

Geoff’s Narrations
The GIST and Treatment Takeaways
Audio PlayerThe Blog
Audio Player
THE GIST
- What’s going on in orthostatic intolerance (symptoms triggered by standing or sitting up) has been well described – from the outside. What’s happening at the molecular level to cause it is another story – no one really has a clue. That’s what the Simmaron Foundation research team took a look at.
- Their recent study revolves around a compound called tetrahydrobiopterin (BH4) that plays a crucial role in several major processes and at high levels has been associated with mitochondrial dysfunction, cognitive problems, immune dysregulation, inflammatory and autoimmune diseases, and chronic pain.
- The researchers dug into a metabolic pathway called the pentose-phosphate pathway to understand what was happening. Multiple tests suggested that low oxygen levels in the cells of ME/CFS patients were triggering one phase of the pathway to become overactivated and the other phase of the pathway to become underactivated.
- It’s always about balance. The downregulated oxidative phase of the pathway likely resulted in increased oxidative stress (check!), impaired lipid synthesis (check!), tissue repair (after exercise???), and pathogen killing (check!).
- Meanwhile, high levels of a breakdown product of BH4 called BH2 were found in ME/CFS. increased BH2 levels – in the presence of low oxygen levels – do a Jekyll/Hyde-like switch on nitric oxide enzyme. Instead of opening blood vessels and reducing inflammation, the enzyme narrows them down, producing inflammation, clotting, oxidative stress, and mitochondrial dysfunction.
- One of the nice things about this study – done at the cellular level – is how well its findings fit with what we know about ME/CFS. High levels of oxidative stress, low cellular oxygen levels, low glutathione levels, inflammation, neuroinflammation, and problems with blood vessel flows and lipids – all these seem to be part and parcel of ME/CFS.
- The authors propose that high-resolution metabolomics study using a large sample size that was informed by machine learning-based network analyses will confirm or deny the role that PPP-induced BH4 plays in ME/CFS. They have the samples but not yet the funding.
- Check out the Treatment Takeaways section for information on those.
What’s happening on the inside – at the molecular level – is more of a mystery. Yes, we know about norepinephrine levels, but what is causing them? Studies suggest that a messed-up renin-angiotensin-aldosterone (RAA) pathway contributes to the low blood volume found in ME/CFS and POTS but no one, to my understanding, has figured out, molecularly, how that has happened.

A new metabolic problem! Yah! 🙂
In”Dysregulation of tetrahydrobiopterin metabolism in myalgic encephalomyelitis/chronic fatigue syndrome by pentose phosphate pathway“, the Simmaron Research Foundation’s small but creative research team led by Avik Roy and Gunnar Gottschalk is trying to get at the molecular roots at them – and in ME/CFS to boot.
The Study
It revolves around a compound called tetrahydrobiopterin (BH4) that plays a crucial role in several major processes. At the right levels, BH4 allows for the production of the feel-good brain chemicals dopamine and serotonin, assists with mitochondrial functioning, and helps the blood vessels to vasodilate or open.
High levels of BH4 have been associated with all sorts of negative results including mitochondrial dysfunction, cognitive problems, immune dysregulation, inflammatory and autoimmune diseases, and chronic pain.
When we last heard from the Simmaron research team, they’d found high BH4 levels in ME/CFS patients with orthostatic intolerance – an outcome that had never been found before.
Another Balky Metabolic Pathway?

Two phases of the pentose phosphate pathway exist. Low oxygen levels appear to be dysregulating them in ME/CFS. (Image by GdenBesten, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons).
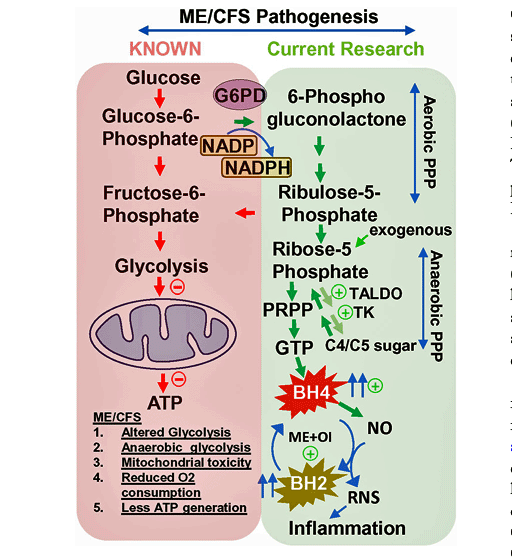
Enter the pentose phosphate pathway (PPP). The PPP is a metabolic pathway that runs parallel to glycolysis and is activated during high levels of oxidative stress – which we know is present in ME/CFS. The pathway produces something called NADPH to regenerate the key antioxidant in our body – glutathione – when glutathione levels dip low. Several studies have made it clear that glutathione levels in the brain are low in ME/CFS.
The pathway can also kick in when glycolysis is not functioning. The status of glycolytic functioning in ME/CFS is unclear, as some studies suggest it’s functioning just fine while others suggest it is not.
The PPP contains two phases: an oxidative (antioxidant enhancing) phase, and a non-oxidative phase that synthesizes sugars and produces something called ribose-5-phosphate – which plays a critical role in the production of BH4.
Finding high BH4 levels in ME/CFS, the Simmaron researchers dug into the pentose phosphate pathway to see what was going on. Multiple tests (gene expression study, real-time PCR, and quantitative ELISA analyses) indicated that the non-oxidative side of the PPP pathway – the side that produces BH4 – had been highly upregulated in ME/CFS.
Low Cellular Oxygen Levels Tagged
And what an interesting upregulation it was. The increased expression of genes involved in lactate production and the non-oxidative side of the pathway suggested that low oxygen levels in the cells of ME/CFS patients were triggering the non-oxidative side of the PPP to gear up.
As that was happening, the downregulated oxidative side of the PPP likely resulted in increased oxidative stress (check!), impaired lipid synthesis (check!), tissue repair (after exercise???), and pathogen killing (check!).
