, the point:
When we look at avian vs swine flus, comparing the location and number of sialic acid a-2, 3 receptors to a- 2,6 receptors in humans, we can see that avian ones prefer one part of the respiratory tract (and, or not, nose) for the a-2,3 receptor density, while swine flus prefer a different distribution area of their own favourite receptor– a-2,3.
What I can tell you, however, from those studies, plus covid studies on all 3 attackers, is that:
All 3 occur all through the vascular system and smooth muscle organs. BOTH the sialic and ACE-2 viruses gravitate to the intestine… in some cases more to the inside, and in another cases more to the outside wall (tight-junction cells)
I can provide a diagram from medical lit.
The nose epithelium is very RICH in ACE2 receptors too !!!
And that’s why dummies wearing covid masks, but leaving their noses exposed to both receiving and spreading covid..
are just that:
dummies.
fwiw: for low-dopamine (like) symptoms (distractable, can’t concentrate or get much done even when sitting at the computer, making you wonder if you have adult-onset adhd), I’ve had really good luck with a liquid mucuna puriens by Banyan Botanicals. It’s a nice low dose that you can titrate easily using the dropper. And for brain inflammation & brain fatigue, I’ve had great results from Apex Energetics’ “Resvero Active” lyposomal resveratrol. Kinda pricey, but (imho) at least it doesn’t taste bad like most of the other lyposomal resveratrol formulas out there.
It has definitely helped me. It’s not a cure all but it has definitely helped with my overall energy level and making it through the day easier with my toddler. The only side effect is a little jitteryness when I started at 100mg then I titrated up with 25mg at a time and the side effects went away. It’s definitely one of the only meds that has helped me. And it was one of Dr. Jay Goldstein’s top recommendations.
Thanks for sharing this info, Cort. My neurologist just prescribed amantadine to me for debilitating fatigue and PEM. Starting at 50mg and titrating up to 100mg. Will update again after I’ve tried it.
Should add that I have ME/SEID.
Did it help you?
Hi, sorry for not updating. I did not notice any improvement over the few months I was on it, so I did not refill/continue using it.
I have taken Amandatine since 1987. I was started on it by MD after testing discovered I did not make antibodies when given flu vaccine. Cautioned to never receive another vaccine I was instructed to start Amantadine at beginning of flu season until end, 100mg twice daily.
Born with CAteye Syndrome, a type of Coloboma caused by gene mutation I am allergic to anti colonergics . I was told to start it on Friday night when I could rest over the weekend when I would experience some double vision. And that by Monday I would be fine. And I was. .
And I have taken it every flu season since. I used to get as sick with the flu as I did with Covid until I started the Amantadine. It works great for influenza A but I still occasionally have a day or two of dealing with influenza B, but only a day or two.
Have never had another problem re anticolonergic effect of double vision.
I do find it helps with MECFS symptoms too so will start it up if I am experiencing a bad flare. And when I had Covid was told to be sure to continue it along with some other medicines. I have been told it doesn’t work for everyone. Only problem was finding doctors willing to prescribe it. When primary retired, new doctor said oh that’s an old medicine. We don’t use it any more. Switched doctors. Found NP in my rheumatologist office who prescribed it for me and followed her when she set up her own independent practice.
The doctor who initially prescribed it told me you’re sick, I just don’t know why. Consulted every researcher he knew, and he knew a lot because he helped found The Arthritis Foundation and was its first president. He also founded their medical journal and was its editor. He told me in 1987 I know what you have. It’s chronic fatigue syndrome. I’m not doing you any favors with this diagnosis. But they will figure it out. He underestimated the politics that would impact this illness. Although he often said it’s going to be terrible for patients in the future.
Also read: Comparison of the effects of acetyl L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: results of a pilot, randomised, double-blind, crossover trial https://pubmed.ncbi.nlm.nih.gov/14759641/ It’s a paid paper, but sci-hub has it
Alcar (acetyl L-carnitine) worked as well as amantadine and was better tolerated.
Amantadine did not work for me, but this could be caused by an underlying immune dysfunction, stuck in that mode associated with inflammation during an acute infection. This causes a loss of blood perfusion control, so that oxygenated blood priority is lost, leading to anaerobic metabolism dominating. This causes a rapid onset of fatigue, raised levels of uric acid as a waste product and, to my horror, gout attacks! Clearly the target for treatment is blocking a chronically elevated cytokine profile stuck in a pro-inflammatory mode for which, sadly, Amantadine is not applicable.