

Geoff’s Narrations
The GIST
The Blog
The “Transcriptional reprogramming primes CD8+ T cells toward exhaustion in Myalgiic encephalomyelitis/chronic fatigue syndrome” study just changed the immune “landscape” of ME/CFS. It grew out of Andrew Grissom and Maureen Hanson’s first single-cell RNA sequencing study, which was published last year.
With single-cell RNA sequencing, we move into a different world. (When you hear RNA sequencing, think gene expression).
This gene expression study used a new, more effective technique – single-cell sequencing.
Virtually all ME/CFS gene expression studies have used bulk sequencing, which assesses the gene expression of all the cells in a sample. Single-cell sequencing, on the other hand, measures the expression of all the genes of each cell in a sample.
Because everything is jumbled together, it’s impossible to determine which cells have gone wrong in bulk sequencing. While single-cell RNA sequencing is more expensive and requires more sophisticated equipment and data analytic techniques, it’s the only way for researchers to determine exactly which immune cells have gone bad—and that’s what happened here.
To give the NIH credit where credit is due, this study was funded by the NIH. Studies like this make it imperative that we move forward there.
The main finding of Grissom’s first single-cell study (“Single-cell transcriptomics of the immune system in ME/CFS at baseline and following symptom provocation“) was the pivotal role that classical monocytes played in the immune dysregulation found in ME/CFS. The study also found an intriguing monocyte-T-cell connection, however. In the ME/CFS patients, the classical monocytes were pounding the T-cells with signals – telling them to activate.
THE GIST
- A recent study from the Hanson lab just changed the “landscape” of ME/CFS by uncovering some immune “scars” we didn’t know existed.
- The study used a new technique called “single-cell RNA sequencing” which is able to uncover the gene expression of single cell types. It’s far more accurate and refined than anything that has been done in this disease before.
- The study focused on T-cell exhaustion. When T-cells are activated, they, like other immune cells, change metabolically in order to produce more energy. If they are asked to do too much for too long, though, they’ll signal the retreat and ramp up their inhibitory networks that prevent them from becoming activated.
- The study uncovered a wide array of markers in T-cells (including epigenetic factors, proteins, transfer factors, “poising”) suggesting that the activated T memory cells in ME/CFS were indeed exhausted.
- The epigenetic finding was particularly interesting, as it suggested that an infection may, at one point, have rewired how ME/CFS patients’ genes were being expressed.
- Rather poignantly, the authors wrote, “Taken together, these results suggest that ME leaves epigenetic scars on T-cells, which leaves them “primed for progression into late exhaustion states.”
- They also proposed that exercise contributes to T-cell exhaustion and potentially contributes to inflammation, as well.
- While memory T-cells got the lion’s share of attention, the authors reported that “notable” alterations in other T-cells were also found.
- Writing that “the induction of T cell exhaustion is a multi-faceted process with various triggers,” the authors made it clear there were no easy answers to explain where the exhaustion is coming from.
- The suspects were familiar; however, chronic infection, inflammation, or a chronically activated stress response (sympathetic nervous system activation) could all be driving the T-cell exhaustion.
- Concerning the stress response, authors stated that a connection “between the autonomic nervous system and T-cell exhaustion “could have important implications for understanding how mental or physical exertion may drive PEM” and pointed to the dysregulated catecholamines (ANS neurotransmitters) Nath found in his recent intramural study.
- Drugs that tackle T-cell exhaustion are available, and the authors wrote that “validating T-cell exhaustion in ME/CFS could open the door to the use of drugs that can return them to proper functioning”.
- The authors also asserted that understanding T-cell exhaustion in other infection-associated illnesses, such as long COVID, will be critical to understanding these diseases.
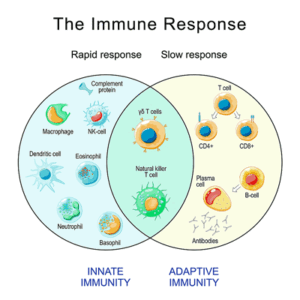
This study suggest that both parts of the immune response – innate and adaptive – may be working in tandem to create problems in ME/CFS.
That’s a very interesting finding, given the hypothesis that the innate immune system (monocytes) is trying to compensate for an exhausted adaptive immune system (T- and-B- cells). It would make sense, after all, for an overwhelmed innate immune system to keep telling T-cells to “c’mon, wake up – I can’t handle this alone”. On the other hand, it may simply be that hyperactive monocytes started off the show – and by continually activating the T-cells – caused them to become exhausted.
Thus far, several studies have found evidence that CD8 T-cells are indeed exhausted (higher fatty acid oxidation rates, lower glycolytic rates, decreased mitochondrial membrane potential, reduced TNFα, and IFNγ production, increased expression of PD-1, and increased plasma levels of TIM-3).
When T-cells are activated, they, like other immune cells, they change metabolically in order to produce more energy. If asked to do too much for too long, though, they’ll signal the retreat and ramp up their inhibitory networks that prevent them from becoming activated. They may also shut down their engines if, in order to save the village, so to speak, they are about to destroy it, i.e., if they become too destructive.
The goal of this study was to use single-cell RNA sequencing to determine which subsets of T-cells had become dysregulated and exhausted, whether or not epigenetics was involved, and to assess protein levels.
Results
The study found no evidence of dysregulation in the naïve T-cells, which had not been activated, but it was a different story in the activated cells.
How ME/CFS-ey this finding was! Things were pretty much OK at baseline, but once the T-cells became stressed by being activated, problems showed up in spades.
Take the transfer factors. Transfer factors are small polypeptides or nucleotides that transfer information from one T-cell to another T-cell or to a B-cell. Instead of urging the T-cells on, the transfer factors in the ME/CFS patients told the activated T-cells to shut down.