The possibility that low oxygen levels, or hypoxia, figure in ME/CFS has been around for quite a while. They tested the hypothesis that low oxygen levels were causing the pentose phosphate pathway to produce increased BH4 levels by creating a hypoxic state and exposing cells to it. The results suggested it was.
Turning a Good Enzyme Bad

Inducible nitric oxide (iNOS) – it’s pretty, but it induces inflammation and may be upregulated in ME/CFS.
In the presence of oxidative stress – which we know is plentiful in ME/CFS – BH4 does something particularly nasty: it turns a “good” enzyme – eNOS – into a “bad” enzyme (iNOS). In its “good” form, eNOS helps to open the blood vessels and keep them healthy.
In its “bad” form, iNOS is associated with the production of reactive nitrogen species (RNS) such as peroxynitrite, activation of the microglial cells in the central nervous system (neuroinflammation), excitotoxicity in the central nervous system, and mitochondrial dysfunction. (Ouch!)
Neuroinflammation Too?
Indeed, when the Simmaron team investigated the impact of BH4 upregulation on the microglial cells and inflammation, they found that under hypoxic conditions, BH4 triggered the production of nitrite – a reactive nitrogen species (a free radical) but only in the ME/CFS patients’ cells. That suggested the elevated BH4 levels in ME/CFS patients’ cells may be triggering neuroinflammation.
Bad Breakdown Product
Further analyses indicated that two byproducts of BH4 breakdown – BH2 and DHPR – were strongly upregulated in the ME/CFS patients as well – and here we come back to the possible molecular origins of orthostatic intolerance.
It turns out that high BH2 levels can produce reduced levels of eNOS – the form of the enzyme that opens the blood vessels. High BH2 levels could also increase platelet activation, cause damage to the endothelial cells lining the blood vessels, and increase the risk of atherosclerosis and blood clotting.
Nasty Oxidative Stress Mix
In fact, it’s worse than that. In the presence of high BH2 levels, instead of producing endothelial nitric oxide, eNOS produces a free radical called superoxide. Instead of opening the blood vessels as it normally does, eNOS does a Jekyll/Hyde about-face and actually ends up clamping down on them.
When superoxide pairs with nitric oxide, it produces peroxynitrite – a dangerous free radical that loves to poke holes in cellular membranes, cause mitochondrial dysfunction, damage our DNA, etc. Peroxynitrite is difficult to measure in the blood, but ample evidence of oxidative stress has been found in ME/CFS. Martin Pall’s 2001 ME/CFS hypothesis proposed that chronically high levels of peroxynitrite were driving the disease, and Bindu Paul and Marian Lemle more recently suggested that oxidative stress plays a key role in it and long COVID.
The high BH2 levels the Simmaron group found in ME/CFS, then, are potentially a big deal.
Treatment Takeaways
The authors did not mention treatment possibilities but AIChatGpt had some ideas about treating some of the potential problems this study found.
Improve eNOS activity
- Eat more dietary nitrates found in beetroot and green leafy vegetables
- Supplement with omega-3 fatty acids, L-Arginine (eNOS substrate – but rapidly metabolized), L-Citrulline (precursor to L-arginine – possibly more effective that L-arginine), antioxidants (Vit. C, E and NAC), nicotinamide riboside, CoQ10, tetrahydrobiopterin (BH₄) (questionable given high BH4 findings?)
- Try statins, metformin, phosphodiesterase-5 (PDE5) Inhibitors (e.g., sildenafil)
Reduce iNOS activity
- Try Resveratrol, quercetin, and curcumin.
- Anti-inflammatories (NSAIDs) and TNF-a inhibitors (etanercept), (anti-TNF therapy) or anakinra (IL-1 receptor antagonist)
- Experimental drugs – L-NIL (N6-(1-Iminoethyl)-L-lysine) and aminoguanidine.
Reduce BH2 levels
- Antioxidants to combat oxidative stress can prevent the oxidation of BH4 to BH2 maintaining the proper BH4/BH2 balance.
- Antiplatelet therapy like aspirin can counteract the pro-clotting environment.
- Supplement with folate to enhance BH4 regeneration from BH2
- Supplement with N-acetylcysteine (NAC) to increase glutathione levels
Potential Therapeutic Interventions
The authors did not mention treatment possibilities, but AI ChatGpt had some ideas about treating the variety of potential problems this study found.
Improve eNOS activity
- Eat more dietary nitrates found in beetroot and green leafy vegetables
- Supplement with omega-3 fatty acids, L-Arginine (eNOS substrate – but rapidly metabolized), L-Citrulline (precursor to L-arginine – possibly more effective that L-arginine), antioxidants (Vit. C, E and NAC), nicotinamide riboside, CoQ10, tetrahydrobiopterin (BH₄) (questionable given high BH4 findings?)
- Try statins, metformin, phosphodiesterase-5 (PDE5) Inhibitors (e.g., sildenafil).
Reduce iNOS activity
- Try Resveratrol, quercetin, and curcumin
- Anti-inflammatories (NSAIDs) and TNF-a inhibitors (etanercept), (anti-TNF therapy) or anakinra (IL-1 receptor antagonist)
- Experimental drugs – L-NIL (N6-(1-Iminoethyl)-L-lysine) and aminoguanidine.
Reduce BH2 levels
- Antioxidants to combat oxidative stress can prevent the oxidation of BH4 to BH2, maintaining the proper BH4/BH2 balance
- Antiplatelet therapy like aspirin can counteract the pro-clotting environment
- Supplement with folate to enhance BH4 regeneration from BH2
- Supplement with N-acetylcysteine (NAC) to increase glutathione levels.
From the study.
Conclusion
One of the nice things about this study – done at the cellular level – is how well its findings fit with what we know about ME/CFS. High levels of oxidative stress, low cellular oxygen levels, low glutathione levels, inflammation, neuroinflammation, and problems with blood vessel flows and lipids – these all seem to be part and parcel of ME/CFS.
A dysregulated pentose phosphate pathway, and the BH4, and BH2 connection provide another explanation for them. Will it turn out? The studies are small and only time will tell if they’re on the right track.
The authors have proposed a way to find out, though. A high-resolution metabolomics study using a large sample size that was informed by machine learning-based network analyses they believe would confirm or deny the role that PPP-induced BH4 plays in ME/CFS.
The small Simmaron research team with their ME/CFS mouse model work, autophagy, rapamycin clinical trial, and now the BH4, pentose pathway, and BH2 findings has been uncommonly productive. They appear to have the samples they need to do the metabolomic project – all they need at this point is the funding. May they get it!
- Coming up – an update on the Simmaron Research Foundation’s ME/CFS Rapamycin clinical trial
Donation Drive Update!

We try and figure it out. If that’s what you want to see, please support us.
Thanks to the over 160 people who have contributed over $14,000 during our end-of-the year donation drive.
This study was a toughie! It’s biochemistry-oriented and took a lot of work to understand – particularly for someone who barely made it through organic chemistry in college (lol) – and for readers to get through. It’s at the molecular level, though, that understanding this disease will probably bring us the greatest breakthroughs. I’m committed to understanding (as best I can) this disease and communicating it.
If that’s what you want to see, please support us!







Thank you for all the great work that you do for those of us with ME/CFS and it’s associated comorbitites. I have donated to you in the past, but unfortunately I am waiting on appeal for my Social security disability claim. If/when I am approved, I will make sure to donate each year to Health Rising. What a valuable service you provide for us.
Thanks! And good luck with disability. I wonder whether you did the 2-day exercise test from Workwell or another exercise physiologist?
The Real Person Badge!
Hi Cort,
I used to believe that hypercortisolism was enough to deprive the body of Aldosterone since the Adrenal glands make both of those compounds. Renin, I believe, is made by the kidneys and affects the Adrenals. Since salt retention is what is managed by Aldosterone’s effect on the kidneys, it follows that failure of Aldosterone would fail to retain salt… and we must lose water and salt in the right proportion to each other. That dehydrates and the dehydration results in low blood volume, which results in low blood pressure, but also in low CELL VOLUME. With low cell volume, the nooks and crannies for receptors may become distorted. I *thought* that was the role for Metformin, that it would restore form to the cell …
The only problem was that I read that Aldosterone levels in CFS blood were observed to be normal. At least in the CFS research that payed any attention to Ald. However, if not all CFS patients have POTS, then it might be directly proportional to not all of them having an Aldosterone deficiency. And I’ll go out on a limb and say that then it would seem most likely the most grave examples of CFS would include POTS, while less severe examples may not–then it would be a subset thing where Aldosterone doesn’t show as deficient until the CFS gets *that* grave.
But please keep in mind that even if Aldosterone production were normal, the types of cytokine storms that likely precipitated CFS and long Covid do damage to smooth muscle, especially vessels and organs, and *especially especially to the kidneys. Kidney dysfunction is one of the most likely organ dysfunctions to occur after such illness, even if others do not appear. Even kidney dysfunction could underprocess the Ald. even if the Ald. were sufficient.
In any case, the next stop in blood pressure is nitric oxide and whether or not enough is produced. Too much can also be the result of certain disease states. The smooth muscle of the blood vessels is neither relaxed or constricted in its resting state. It is somewhat constricted and can become even more constricted, but nitric oxide’s job is to relax the constricted vein in response to the systolic pump of the heart. In diastolic, the ambient medium constriction should resume.
To apply that to POTS etc… when one gets up the brain ought to have relaxation of its vessels while the blood is shunted there from vessels that do not need it as much at that moment: like the intestine’s vessels. The blood pressure should be higher down below but lower in the brain. Since it does not happen, dizziness results. A nitric oxide imbalance seems like enough to explain that. But I wonder whether it is a renin dysfunction or an Aldosterone dysfunction that precipitates the problem. Or is it all one big clusterf…oops, typo.
Well…
1. if blood volume is too low from Aldosterone production deficiency, or from kidney inefficiency in responding to it with salt retention
then
2. blood volume will be too low
and
3. blood pressure will be too low even if vessel constriction is business as usual.
…But I do not think that vessel constriction *would be business as usual. Because in Covid we know that ACE2 receptors are blocked by the virus and unable to enzymatically break down the angiotensin, with a cascading message to the nAChR eceptors which should then tell the mitochondria to make more antioxidant (NAD..something). This may be the brain portion of the problem. Not enough dilation.
And please remember that in addition there has been a massive replacement (remodelling) of endothelial SMCs (smooth muscle cells) because *there is no time* during cytokine storm damage to them to replace like with like. Instead, more rigid (collagenic) cells are the ones to remodel the more flexible (elastinic ) cells. So even if the nitric oxide response were normal, the number of flexible endothelial cells remaining to do it are diminished.
You may be wondering: what if I didn’t get long covid, but rather had a pandemic Flu? Well, flus have a cytokine storm because of the blockage of Sialic Acid receptors by the flu viruses. And they are resident in the same smooth muscle locations. This I had to deduce by myself, but I can document it.
But there is one grey area in all of this:
The textbooks say that in a cytokine storm the endothelial flexible cells are not replaced like with like.
However,
we never cease to have EGF– endothelial growth factor for other situations.
So maybe the message is only that the EGF is not used for cytokine storm management, but not that it can never occur, nor never repair in the future.
This opens a new treatment avenue: stimulating EGF.
One of the best — or only — vessel repair compounds is the citrus bioflavonoid. There are 5 or 6 types/names but only 5 fruits in which they seem to occur. Tangerines and tangeritin seem to be the most potent.
I can document an NIH meta-analysis of citrus bioflavonoids both protecting, during, and healing capillaries, after, a massive cytokine storm. But also, Omega-3 helps to also protect, during.
Because you see: we are focussed on ROS. Well, the effect of ROS is upon the endothelial cells. But if we have antioxidants there to bind with them, they can be neutralized and much less damage can occur.
Okay, I’m done. But don’t tell me I never gave ya nuthin:
Polyphenols…antioxidants….bioflavonoids….
are all
antioxidants.