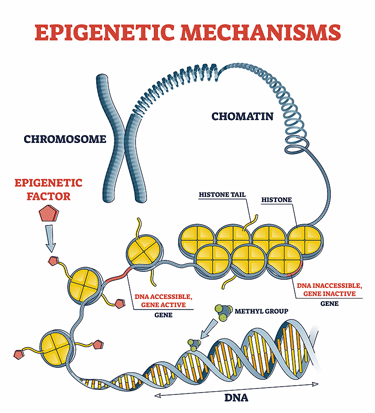
Epigenetic changes – possibly triggered by an infection – put the T-cells from the ME/CFS patients on a road to exhaustion.
An epigenetic analysis found the same thing – epigenomic factors that controlled gene expression were “rewired” to produce T-cell exhaustion. The epigenetic finding was particularly interesting, as it suggested that an infection may, at one point, have rewired how ME/CFS patients’ genes were being expressed.
A lot of exhaustion seemed to be going on. A loss of “regulatory poising” in activated T-cells suggested T-cell exhaustion was present. Regulatory poising refers to what the immune system is “poised”, or ready, to accomplish. The fact that ME/CFS patients’ activated T-cells had lost poising about genes involved in cytokines, proliferation, and cytotoxicity indicated that they weren’t in any way ready to pounce on any pathogens, leaving them in a hypofunctional, or exhausted, state.
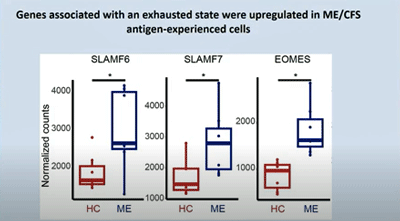
The elevated expression of several proteins (SLAMF6, TOX) also signaled that the ME/CFS patients’ T-cells were exhausted.
While exhausted memory T-cells don’t produce as many pro-inflammatory cytokines as normal T-cells, they can produce them constantly. Plus, they secrete chemokines that recruit inflammatory monocytes.
While memory T-cells got the lion’s share of attention, the authors reported that “notable” alterations in other T-cells were also found. Perhaps tellingly, the γδ T cells that bridge the gap between innate and adaptive immunity exhibited “substantial dysregulation”.
Plus, CD8+ TN_NN cells, which play a key role in defeating intracellular viruses and ridding the body of cancer cells, were overly activated as well – suggesting that something was chronically stimulating these cells as well. Nancy Klimas’s statement, “If any diseases show chronic immune activation, ME/CFS and long COVID do” rang true in this study.
Cause?
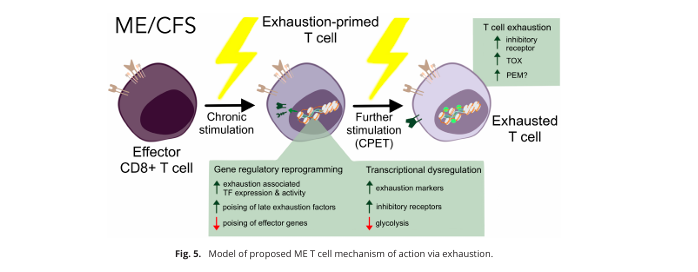
A model of T-cell exhaustion from the paper.
Then came the big question: where is this T-cell exhaustion coming from, and what does it mean for ME/CFS? Unfortunately, there are no easy answers. The authors wrote that “the induction of T cell exhaustion is a multi-faceted process with various triggers”.
Because infections are a prime source of epigenetic shifts, the epigenetic “scars” found in the ME/CFS patients’ memory T-cells could, however, very well be the result of the infection that triggered the disease.
The authors also were unclear about whether the T-cell exhaustion found is “driving (the) pathology” in ME/CFS or is a consequence of some deeper problem. They noted that a chronic infection, inflammation, or a chronically activated stress response could drive T-cell exhaustion.
In fact, for all the attention given to infections, a chronic infection may have nothing to do with the T-cell activation and exhaustion seen in ME/CFS. The authors stated that a connection “between the autonomic nervous system and T-cell exhaustion “could have important implications for understanding how mental or physical exertion may drive PEM”. They pointed to the dysregulated catecholamines (ANS neurotransmitters) that Nath found in his recent intramural study.

The T-cells are involved, but it’s unclear who’s in the driver’s seat in ME/CFS: the T-cells, monocytes, B-cells, autonomic nervous system, mitochondria?
An Autonomic Nervous System Connection?
The authors didn’t dwell on the ANS aspect, but it’s worthwhile looking at it further. Sympathetic nervous system activation—which appears to be present in ME/CFS—can promote a microenvironment that favors T-cell exhaustion by suppressing the effector T-cells this study found dysfunctional.
Chronic activation of the stress response can also impair mitochondrial functioning, increase oxidative stress and inflammation, and produce harmful epigenetic changes—all of which can contribute to T-cell exhaustion. A recent Nature Highlight article, “Stress can be exhausting for T cells,” notes that immune checkpoint inhibitors are not always helpful in reviving T-cells, and point to the autonomic nervous system for answers.
With their ability to narrow blood vessels and tamp down blood flows, autonomic nervous system receptors called B2 ARs have generated great interest in ME/CFS. Dysfunctional B2 ARs can also promote T-cell exhaustion in a variety of ways. Narrowed blood vessels, which result in hypoxia and metabolic stress, can drive T-cells to exhaustion. Wirth and Scheibenbogen have outlined the role B2 ARs could be playing in calcium signaling and the mitochondrial problems in ME/CFS.
Treatment
In the end, it’s all about treatment. While this study does not by itself appear to open the door to a drug trial, this is how, molecular upon molecular finding, we get there.
Last year at the IMEC conference, Dr. Hanson stated that it takes a lot to convince immunologists that T-cell exhaustion is present. I don’t know if we’re there or not, but the data is accumulating, and data is what you need to get to try drugs that target T-cell exhaustion. The authors wrote:
“Validating T-cell exhaustion in ME/CFS could open the door to the use of drugs that can return them to proper functioning.”