I have documentation that polyphenols in dark fruits like
blueberries and pomegranate can
block:
a) covid injection by ACE2 receptor
b) viral replication by ribosomes
c) viral release by other transporters.
2 Post-Thoughts:
P.T.s
by Chris D.
1. I often describe endothelial damage incorrectly since it is confusing. The inner, or endothelial cell processes the renin/angiotensin. Then it ENCOURAGES the creation of nitric oxide in the smooth muscle cells that are flexible. My apology. This means that massive remodelling that loses these endothelial cells means not enough of them telling their nearby SMCs to produce nitric oxide to relax. The normal dilation reaction is said to be a response to vascular shear pressure, caused by the systolic pumping of increased blood volume.
I guess it must be a baroreceptor
2.
Since Cortisol is the stress-management hormone, it is natural for it to become too high during a crisis like flu or covid…or sepsis…or a serious serious bacterial infection… and then to fluxuate between higher and too high for the rest of CFS, until you alleviate CFS.
But Adaptogens such as Ashwagandha (used for 3000 years in traditional Indian (India) medicine to regulate cortisol levels), Ginseng (used in China and Japan for 2000 years)
https://pmc.ncbi.nlm.nih.gov/articles/PMC7322748/ — ginseng
“Panax ginseng occupies a prominent status in herbal medicine for its various therapeutic effects against inflammation, allergy, diabetes, cardiovascular diseases, and even cancer, with positive, beneficial, and restorative effects. The active components found in most P. ginseng varieties are known to include ginsenosides, polysaccharides, peptides, alkaloids, polyacetylene, and phenolic compounds, which are considered to be the main pharmacologically active constituents in ginseng. P. ginseng is an adaptogen. That is, it supports living organisms to maintain optimal homeostasis by exerting effects that counteract physiological changes caused by physical, chemical, or biological stressors. ”
….adaptogens… can regulate the cortisol response. It may be good to have a sharp response, which dulls the immune system also, not what I might expect.. but yes, if we are already reacting sharply to a disease, we don’t want the system to become even more militaristic and kill us too.
“Mineralocorticoid receptors
The MR is stimulated by both aldosterone and cortisol, but a mechanism protects the body from excess aldosterone receptor stimulation by glucocorticoids (such as cortisol), which happen to be present at much higher concentrations than mineralocorticoids in the healthy individual.”
” The aldosterone mineralocorticoid receptor (MR) complex binds on the DNA to specific hormone response element, which leads to gene specific transcription. Some of the transcribed genes are crucial for transepithelial sodium transport, including the three subunits of the epithelial sodium channel (ENaC), the Na+/K+ pumps and their regulatory proteins serum and glucocorticoid-induced kinase, and channel-inducing factor, respectively.”
“The MR is stimulated by both aldosterone and cortisol, but a mechanism protects the body from excess aldosterone receptor stimulation by glucocorticoids (such as cortisol), which happen to be present at much higher concentrations than mineralocorticoids in the healthy individual. The mechanism consists of an enzyme called 11 β-hydroxysteroid dehydrogenase (11β-HSD). This enzyme co-localizes with intracellular adrenal steroid receptors and converts cortisol into cortisone, a relatively inactive metabolite with little affinity for the MR. Liquorice, which contains glycyrrhetinic acid, can inhibit 11β-HSD and lead to a mineralocorticoid excess syndrome.”
Good to know… while sealing your gut with licorice.
After ACE2 triggers cytokines, the immune system may mistakenly attack the lining of blood vessels (endothelial cells), causing damage to collagen and making it difficult for the vessels to repair themselves. Over time, this could result in conditions like APS and lupus. High levels of a clotting protein (vWF) and an increased tendency to form clots further worsen the damage. Ongoing inflammation and scarring (fibrosis) make the vessels stiff and less functional. To restore healthy blood vessels, only cortisone treatments are effective.
That is conjecture. If your source is what you included below, it does not argue those things.
Lupus and APS are way down the road from long covid. What you said us “may” and “could” but the immune system is not making a mistake when covid virus information is found in some tissues 8 or more months after infection.
If you read what I said most recently you will see that there *are other treatments for inflammation, and saying “only cortisone is effective” is a death sentence because you can’t endure ling-term cortisone.
Your arguments do not follow.
“Lupus and APS are often thought to develop much later or as separate conditions from long COVID.”
Unfortunately, that hasn’t been the case for me. After contracting COVID-19, an ELISA test in 2021 confirmed that I have Lupus and APS. I also have a genetic predisposition for von Willebrand disease types II and V.
I think it’s worth revisiting the research on medications and supplements that people with Lupus should avoid, as this could be valuable for others in similar situations.
On top of this, I’ve been taking hydrocortisone for the past 10 years due to adrenal insufficiency.
Your piece from 2 1/2 years ago makes a nice companion piece to this one: https://www.healthrising.org/blog/2022/03/27/novel-approach-mitochondria-chronic-fatigue-syndrome/
Sorry, I forgot to include: “Endothelial inflammation in COVID-19”
https://www.science.org/doi/10.1126/science.add2962
Were unusual levels of BH4 found in the new pre-print study, “Replicated blood-based biomarkers for ME/CFS not explained by inactivity”?
I must be a bit dense, but I can’t find the list of biomarkers in the pre-print document to search!
As I mentioned the importance of “Catecholamines (neurotransmitters and hormones)” in the Phoenix form in February 2024, please keep in mind that there could be an error in the gene GS224 tetrahydrobiopterin.
Hi Cort:
May I have your permission to post this on BlueSky?
Thanks for your answer. SWA
Of course! Please do so! 🙂
The Real Person Badge!
Hi Sieglinde,
I am now not sure who it was that wanted some treatment options that they could try now, you or another person. But I will pretend it was you and answer here.
First let me say that I advocate healing foods and nourishing foods as opposed to medications, but I do include supplements. But also that anything I might mention is something to not rush out and try, but rather to discuss with one’s doctor. And I strongly suggest you get a doctor of functional medicine if you currently see only a GP.
Since I started researching from texts, studies and papers for the causes and treatments for CFS, I have found a handful of major facts to consider: Leaky gut is most likely the greatest source of inflammation in any syndrome. Compromised Blood-Brain-Barrier (BBB) happens for the same reasons: unwelcome substances, the trigger of zonulin. Dr. Tom O’Bryan asked, in role play, “So if the same substances that causes leaky gut are also in my BBB , does that mean I might also have Leaky Brain?” To which his answer as an actual Doctor of functional Medicine was “YES !!” So that’s Leaky Gut and Leaky Brain to attend to. Then consider that you will have excess inflammation and that you do have vascular endothelial cell damage, and usually would have had a period of hypercortisolism which depleted your Adrenal glands so much that now you even struggle to produce the cortisol, not to mention that adrenaline and Aldosterone production are being sacrificed.
My suggestions are then: to seal and heal the Leaky Gut as well as the Leaky Brain because the brain inflammation you know about is caused by unexpected guests. You need to counter inflammation with foods and herbs but also bring down your cortisol fluxuations near the normal homestatic range. The way to do that is with Adaptogen herbs that have been used both in China and in India for centuries.
If you work on relieving your Adrenals of such an inappropriate demand for cortisol, then you can hope to regain proper Aldosterone production, and Adrenaline production, leading to proper blood volume and proper cell volume. This will help to correct your Renin/Angiotensin/Aldosterone ratio and that will aid the restoration of vascular pressure function to some degree.
Lastly, you know that the mitochondria in muscle cells are of the wrong phenotype, intended for the innate immune response and production of more cytokines. You need to encourage mitophagy so that the incorrect mitochondria will be replaced.
In my following message: what foods and supplements will do those things.
Hi Christopher D,
Thank you for your thoughtful and detailed post. While I appreciate your advice, I’d like to share that I’m very well-informed about supplements—their benefits, but also their limitations, particularly when it comes to rare, or rarely diagnosed illnesses. Since 1979, I have been following medical advice and consulting with specialists in Germany, USA and UK across all related fields to better understand the role of supplements in health management.
If you’re interested, I’ve shared a post that outlines my journey and has been confirmed by specialists. Feel free to take a look: “July 18th – It is official” https://forums.phoenixrising.me/threads/a-new-way-of-thinking-and-searching.88045/#post-2406399
Although the connection between post-polio syndrome (PPS) and pituitary dysfunction is indirect, it’s important to note that chronic stress, inflammation, and autonomic dysregulation associated with PPS can disrupt the hypothalamic-pituitary-adrenal (HPA) axis. This disruption can lead to low cortisol levels, amplifying hallmark symptoms of PPS such as fatigue and poor stress tolerance.
I’m sure you’re familiar with demyelination, which refers to the degradation of the myelin sheath—a critical structure made of cholesterol and enriched in glycolipids. This process can occur due to viruses, bacterial infections, chronic inflammation, or even certain medications. The consequences include denervated muscles, often detected through electromyography (EMG) which I had twice. While this is well-documented in conditions like amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and post-polio syndrome, it is unfortunately often overlooked or avoided in testing for ME/CFS and, most notably, in post-polio patients.
It’s worth mentioning that no vitamin can directly stop demyelination. For instance, rebuilding the myelin sheath depends on specific dietary sources such as beef, dairy products, or fatty fish, which are rich in essential lipids. Unfortunately, due to the presence of high phytanic acids (very long-chain fatty acids, VLCFA), I am unable to consume these foods.
The challenges of post-polio syndrome extend far beyond preliminary diagnoses and immediate symptoms. One of the critical gaps in medical science today is addressing the recurrence and progression of long-term illnesses like PPS, which demand deeper investigation and long-term strategies for management.
Greetings, SWA
Thank you SWA.
I couldnt possibly argue with that. I assume it was someone else wishing there were treatment options to begin now, and after a separate blog.
No, there is no vitamin to remyelinate. But L-Theanine, found in Green Tea, and also extracted from it stimulates the Oligodendrocytes that manufacture the myelin. There is almost nothing else known to do that when the myelin maintenance system is hurting.
And I am indeed aware that in addition to the myelin at issue in the brain (white matter) central in MS, there are also myelinated nerves along with naked ones in the body. For examples, the sympathetic nerves lining the outside if blood vessels or in other locations are of 3 types: 2 myelinated and 1 unmyelinated types of peripheral nociceptive Afferent nerves. Meanwhile tgere are the myelinated Efferent nerves which send motor instructions to the peripheral body. Indeed, the vagus nerve is myelinated, and demyelination could cause huge problems to its function. All the more reason to try L-theanine which comes naturally in Green Tea.
I will certainly read your story since everthing helps me to learn more about these same parts of the brain and body.
For your interest only: one of my best friends, who was older than me, whom I have not mentioned here before, had polio as a young person and Fibromyalgia for the rest if her life.
My personal view of CFS and of all of the other syndromes we know by by name here on H-R is that they are all long-hauler conditions. There is data even from after 1918 Spanish Flu of long-haul patients. Of all of the pandemics. I read the data, which is why I hold this view. I also believe in Long-Haul Polio, long-haul Hong Kong Flu, long-haul Post-Gulf-War Illness etc
For this reason we are learning from other chronic conditions about our own conditions of concern.
Yes, post-Polio must include HPA Axis dyshomeostasis. but also I concluded in about November 2021 that CFS includes this, before finding H-R, . But Cort has covered this same dyshomeostasis here.
I find commonalities as useful as differences between syndromes. We are all human bodies. All the same functions can suffer, but all the same functions can be healed. I think it is best no to silo one’s health simply because medicine has siloed one’s diagnosis.
Please remember that even though I am writing to you and you are very knowledgeable, if an important topic arises I might give detail that you do not require since others are also reading.
Respectfully,
CD
Dear Christopher D.,
I want to clarify that I am not trying to argue but rather to seek out and consider the facts as they are available. It is well-known that even published results can sometimes be retracted due to bias or misinterpretation. Nevertheless, properly conducted tests remain one of the most reliable tools we currently have. That said, it is ultimately the patient who knows best whether a medication or supplement is effective or causing adverse reactions.