Since the NIH has been funding much of the T-cell work, one would hope the data would lead to an NIH-funded trial.
If a jacked-up autonomic nervous system is the culprit—and we don’t know that it is—ChatGPT stated that “modulating ANS activity may help restore T-cell function in conditions like HIV or hepatitis” and pointed to several approaches: non-selective beta-blockers like propranolol, checkpoint blockade therapy, immune checkpoint inhibitors (e.g., anti-PD-1), and stress management (meditation, mind/body practices).
Conclusion

Multiple lines of evidence are converging on the T-cells.
For the first time that I can remember, an epigenetic analysis indicated that the triggering infection could have rejiggered ME/CFS patients’ genes in such a way as to leave their immune cells exhausted.
“Our epigenomic profiling of ME CD8+ T cells revealed multiple lines of converging evidence indicative of T cell exhaustion in ME.”
Uncovering the T-cell problems, particularly on the epigenetic levels, was a real achievement, but what really stuck out for me were the many potential connections. There’s a potential monocyte-T-cell connection, a memory T-cell-B-cell connection (memory T-cells activate B-cells), and an autonomic nervous system-T-cell connection. Being able to make connections suggests researchers are on the right track.
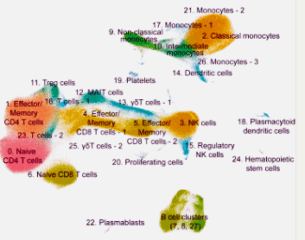
A possible monocyte-T-cell connection exists. Monocytes (upper right) and T-cells (middle left) showed up big time in Grissom’s first paper.
Plus, one would think this study would open the door to studying the other immune cells (B, NK) that appear to be exhausted in ME/CFS as well.
The authors clearly hoped this study would provide a template for studies in other conditions, including long COVID.
“As a major study profiling the epigenetic landscape of purified ME T cell subsets, we provide a critical resource not only to understand immune dysfunction in ME but to inform comparative studies on other infection-associated chronic conditions (IACC), including LC.”
Indeed, nothing even remotely like this study has yet been done in long COVID.
Last Blog of Health Rising’s BIG (little) Donation Drive

If you can, please push some support our way. 🙂
Thanks to the hundreds of people who have supported Health Rising to the tune of about 56K.
The last blog of the donation drive ends on a nice note: converging lines of evidence indicate that T-cell exhaustion – something hardly dreamed of 10 years ago – may not only play a significant role in ME/CFS but could result in drug trials.
Health Rising has always prioritized reporting on the molecular evidence emerging in ME/CFS and other diseases. If that’s important to you, too, please support us in a way that works for you.






Interesting article. Most seem to be assuming the T cell exhaustion is coming from a chronic, or reactivated virus. But you are questioning that.
The viral cause is tidier. And possibly easier to treat if it’s correct (eg. Antivirals as per PolyBio trials).
What came first, the chicken or the egg?
It’s obvious multiple systems are involved. So I guess in terms of treatment all systems need to be worked on. The classic holistic approach. Although didn’t Nath’s study suggest it starts with the immune insult? And isn’t Klimas saying treat inflammation / immune factors first and THEN the HPA?
Gosh, complex.
I guess until the research and trials progress further, we need to take control of our own lives and health and work on things holistically with natural treatments (and some pharmaceutical) and approaches. Including mind / body
Maraviroc (from the PolyBio trials) is targeting the T-Cells with blocking the CCR5 receptor and though reducing the recruitment and activation of T-cells which might indirectly help in preventing T-cell exhaustion by reducing chronic immune activation
Well, there’s a few parasites that harm people but there are litterally thousands of MICRO parasites that can’t be seen.
Maybe that’s why they can’t find anything.
Surely the researchers know this
Re: autonomic nervous system impact on t cell exhaustion, this is fascinating:
https://www.salk.edu/news-release/reducing-stress-on-t-cells-makes-them-better-cancer-fighters/
Beta blockers a potential option
Good find, Matthias! Thanks for sharing
Nice! Thanks. This appears to be a new and growing field and the stress side seems to make so much sense in this oh so stress sensitive disease.
From day one I said to everyone I know including medical people, there is something wrong on a cellular level. That is before I even knew what M.E involved. I felt it, deep within.
My own experience I had loads of viruses every year in winter season in UK, probably 10 years running, before I got M.E. So, my immune system was active a lot. The last virus I had I also said, something is not right, I felt deep within that last virus was different, i didnt feel right, and I never recovered. Gone on to get severe M.E.
The journey so far has changed over the years in some ways but remained same in others. I’ve not managed to improve much at all for the last 10 years of the 13 years I’ve been I’ll, but I’ve adjusted to this level of functioning. It’s obviously in my case the ANS and Immune system plays a huge role.
If I over do it slightly, just talking too much I get flu like symptoms that can last days, weeks and it’s like having a virus, runny eyes, nose, aches and pains, sneezing, sore throat etc yet I havent got a virus, not had one in 13 years. Equally I my ANS gets activated, i get insominia entire night, it’s like I’m wired just from over doing it a bit, too much stimulation, not even stress as such, just talking too much, or over doing it cognitively, then my sympathetic nervous system just be activated as I dont sleep, and feel like I’m running of adrenalin.
I’d say this research is on the right path, but it seems complicated. Great article.
Hi Loz. I don’t know if you take any supplements but most of your symptoms also match those of of having too many cytokines. See my post below. Aches and pains are definitely cytokines while your other symptoms could be but they also sound like mast cell issues. Which could both happen at the same time, due to physical stress. There is physical stress like any time you work any cells in your body, even talking, swallowing and thinking. Then there is emotional stress which we know is bad. If you haven’t looked into these I hope you will. It won’t cure your CFS but it may help decrease the symptoms you mentioned.
Appreciate your response — I am always amazed that over-talking exhausts me more than anything. Seems so strange. Glad to hear I am not the only one!