There have been numerous instances where treatments were recommended without adequate testing or understanding. For example, how many cases have been documented where doctors recommended Vitamin K without first checking if the patient had Von Willebrand Disease (VWD)? This highlights a critical gap in clinical evaluation.
Another important reminder is the historical use of graded exercise therapy for patients with ME/CFS, which often resulted in worsening symptoms and further debilitation. This neglect stems from a lack of clinical understanding of key physiological factors—such as disruptions in the neurotransmitter acetylcholine, which has been recognized for years as playing a diverse role in transmitting signals between nerve cells and muscles, especially in individuals with post-polio syndrome (PPS). My research over the past two years on PPS and its similarities to other muscular illnesses and conditions, such as SMA and ME/CFS, is expected to be completed by the end of January 2025.
As I mentioned, it is crucial to use appropriate testing before starting any treatment. Even Dr. Jarred Younger emphasizes the importance of testing, as he explains in his talk: https://www.youtube.com/watch?v=Z9uwzkl9uMU.
SWA,
while no one vitamin can directly prevent myelin degradation,
one mineral and electrolyte is crucial to myelin’s formation and that is magnesium.
https://pmc.ncbi.nlm.nih.gov/articles/PMC8385315/
Most north americans are deficient in magnesium at one time or another, if not constantly, because of farming practices that have depleted soils.
50% or more if the world us also chronically deficient in D3. D3 is not a vitamin either. It has the structure of a steroid and has been purposefully left misnamed as a vitamin. D3 is a steroid hormone. It helps to limit inflammation and rebuild the gut lining, it saved a large proportion of high-risk covid patients from hospitalization in a Spanish study using high doses of D’s active form , and in the same study it saved those patients who still had to go into ICU (far fewer) from death.
I mention D3 because, like magnesium, its deficiency in our populations is glaring.
I guess sufficient sunlight is not a vitamin either…
Christopher D,
You are right “I guess sufficient sunlight is not a vitamin either…”
I’m deficient all my life. This is the reason why I’m taking Vit D3.
You may like to look at Mineral Imbalance Effects
https://swaresearch.blogspot.com/2024/06/mineral-imbalance-effects.html
Hi again SWA 🙂 I read both your Phoenix Rising posts and the Frontiersin PPS article/analysis. I understood the latter.
I am not saying that I did not understand your own statements though.
I will try to be relatively brief.
I am interested to know what makes a person susceptible genetically to ALS since your relative contracted it plus the statement in the article that it may be related to PPS in its genetic predisposition.
One thing I would like to say about genes is this: Epigenetics. You probably know the subject, but to be sure it is covered: genes in one person or family definitely can more susceptible to harmful changes in their environments, the one factor I would focus on being toxins. Cytokines are toxins. Epigenetics states that genetic changes brough by toxins can also be reversed by the removal of such toxins plus other types of healing.
What I gathered from your descriptions of PPS and also from the article was this: Polio seems to attack and damage nerves, but also the CNS. And that would include motor centers in the brain. Polio can cross the BBB. So can Omega-3 oil, by the way. Is it, by itself high-phytanic?
What we also know about EBV and Herpes viruses is that they reside in nerve cells. An example is HSV-2 which, when the nerves near the area of skin eruption become iritated, the irritation is passed on to the live skin cells.
I can completely see how nerve damage including demyelination would lead to muscle atrophy. The challenge therefore would seem to be to treat the nerves and the CNS. Muscles atrophy when they do not receive enough stimulus or “challenge” from nerves. I learned from the Scapula condition that you mentioned.
Something that really stood out was that you said you have always been cold since Polio infection. Sepsis is (obviously) a toxic amount of cytokine, chemokine and possibly bacterial waste products also.
But we need to compare Fever with Sepsis. In the innate immune reaction, if fever occurs the person is hyperthermic. This not only derails a lot of cell functions which are optimized for 97 o F. It deprives a microbe of energy by favouring anaerobic glycolyis (about 1/12th of the normal ATP creation by muscles) and also increases cytokine levels because the mitochondrion fails, by nature of the chemistry, to create as much antioxidant as during aerobic glycolysis.
In CFS it seems to be a unanimous agreement at this point that the mitochondria are stuck in a more innate immune reaction. I mentioned to Cort before that the purpose of Itaconate is to — divert ATP to MORE cytokine production. So Rob Phair’s Itaconate shunt hypothesis of why fatty acid metabolism is likely failing in ADDITION to the yield from glucose being 1/12th of normal,makes a logical conclusion that only proteins could then be catabolized for ATP, but so few ATP would be yielded that (my conclusion is: ) the cell would go into a glutamate/calcium loop that finally ends in apoptosis due to lack of ATP for removal of compounds and due to the fact that high calcium levels in the wrong part of a cell — in the cytosol, trigger apotosis.
So… if a person were one of the only 5% of humans who react so strongly to Polio that they are left with PPS… or if a person were one of the only 1-2% (of reported) Americans who react to strongly to (Flu, Sepsis, Bacterial overgrowth) that they develop CFS (probably with a life-stressor thrown in to exacerbate the reaction in later life….) OR one of the 1-2% worldwide of people who end up with Long Covid…
I feel that they would be in the same boat regardless. It is really a matter of what a specific pathogen attacks most therefore where the most damage is done by unregulated immune reaction.
I would like to stress again that L-theanine stimulates Oligodendrocytes in the brain (and Schwann cells in the body) to produce new myelin. Dr. Terry Wahls is a functional medicine doctor who turned her MS around using a paleo diet that she modified plus fine-tuning supplementation. The diet is about a LOT of exclusion.
I know you already do a lot of that.
Finally, the person who remains as the 5% or the 1-2% can not stay in a fever state. In hospital many patients who contract diseases WHILE in ICU develop SEPSIS consequently.