You are certainly not alone…:)
One loses a lot of CO2 when talking.
Or when ones breathes through the mouth instead of the nose,
Etc.
Maybe the one thing I can say for sure with me is that the autonomic nervous system seems like it has to be involved. I don’t think I’ve had a relaxing day in 40 years. It’s all “wired and tired” which intuitively just has to do with the stress response, At least that’s how I see it.
There’s no way in hell I could do what Cort does…running this show
Bot being able to relax is one way the organism respond to not having enough energy. Over-excitation of the cell.
Cort, have you ever tried *proper* thyroid supplementation?
That is, physiological doses of T4 and T3?
This is from Dave W (whose comment got hung up by our automatic system)
Loz, I have sensitivity symptoms very similar to those that you described above – in response to even low levels of virtually any kind of physical or mental stimulation. Even light in my eyes, sound in my ears, or a drink of water can sometimes be more than I can handle. We seem to be delicate and fragile to any kind of stress/stimulation.
When I first got seriously clinically ill with CFS at age 30, it started with what seemed to be a significant immune system activation. (Ie. My nose ran nearly constantly and profusely, sore throat, constant fatigue, loose bowels, some urticaria (skin swelling/welts) and frequent angio-edema like lumps – in deeper skin layers only on the soles of my feet (simply from my body weight I presume).
However, in less than a year my symptoms abruptly changed; My nose (and bowels) suddenly became unusually dry (instead of constantly wet), the urticaria became quite infrequent, but the angio-edema kept coming and going in recurring bouts. The sore throat became periodic rather than chronic. However, the chronic fatigue never let up. I now interpret this abrupt change in my symptoms to quite possibly reflect a transition from a chronically (partially) up-regulated immune response – to an exhausted or down-regulated immune response. This stage has never switched back again to the initial stage that I first experienced.
After seeing multiple doctors and specialists in the first months of my illness (all of whom were perplexed by my symptoms) – I recall breaking down and weeping one day when I was by myself, as (like you Loz) I just came to the realization that whatever was wrong with me – it was deep, metabolic or basal, (or perhaps as you described it; “cellular”).
During that cry, I came to terms that it would likely de-rail a normal life (and the quantity and quality of life’s potential joys) for the rest of my life. I am 69 now, and that epiphany was (unfortunately) valid. I will never forget that moment of realization, but somehow – due to the way this (CFS) illness made me feel – I just knew that it wasn’t transient. I still kept vigorously looking for explanations and treatments for decades, but only because hope is the last thing that we ever want to give up. (Never say never).
Great article (again) and thank-you Cort. This research (while possibly quite explanatory of cause) made me ponder the question; If epigenetics has altered our gene expression (forever thereafter) and deregulated our immune response in the process (causing all of our symptoms) – can such an epigenetic change ever be (realistically) hoped to be reversed?
It’s interesting how our symptoms can change over time. Like Dave I had a chronically sore throat for a long time – and mine cleared up. After about 15 years multiple chemical sensitivities showed up – bit time! They really were a game changer. They are better now but still problematic. Over the last year or two my long time burning muscle sensations after exertion disappeared (and wooziness appeared).
Epigenetic changes can be reversed! That only makes sense – since they pop up at one time – they should be able to “pop” down I would think. They are the way our bodies adapt.
Dr Liisa Selin has also studied immune exhaustion; in fact she was actually the first one to study it. You can read her studies here— https://www.umassmed.edu/selinlab/
Yes and plenty have alluded to it over the years.
The 2017 study from Mady Hornig et al showed early activation, followed by exhaustion.
I think it is unequivocal now that t cell exhaustion is a core feature of ME/CFS.
As Hanson and Cort allude to, the question now is what does it mean? Is it primary or secondary? Or a bit of both? How clinically significant is it?
That study seems to loom ever larger doesn’t it? 🙂
This great news! I thought of 2 things while reading it that stood out. First is cytokines. I took cytokine suppressors (as well as numerous other supplements) daily for 6 years before recovering to a more normal state. It took 4 years to recover from the Lyme symptoms and another 18 months for the fatigue of CFS. I still take them on and off today. I followed DR Ross suggestions here: https://www.treatlyme.net/guide/cytokines. Maybe they did have an effect on my recovery? The second thing that dawned on me was the reason DNRS (Dynamic Neural Retraining System) works for some people. I suspect it’s because it’s a way of getting people to do a form of “meditation”. It’s pretty much just repeating a mantra to yourself from what I’ve seen about it. I also meditated daily during my period of recovery. I’m starting to feel more tired so it’s time to start up again since I know I’m susceptible to stress. I start feeling poorly pretty quickly. And all it takes these days is turning on the computer, tv, or radio. Namaste.
I do think mind / body stuff can help many people. Not a cure, but with several other things can really help
Absolutely! If you haven’t read John Sarno’s book, “The Mind Body Connection” definitely do! Some physical pain (including Fibromyalgia) is caused by your brain to distract from mental/emotional pain. This is done by shutting down oxygen supplies to the area which increases nerve pain. The cure is easy. Just think to yourself, “I know there is no reason for this pain so either it stops or I’m going to think about_________.” And then focus on an issue that causes you distress. It only takes a few seconds once you’ve done it several times. To start just think about the topic, any topic that raises your BP for about a minute and then do something else. Wait a few minutes and see if the pain level has changed. Repeat a few times and if it not actually a real illness or injury the pain should disappear. The hardest part of the whole thing is just remembering to do it! I’ve seen it work too often to count and sometimes it works to decrease pain levels on actual injuries too. The brain is more powerful than we know.
Thanks for the resource, though it’s a bit much for me to read right now… May I ask, just to get an impression what you mean, could you give examples on what you took that worked as cytokine suppressors? Many thanks! JR
1. Liposomal Curcumin 500 mg 1-2 pills 3 times a day. I used regular curcumin taken as “golden paste” or high fat meal.
https://www.turmeric.com/recipes/golden-paste
These may also help in supporting the body
2. Liposomal Glutathione 400-500 mg 1 or 2 times a day. Or, what I took are its precursors:
Alpha-Lipoic Acid 350 mg 1 pill 2 times a day.
N-Acetyl Cysteine 500 mg 1 pill 3 times a day.
Also:
Quercetin 250 mg 2 pills 3 times a day
Cytoquel by Researched Nutritionals has a combination of the above. I never used it because I like to manage my own dosing, at least to start. DR Ross has very good prices at his store and he doesn’t sell “his” brand name products!
Hope this helps!
Oh that’s great, thank you so much for taking the time to reply! With these products, did you need to take them for some time to feel some improvement, or could you tell directly when you took them that they made you feel better? I’m asking because I always find those products a bit challenging where you need to have lots of patience to find if they do something for you 🙂
I understand what you saying but I suspect it’s pretty much an individual thing. Curcumin is the place to start. It’d not a “drug” so feeling a huge difference within hours is unlikely. 24 hrs you should feel a little better? But as you know, there is no way of knowing if the pain is only from cytokines, and which cytokines? But if nothing is happening after 5 days I’d be surprised. The lipo-somal Glut should help same as curcumin. But you should take it after a washout period with the curcumin because otherwise you won’t know if it’s working. If you take the others instead of the Glut you won’t see them working for awhile, like a month maybe? They are rebuilding your system and microbiome which can take a long time. Years? Hope this is a little helpful. This whole thing is looking like a lifetime project.
This is very helpful, thanks a lot! I actually don’t have too much of a problem with pain, but i guess cytokines can cause symptoms other than pain. Some of these supplements I already tried a couple of years back, but never in the liposomal form and I have much more inflammation today as I progressed to severe grade. – So I’ll put them on my list to try! Thanks a lot again!
Here’s a good, easy reference on the different types of cytokines. https://teachmephysiology.com/immune-system/innate-immune-system/cytokines/ I just saw a article somewhere on different supplements effecting different cytokines. Maybe here? Cort? I’ll see if I can find it but it’ll be a few days. Curcumin is the best place to start anyways. I take it 24 hrs and 12 hrs before a vaccine and it has seriously decreased the side effects! 🙂
I really hope a study like this is conducted for fibromyalgia as I am very curious if we have immune cell exhaustion features, too. It stands to reason we likely do based on the ANS and constant stress response issues in FM.
There’s been a lot less immune research in FM but both T-cell and NK cell dysregulation has been found as has chronic inflammation which could be the driver of all of this. Plus more mitochondrial work has been done. If the mitochondria in the immune cells can’t keep up the pace the cells will become exhausted.
All in all, what we really need is for all these diseases to be studied together which is what an NIH Center could do.
So many great studies fo ME/CFS recently! It feels like nothing can remain a mystery as long as there is enough funding for proper research.
I can’t help but feel a bit envious that the same is not happening for fibromyalgia, as someone suffering from this illness.
When I started reading this blog, it gave me a lot of hope that a real solution would be found during my lifetime, but I’m starting to feel like that will unfortunately not be the case.
There are so many biological overlaps between all these diseases (ME/CFS, long COVID, fibromyalgia and others) that I do think that Avindra Nath is probably right – that if you solve one you will probably solve them all 🙂
One can only hope, the impact would benefit an incredible amount of people around the world. So many of us would get their lives back!
Have been taking 1mg Klonopin at night for 4 years. Tapered down to .5mg per night all of last year and found 2024 to be my worst year out of the last few. Starting this new year I plan to go back up to the 1mg dose.
Fully aware of the risks associated with benzodiazepines, I thought I was doing the right thing coming down/off. But with a chronic illness this bad, I’ve made peace with knowing the therapeutic benefit and quality of life outweigh the cons.
That said, this article came at the right time because having Klonopin calm my ANS all these years has been helpful. No wonder I had more energy at 1mg!
After reading this I wonder about adding a beta blocker too. The science is compelling.
My only hesitation to increasing my dose has been the possible release of TNX-102 SL toward later 2025.
I will contact those researchers I link to above.
Apparently a side effect of beta blockers can be fatigue….
I did really badly on propranolol. In fact, it may have caused my first crash… I don’t understand the mechanism but Dr Teitelbaum mentions somewhere that it can pull the rug out from under m.e/cfs people too – or words to that effect.. 🤔 Interestingly I seem to tolerable Bisoprolol better… but not taking either presently.
Propranolol may also contribute to more insomnia and weight gain in some people.
Do you know which beta-blocker they used? The research article is in Nature and behind a (very expensive) pay wall. I hope they respond when you contact them!
Great idea! Thank you!! Very smart to point out they could increase fatigue.
I’m on beta blockers…they work fine for me.bits gonna be Individual. I’ve also heard they possess antiviral properties
If low dose benzodiazepines help this terrible disease, I would say take them, even if it means a lifetime. Many people also take antidepressants for life.
I know people who have been taking these medications for over 20 years without any problems. In other diseases such as rheumatism or cancer, patients take drugs that are much worse and more dangerous, but they still take them because they are sick. For ME/POTS ANS type ME patients, these medications can be useful as can beta blockers. Start with a very low dose and try different types of medications if it doesn’t help. And if it doesn’t help, stop.
Gijs, You are a wonderful person. Thank you for taking the time to reply to a total stranger with such sensible and thoughtful words. With all the decisions we have to constantly weigh out over the potential consequences, I truly appreciate your calming my trepidation on this.
It’s invaluable to have resources and engagements like this.
Thank you for helping to bring me peace. You’re very wise. Wishing you health and happiness!
Thank you Shea for your kind words, i wish you the same!