If SEPSIS does not kill one, but if it does persist,
it results in HYPOTHERMIA. This is why you being cold
all the time, but also many CFS patients too,
is very significant to me.
If the nerves and CNS are not just damaged, but rather
continually inflamed… cytokines are a given. You would have to check which cytokines are still produced hypothermic sepsis vs fever, but I kind of recall it being about 5 types of cytokine produced in fever vs 2 types in hypothermic sepsis.
I think the stumbling block for people thinking of hypothermia in CFS and Sepsis is: but I didn’t get dropped in the ocean …. I wasnt frozen in winter…..
Hypothermia in Sepis is *by design* to keep up the fight against the perceived threat, but at the cost of energy production.
If I am not mistaken (which I may be) the two main cytokines produced during hypothermic Sepsis are:
NO and Bradykinin. That’s Nitric Oxided and Bradykinin.
Here is what is weird: those are both intended vasodilators in the RAAS blood pressure system, but they are also meant to be -ased soon after dilation. They are not meant to hang around in great volume. Both are cytokines but both are needed vasodilators.
Bradykinin is also a facilitator of PAIN. Bradykinin plus substance P. Unfortunately, Bradykinin
ALSO:
discourages the Kidneys from retaining salt (despite Aldosterone)
AND
makes blood vessels more permeable and more likely to internally bleed.
I think this last point might be very important in your case.
Hi again,
Thank you for taking the time to review my writing. As you may have noticed, I’m already familiar with many of the suggestions you’ve shared. For example, I’m aware that cytokines can disrupt the metabolic system by continuously attacking T and B cells and launching persistent assaults on various regions of the body.
However, what is less commonly discussed is the root cause of muscle weakness, which no current supplements seem to effectively address or alleviate. The key factor here is the destruction of Anterior Horn Cells—the same damage associated with conditions like polio, spinal muscular atrophy (SMA), amyotrophic lateral sclerosis (ALS), and other similar muscle-weakness disorders.
Unfortunately, research into ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) has rarely explored Anterior Horn Cell Damage or conducted essential diagnostic tests such as electromyography (EMG) or nerve conduction studies (NCS).
If you’re interested in exploring this topic further, I recommend these two presentation: https://www.youtube.com/watch?v=aeYxbBuRIpA&t=969s
https://www.youtube.com/watch?v=MJECqcYJhmo
Part II: actual names. Not only may the gut be (likely) leaky, but also in such a state of inflammation it is likely to be underproducing Butyrate (gut fuel) and Acetate (for acetylcholine and for Acetyl CoA — coenzyme A) which is so important in the mitochondrial TCA/Krebs cycle of producing ATP.
Warning: further down I will mention detoxification since cytokines are toxins and so are many of the things that unwanted bacteria excrete in the gut. But the key to remember is that you shouldn’t just jump in both feet with detox because your detox pathways in your body need to be clear and ready. That is your lympathic drainage pathways. You really want to be seeing a doctor of functional medicine for your timing in this area. And detox too quickly feels: sick.
These are facts but not advice !
Some of the largest causes of Leaky Gut are wheat gluten, barley gluten, lactose, and milk casein. Cows were evolved to eat grass… we were not. Cow milk is no longer seen as appropriate for us. GMO wheat has 2x the gluten content since bread and pasta makers like stretchy dough. That makes gluten sensitivity twice as bad or worse. You can safely give those up yet find the same nutrients in other foods. Wheat gluten is identified by Toll-like Receptor 4 as being similar to bad bacteria. It is molecular mimicry, but it is still a fact. Going back to natural foods is the simplest way to say “stop eating packaged foods full of additives. ” Another thing you can do is filter your water with devices like ZeroWater… or switch over altogether to distilled water. Distilled is literally only H20 because the dissolved materials do not evaporate at the temp. where water does. This will greatly decrease your exposure to chlorine, bacteria and even teflon and hormones… to name only a few.
Once we stop irritating our gut, or *while* we are ceasing this practice, we can seal it. Bring back health to the tight-junction cells which should allow out nutrients, but should not allow out complete gluten particles, for example, and not zonulin. Check with your doctor, but there is a peptide named BPC 157 which tightens up the junctions again. Then, to nourish the gut tissues we need resistent fiber as pre-biotic fuel, plus pro-biotic foods that contain the bacteria we need most: so that is whole vegetables, legumes, leafty greens, whole fruits, and then probiotics such as sauerkraut, there is both dairy and non-dairy Kefir and yogurt, Miso soup, as well as Kimchee and Kombucha.
In the process of soothing your gut, we can supply foods, herbs and spices which thwart bad bacteria and parasites (cloves, ginger, oregano, licorice root) and which give a boost to our natural mucosa by providing their own mucillage (Marshmallow root, Slippery elm, soaked Chia Seeds). Lastly, ground flax seed stirred repeatedly into hot water also lines our tissues and is an anti-inflammatory. Curcumin from Turmeric seems to be the Queen of all anti-inflammatories and will help the bloodstream as well as the gut. Lastly, Glutamine heals the tight-junction cells as well as that peptide mentioned earlier.
In order to stabilize our cortisol function, to bring our levels down and our fluxuations down, we can take a very safe herb called Ashwagandha (India) which is an Adaptogen to stressors (toxins, illness, life stress, injuries, cold, lack of rest). Another of these Adaptogens is Panax Ginseng. But in tandem we need to also lower the causes of inflammation around the body by binding toxins and ROS (reactive oxygen species). The following are excellent antioxidants/anti-inflammatories: Vitamin C in large doses, throughout the day, ground flax seed, Omega-3 fatty acid of the EPA kind. A very special one which an NIH meta-analysis of studies proved both protects capillaries especially, and vessels second, and also HEALS THEM AFTER damage to some extent, and can eradicate new Hypertension in approx. 2-3 weeks: is Citrus Bioflavonoids found in Citrus fruits, moreso in the white pith than in the pulp. I can provide references on all of this info. It has also been found that vitamin B3 is an anti-inflammatory. It is one of the small number of things to which an a7 nicotinic acetylcholine receptor will respond: Acetylcholine, Vit B3 (nicotinamide, Choline, Nicotine. Nicotine has been proven to be a nutrient in things like Cauliflower… but you wouldnt smoke a cauliflower, now would you? So why smoke tobacco. There are many more antioxidants, but I want you to know the bang for your buck, and one that can not be omitted is Polyphenols in dark red and dark blue/purple fruits.