Shea like you I have been taking Klonapin(Clonazepine) for many years after Dr Cheney recommended it in the 1990s’. My CFS specialist here in Australia sadly died then it was CONSIDERED I could get addicted so after seeing an addiction specialist I slowly lowered the dose like you to 0.5mg at night. I agree that it almost certainly calmed my ANS. I am much more tired and wired now. I would much prefer the bigger picture inform medical decisions than singling them out
Hi Maggie, Well said! I couldn’t agree more. It’s comforting to know we at least have each other to share things like this with and helps tremendously to learn from one another. It seems our experiences match.
May I ask how long ago you reached the lower dose of .5mg?
May I also ask if you’re thinking of going back up?
Similar to you, my prescribing doctor retired so I tapered my dose concerned about the possibility another doctor would not prescribe in the future. However, since then I’ve learned more doctors than I thought are on board with prescribing Klonopin for ME and have grown comfortable with going back up (which I’m about to do).
Hi Shea, I reached the lower dose a couple of months ago. However I have awful early morning panic attacks lately and am beginning to wonder if it is the lower dose. You have encouraged me to give it a go and see what happens
Maggie, I’m so sorry about the early morning panic attacks. It’s possible those could be attributed to lingering withdrawal that might end up clearing and going away after a while.
However, the wired and tired sounds more like going back to not getting as good of sleep.
I think you and I might be on the same schedule of holding steady at .5mg for several months. I’ve definitely steadied and adjusted to this dose and, given how much less energy I have, think it best to go back to what was actually working for my body.
Please continue to comment back and let me know how things go with going back up. I’ll do the same!
Hi Maggie, I am in Australia too and used to take klonopin years ago, curious who your CFS specialist is? I feel like it was helping but then I stopped seeing the psychiatrist who was prescribing it to me.
Hi Jacinta, I was seeing Dr Don Lewis in Melbourne who sadly died 6 years ago. My GP is happy to prescribe it at a low dose
This is the most horrific thing I’ve read. It mean that our cells are reprogrammed? How could we get out of that?
It’s depressing as hell.
It’s the gene expression of the cells that have been reprogrammed – and it can be reversed. Epigenetics changes what genes are getting expressed all the time – it’s our way of adapting to the environment – so epigenetics is modifying epigenetics if I have it right. That versatility means it is possible to reprogram our epigenetics…
Cort. Thank you so much for a great article and for spreading hope even here in the comments. Means a lot!
Id like to second that cost and apologize. A couple of times in the past I’ve been bellicose.
Unfair of me. You do great work and I’m very grateful for this resource
How could we reprogram our epigenetics? I suppose it’s not an easy thing to do, am I wrong?
Epigenetics is still a relatively new field. You can get a popular science book on epigenetics, it’s quite fascinating. Epigenetic regulation is everywhere in the body. Genes text gets “read” in the cells and translated into the proteins it encodes (that’s called “gene expression” I think). Epigenetics is about a “second layer” of mechanisms above the genes that can switch genes on and off, or up- and downregulate how much of the encoded proteins is going to be produced/made available from the genes. It is also a mechanism of the body for adapting to all kinds environmental conditions. But as Cort says, there’s also a chance of reversal as epigenetics is a flexible mechanism. I’ve long thought that ME/CFS is likely to have an epigenetic component, both due to the wide range of “environmental” triggers (infections, stress, chemical exposure etc.) that are known to initially trigger the disease, as well as due to the waxing-waning nature of crashes in ME/CFS and the fact that some patients do recover. If it’s epigenetics, it’s probably better news for the chances of recovery than if it were say permanent tissue damage/degration (i.e. ME/CFS being a complex dysregulation problem in the body rather than tissue damage offers hope that once the root cause is found, and a cure developed, patients can really get better again).
I find that another hopeful perspective is that epigenetics could offer opportunity for developing a completely new class of therapeutics. If an epigenetic mechanism is are at the core of ME/CFS and once it’s really understood, modern biotechnology may in the best case allow to design specific therapeutics tailormade to that mechanism.
Look for example at this article about Prusty’s research at his former university https://www.uni-wuerzburg.de/en/news-and-events/news/detail/news/herpesviruses-awaken/ (Prusty is a herpesvirus and ME/CFS researcher): The mechanism discovered is an epigenetic one (microRNAs) and the article states that: “For the first time, this work from Würzburg shows that a microRNA can directly regulate the maturation process of other microRNAs. This also opens up new therapeutic possibilities: Artificial small RNAs can be designed to specifically switch off individual members of microRNA families.”
I hope this relieves your depression a little 🙂
Hi Cort. Yes, progress has been made (thank goodness), and there is evidence that autonomic nervous system (ANS) dysfunction can be linked to viral, bacterial, and fungal infections. Additionally, the connection between T and B cell malfunction and muscle weakness or exhaustion in dysferlin-deficient muscular dystrophies is tied to the DYSF gene. This gene encodes dysferlin—a well-documented protein crucial for muscle membrane repair. Abnormal methylation of the DYSF gene has also been implicated in conditions such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
This dysfunction can affect nearly all muscles but predominantly impacts skeletal muscles, which play a critical role as a key “organizer” of vascular tone and overall autonomic regulation. Since skeletal muscles are integral to circulatory and autonomic processes, impairments in these muscles can have widespread effects on the ANS and overall health.
Is gene editing the only solution?
https://www.nature.com/articles/s41467-024-55086-0Is this the only solution?
I think gene editing and stem cell therapy really need backing personally
Yes, you are absolutely right. CRISPR-Cas9 has had my full support (if needed) ever since Jennifer Doudna’s groundbreaking work was published.
Just imagine how much pain and suffering could be alleviated if issues like methylation in SMA (spinal muscular atrophy) and the DYSF gene (Limb-Girdle Muscular Dystrophy) could be corrected, allowing these genes to function as intended. The potential to restore proper gene expression could be life-changing for countless individuals and offer hope for treating or even curing debilitating genetic disorders.
Hi JR,
Greetings from Germany/Bavaria!
I’m sorry the original link didn’t work—here it is again: https://www.nature.com/articles/s41467-024-55086-0.
I’m also sorry to hear about your limb-girdle dystrophy. I too, suffer from the same epigenetic condition. A shorter article in German is available here: https://www.charite.de/forschung/themen_forschung/2024/muskelschwund.
As you’ll see, the Max Delbrück Center has successfully remove two DYSF gene mutations. These mutations may impair the function of the dysferlin protein, which is critical for muscle repair.
Please note that I don’t hold a PhD, but I’ve been studying genetics since 2010. I also wrote a short article titled “What Pathogens Could Be Involved in DYSF-Gene Methylation?” that you might find interesting: https://swaresearch.blogspot.com/2025/01/what-pathogens-could-be-involved-in.html.
Take care of yourself, and try not to overexert your muscles.
Best Sieglinde
Hi Sieglinde, a hello from Germany. The link to the nature article does not work, may you please copy it again or include the title of the article?
It seems to me you’re possibly a professional in this field due to your specific knowledge… may I ask you as a lay person what gene editing means? (This is different from therapeutics adressing epigenetic mechanisms, right?)
I have severe ME and have a friend with limb-girdle-dystrophy who works for Helmholtz centre for infection research.
Thank you and have a nice day!
I mean, do I get this right that abnormal methylation (that’s epigenetics, right?) could not be adressed by gene editing? Thank you!
To JR:
Here is one more direct link to “Developing a CRISPR therapy for muscular dystrophy” https://www.mdc-berlin.de/news/press/developing-crispr-therapy-muscular-dystrophy
I hope this give you more details.
Best, Sieglinde
Thank you, it looks very interesting!
Thank you for this exciting news. The article linked to at the beginning of the post was published in January 2024. Is there a link for the more recent study that this post discusses? Thank you again!
I hate it when I put the wrong study in there! Thanks for letting me know – it’s been changed and here’s the right link 🙂
https://pubmed.ncbi.nlm.nih.gov/39621903/
How many of you have actually had your T cells and B cells tested? My guess is not many.
Because I was diagnosed in 1984 by two immunologists doing research at our local university, I had the full complex of immune tests including the 8-way multi-test. In the 8-way test, I was only reactive to one thing.
I only had 372 total T cells and a complete reversal of T-4/T-8, but was HIV negative.
No one ever questioned how sick I was because of these tests and I was put on experimental treatment immediately.
T cell exhaustion…how about some “new” news?
I have. I dont know if there are different test methods etc. But my immunologist tested a full panel of T cells (number, ratio etc.)
They are all within range…
Hi,
I’ve been ill for 37 years.
My lymphocytes (T-cells) have been tested many times (20+ ?). Not a single time in 37 years was I between the normal range. I’ve always been below the lower limit of the range, sometimes by 50%. I was told, “It just means this is how you are, otherwise you would be constantly ill”. I have severe ME, cannot cook or take a shower everyday. My BMI is 15. If this is not being “constantly ill” I cannot imagine the kind of life (or hell) it would be… Anyway.
My CD57 were once tested (in 2019) at 22 (normal range: 60 – 360). My IL-6 serum at 447 (normal range: 0 – 5). My IL-8 serum at 531 (normal range: 0 – 15). My TNF serum at 36 (normal range: 0 – 6).
I had several EBV reactivations over the years. I was also tested low positive for toxoplasmosis for 3 years.
I was also diagnosed with EDS. I believe I have cranio-cervical instability. My plan is to see Dr Gilete in Spain end of the year.
*correction: it is my lymphocytes that have been tested, not T-cells specifically.
Great blog Cort! You nailed it this time! The (overactive) ANS and immune system that’s where the action is. In my opinion, the disease mechanism that allows this disease to persist. So, how we can fix this? By dampening the overactive sympathetic nervous system. That is what treatment should be aimed at. The immune system follows naturally. Much more in-depth research into the autonomic nervous system should be done. Why does it remain in the active position? There lies the answer to the cause of our disease.
🙂 I think dampening the ANS is a great start! The ANS is involved in blood flows, pain sensitivity, digestion, etc. and it regulates the immune system as well. The list just seems goes on and on.
Some murmurings that stellate ganglion block might be a treatment option. Have you heard anything about this?
A small study on its use in long covid suggests potential benefit:
https://pmc.ncbi.nlm.nih.gov/articles/PMC10498998/
Hi Cort, Before you dampen anything, you need to find out why it is revved up in the first place. The body has wisdom that we ignore at our peril.
And more importantly, I would like to know how many people commenting on this blog have been tested for HHV -6 A (not HHV-6 B) which I believe is the cause of ME/CFS and reactivation in Long Covid.
If HHV-A is not implicated, why are they studying metformin’s ability to reduce its replication in T cells?
https://hhv-6foundation.org/antiviral-treatments/metformin-reduces-hhv-6a-replication-in-t-cells-by-activating-ampk
Yes metformin might possibly help on several fronts. Also, PolyBio are studying Truvada (anti-EBV, but not sure about HHV-6)
Mattias, Truvada is an anti-HIV medicine. I haven’t seen anything about it being anti-EBV. The fact that they are looking at in ME/CFS speaks volumes.
Not quite right;
https://pubmed.ncbi.nlm.nih.gov/32409608/
Hi Mattias, Truvada is not the same as tenofovir. It is a brand-name medication that contains tenofovir and emtricitabine. Like Truvada, tenofovir is also used to treat HIV. HIV may reactivate latent EBV infection and reactivated EBV can cause a worse prognosis in immunocompromised patients with HIV.
Recently, a team from MIT and Harvard have shown that the Tenofovir prodrug tenofovir disoproxil fumarate (TDF) in Truvada and tenofovir alafenamide (TAF), targeting the EBV DNA polymerase, are highly potent inhibitors of EBV lytic DNA replication (in EBV reactivation).