Now sealing the BBB. I could have put this right after sealing the gut. A doctor can advise better. Cort has made it clear to us that CFS includes a high amount of brain inflammation. We don’t want body antibodies in our brain, and we don’t want food in our brain either. Omega-3 can cross the BBB even when it is healthy and not leaky. The compounds known to seal the BBB are resveratrol extract (comes from red grapes) and … the next finding needs to be cross-examined by you: the reddish fruit on the outside of coffee beans has been more recently claimed to be EVEN MORE effective than resveratrol in sealing the BBB.
Let’s try to put this into a better context:
no, that does not mean just drink more coffee. If you happen to know that the difference between “hard coffee” and “soft coffee” was once the option of either peeling back the reddish fruit but leaving it attached to the seeds before fermentation, or of removing the fruit before fermentation, that’s great. But I am talking about the fruit only, and not the “coffee.”
There are specific nutrients for helping your Adrenal glands to stabilize and to be nourished, and while I have that info somewhere, it might be better to make reference to Dr. Amy Myers in Texas who explains this recovery in detail. She is very careful not to rush one aspect of recovery if another has not yet been achieved. She spoke of resveratrol. I found coffee fruit elsewhere.
This leads to the topic of kidney damage and dysfunction from the trigger of your CFS state, whether that was a pandemic-grade influenza, or sepsis, or a grave bacterial infection, or even Covid 19.
In all of these occurrences, there tends to be significant kidney damage.
I will not presume to provide information on Kidneys. But it is definitely out there on the internet.
Finally, fasting and getting the body into a ketoacidic state by fasting, or by other means in the Keto-type-diet encourage MItophagy, in which the cell eats the mitochondria if they are not appropriate to the current cell state. One substance for achieving this state is Urolithin-A. It has been explained that Urolithin-A induces mitophagy of *damaged mitochondria*. This was a key topic in one of Cort’s most recent blogs: damaged mitochondria.
The cell can actually rid itself of dysfunctional mitochondria. It is not as if once they are damaged you are stuck with them. You might think: “the mitochondria have their own DNA, so if the DNA is damaged, then the mito are irretrievable’. It does not work that way. If one cell contains damaged mitochondria, then the checks and balances of cell division and apoptosis may deem that this cell is not healthy for division. In such a case, the cell will be destroyed and a healthy cell will sense the vacuum next to it and divide to fill that.
But the cell having mitochondrial damage is not necessarily irretrievable. Mitophagy is often possible, along with replacement by an appropriate mitochondrion pheno type, not for cytokine production, but for ATP/exertion.
Hi, huge thankts Cort, to your interesting and helping work.
What do you think is the best way to reduce oxidative stress in a ME patient.
That;s a good question and I don’t know. I would think NAC would be a natural but I’m really hoping that we’ll get some new and better antioxidants in the not too distant future.
The Real Person Badge!
Thanks, Cort, for another interesting article.
If this disease process “turns a ‘good’ enzyme – eNOS – into a ‘bad’ enzyme (iNOS)” though – I do wonder if increasing eNOS levels by taking beet supplements as ChatGPT suggests is really a good thing, or could it be a bad thing?
I would be curious what others who are more gifted in understanding metabolic processes than I, think about that.
If it could selectively promote eNOS and tamp down iNOS I think it would be a good thing. I imagine that given the right environment that the NOS enzyme stays in its healthier form and that things like beets help provide that environment.
Thank you!
The Real Person Badge!
Hi Court, thank you for your dedication in reporting on the latest developments in MECFS. I agree that there is a problem with the PPP but am surprised about the findings of increased BH4. Isn’t Ron Davis researching low BH4 in his latest study? I have a G6PD mutation which leads to a low G6PD enzyme and impairment of NADH production and low levels of glutathione as a result. Some of the AI suggestion for treatment also includes BH4 supplementation. How does that sit with the theory of high BH4?
Right – these two findings are at odds. It could be that both are found in ME/CFS; that is some people with deleterious mutations have low BH4 and other people have higher BH4…or one of these studies could simply be off.
The Real Person Badge!
I’ve had multiple tests where my renin level is several times above the maxim norm. Aldesterone remains completely normal. Usually this would be the sign of a tumor but imaging shows nothing. Blood pressure is normal with minimal medication and while the medication might skew the tests. nothing I’ve read says it would produce results to wildly high. Notably, a low histamine diet showed a long term drop in my blood pressure. I have no tachychardia or fainting issues, but plenty of the “must go horizontal” POTS.
What surprises me is the complete lack of interest by any of several doctors. Sometimes those outliers, as with those few people naturally resistant to HIV, can provide some substantial clues.
I have come to a similar conclusion on my own but my view of the root cause is a little different.. Its like we have a chronic form or G6PD deficiency and can’t make NADPH which is required for so many interactions including glutathione. That is why we elreact so much to medications and chemicals. Our red blood cells are lacking in the protective glutathione. This in itself will cause the endothelial inflammation and downstream effects. So I gave been on the hunt to find anything that will increase NADPH. I have tried various things to counteract the effects of peroxylnitrate and superoxide as I found that was happening too. Melatonin is supposed to help but that can be rough and flavinoids. I also wonder what is underlying this G6PD deficiency. Could it be the herpes virus? I tried medication for that too and that was a no go… Anyways there is all my research of 4 years from morning to night without a day off snd only meal breaks from my bed in a nutshell. Its so great to see this article.
I periodically dabble in exploring my epigenetics. Recently I’ve been using a great website geneticlifehacks.com. They have an article on BH4 and if you’re a paid member with your raw data, you can get a snap shot of your BH4 related genes.
https://www.geneticlifehacks.com/bh4-tetrahydrobiopterin/