If this is the case, the hope that solving one of these diseases means solving them all would certainly be even more realistic. POTS, ME, SFN, Fibro all seam connected via the ANS
How can we dampen our overactive ANS currently?
Mast cells are definitely implicated here too
I’m just not sure if the overactive ANS is a cause or rather a consequence… in daily life, for me it often feels like the body tries to compensate energy/fatigue problems by revving up the ANS to create more drive for action (like imagine if a car were stuck with handbrake engaged, you’d need to revv up the engine extra hard so it makes a jump forward). But it may well be a vicious cycle, like: the body compensating cellular deficiency by revving up the ANS, but highly acticated ANS in turn contributing to cellular deficiencies…?
I think vicious cycles are big in ME/CFS!
I guess, in theory, if you can address one part of the cycle effectively that could break down the vicious cycle?
I guess it should do so, or at the very least dampen it down! I believe ME/CFS is a cascade dysregulation in the body, of which many puzzle pieces have already been uncovered by research, but the root cause (so-to-say the main switch :-)) is still to be discovered…
JR i feel the same and agree it could be compensation. Still it remains the one million-dollar guestion… 🙂 After almost 3 decades of this disease i still don’t know the answer.
Yeah it’s kinda similar to an unsolved murder. The suspense is killing me/us
I still can’t comprehend why this is taking eons to figure out.
The research dollars seem to be flying out the window on useless studies with little concrete findings.
With all this new cutting edge technology I assumed I’d be well…that wS 10 years ago 🥱
In my view there is brain inflammation in ME/CFS. The longer and intenser the episodes got the less able I was at regulating distress. And it was wild and bordered paranoid psychosis in the worst moments. But it seemed totally normal to me given my understanding that I had (herpes – HHV-6b?) brain inflammation. From my experience I find it a bit odd that other patients think they have nervous overactivation without a good cause.
When I was denied acyclovir for a third round that had immediately stopped the inflammation process twice before I realised I had to learn to rest and pace perfectly in order not to trigger any other episode.
I have become almost perfect at managing my illness and I am doing a good recovery process.
There is any proof for an overreaction of the nervous system but there is more and more proof of an inflammatory process possibly due to different types of herpes.
What I used to calm my nervous system when I was not able to regulate it myself anymore during the worst episodes with walking, yoga and meditation was SHIATSU treatments. Because it is a very gentle treatment I can recommend it even during an episode for ME moderates. It was only later when I was more stable that I took up acupuncture with needles which “makes more waves” in the energetic body towards healing. What can be too much when you have developed serious ME/CFS. My first treatment directly lead into a crash.
From there on I knew that I had to tell my doctor to go easy on me. And when my reactions were too intense I went back to my shiatsu practitioner for some time for rebalancing. : )
Wow this gives a lot of hope. Not only for future Drug trials but as far as I undestand patients could also be tested for present T cell exhaustion, right?
That would mean that patients who would benefit can be easily identified and there would not be as much risk of getting worse by treatment. ( one of my biggest Concerns as this disease seams so diverse )
This seems quite significant.
Is this the type of study that could be shown to an immunologist, with the hope they might run some tests and prescribe some drugs to address the T-cell exhaustion?
And does anyone know how this would tie-in with OMF nano-needle study from a couple years back, where they showed (with a p < 0.000001) that there was a problem with the plasma from ME/CFS patients?
I think I’ve read that Low Dose Naltrexone, which helps many ME/CFS patients, also has effects on T-cells (you’d need to google which).
It also has an effect on natural killer cells, about that this article https://www.tandfonline.com/doi/full/10.1080/21641846.2019.1692770#abstract says that “Recently, LDN was shown to restore the impaired transient receptor potential melastatin 3 ion channel function in natural killer cells of ME/CFS patients”. I think the nano-needle study was about ion channel function, too.
Hey, off topic, but relevant to the recent oxaloacetate article…
My daughter has been found to have a biotin deficiency. I guess we will try a biotin supplement and see if that helps. It might not be meaningful.
But apparently biotin is necessary to generate oxaloacetate, which was found to be low in a 2017 study on ME/CFS, and which was the foundation for its potential use in ME/CFS.
Just wondering if anyone out there has had biotin deficiency.
Biotin supplimentation can interfere with blood lab results
In March 2024, I raised the question: Why are the white blood cells (T and B cells) becoming exhausted?
Is it due to the thymus, the bone marrow not producing enough white blood cells or an issue with the lymphatic system?
RBC and WBC from the bone marrow to the vascular system https://swaresearch.blogspot.com/2024/02/rbc-and-wbc-from-bone-marrow-to.html
Sepsis Management Overview and CLINICAL ASSESSMENT
https://swaresearch.blogspot.com/2024/10/sepsis-management-overview.html
Enlarged Thymus Gland https://swaresearch.blogspot.com/2024/03/enlarged-thymus-gland-symptoms-causes.html
This is exactly how it feels doesnt it ! T cell exhaustion. I notice that the cells produce cytokines … which means inflammation. Something the body does not need. …. It is unfortunate for us … that the subject of ME research is so complicated for an ME patients exhausted mind to follow and understand … and on top of that … the absolute essential care and attention ME patients need to pay to their bodys functionality … in order to help the body function on the most basic level. …………………………. I dont know if I will succeed with a new intention that I have … but the inflammation being constantly created at the cellular level, might be mitigated by simple grounding of the body to the planets natural flow of free electrons? …………………………………… Quite simply placing bare feet for a good 30 mins or more … upon the bare ground or grass … to allow free electrons to flow into the bodys system to bond with the free radicles and calm things down ? ………………………… I tried this yesterday with good results for a while. : )
So thankful to have found others like me. No amount of explanation can make people understand what this is like. I can do so little. Socializing is usually out of the question as I am never sure I can get there and back. It’s like living death. So glad the medical community is really working on it.