Geoff’s Narrations
The GIST
The Blog
The GIST is at the bottom right of the page.
Three months ago, Health Rising reported on the Simmaron Research Foundation’s Rapamycin ME/CFS clinical trial. It’s already time for an update—Simmaron’s research team moves fast.
A Little Background
The mTORC1 complex.
Seeking to create a mouse model for chronic fatigue syndrome (ME/CFS), the team gave mice a compound that inhibited autophagy, a cellular cleanup process crucial to mitochondrial functioning. When autophagy breaks down, it can impair oxygen consumption and mitochondrial activity, affect immune functioning, turn cells into pro-inflammatory generators, and lead to clumps of proteins that can damage all sorts of cellular processes.
The mice responded with high levels of a product called ATG13. Increased ATG13 levels are usually associated with increased autophagy but can also be the result of a compensatory response to a downstream blockade of autophagy. Indeed, the mice responded by looking like they had ME/CFS. Interestingly, the female mice were much more likely to become ill, quickly became fatigued when asked to exercise, and their grip strength declined.
Moving to humans, the Simmaron team found high ATG13 levels in the serum of ME/CFS patients. Then, when the serum was applied to cultured microglial cells, it appeared that the cells’ mitochondria had indeed imploded. Clearly tweaked by something, the microglial cells spewed out free radicals and produced inflammatory factors.
Further study suggested that the high ATG13 levels in the ME/CFS patients were activating receptors on the microglial cells called RAGE, which trigger signaling cascades in cells that cause inflammation and the generation of reactive oxygen species (ROS). This seems to be a nice fit for the microglial hypersensitivity problem that appears to be present in ME/CFS and could be affecting many symptoms.
When an antibody to neutralize ATG13 was applied to the serum and the microglia didn’t respond so negatively, the Simmaron researchers concluded that high ATG13 levels were the culprit.
With autophagy as a target, enter a drug – rapamycin (Rapumune, sirolimus) – that can upregulate autophagy and, if I have it right, clean up the damaged proteins and organelles produced by the upregulation of RAGE. Rapamycin may also be able to reduce RAGE activation.
Phase I Trial Update
The Rapamycin clinical trials team.
I recently communicated with Simmaron Research Foundation CEO and Translational Science chief Gunnar Gottschalk, Ph.D., about the Rapamycin (Rapamune, sirolimus) trial. (Gottschalk is the primary investigator for the trial, and David Kaufman, MD, is the clinical trial lead.) With most of the Phase I trial complete, Gottschalk was able to talk about some of the preliminary results.
The phase I trial used a variety of questionnaires (MFI, SF-36, sleep questionnaire, symptom inventory, Bell Activity Scale) to assess symptoms and functionality, a biomarker for autophagy activity called beclin-1, and some lab tests to assess safety (CBC w Diff, Chem 14, Lipid panel, Hemoglobin A1C, HS-CRP).
The study aimed to raise autophagy levels to increase energy production, reduce inflammation, and improve symptoms.
Differing dose regimens were used. I didn’t see the starting dose, but it may have been 5mg, after which doses were ramped up from 1-6 mg/week, depending on the patient.
The participants were assessed two weeks before the trial started, six weeks after taking Rapamycin (on a ramping-up dosing schedule), and then 30 and 60 days later. The patients were stratified according to onset type, duration, disability, and gender. There is no placebo-control group.
Results
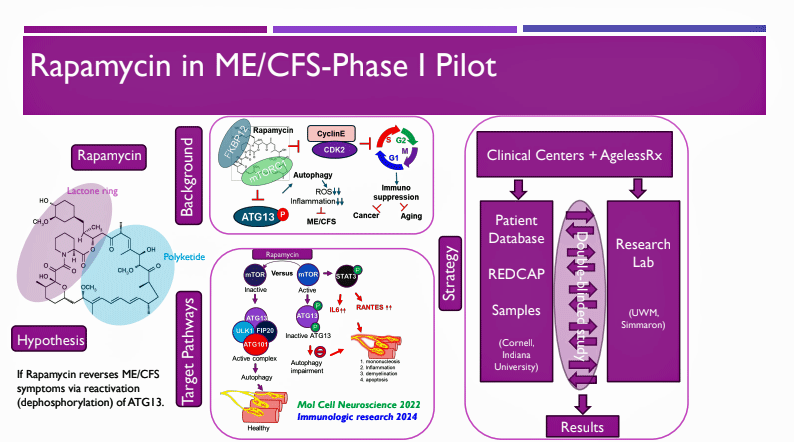
Rapamycin ME/CFS Pilot Study.
Gunnar reported that with most of the results in, a significant percentage of patients were considered responders. He stated that the responders “were seeing a significant number of changes in key symptoms, including reductions in PEM, improved energy, and reduced brain fog.
THE GIST
- Just a few months ago, Health Rising posted a blog on the SImmaron Research Foundation’s rapamycin clinical trial for ME/CFS. Simmaron has been moving fast: the Phase I part of the trial is almost complete, and with good results in hand – and with half the funding they need – they’re moving to a larger Phase II trial.
- The trial tested the idea that a drug called rapamycin – which is being investigated in longevity circles- might be able to restore a process called autophagy in ME/CFS.
- Autophagy is a cleanup process cells use to vacuum up damaged mitochondria and other organelles. Problems with autophagy can lead to problems with energy production, inflammation and other issues.
- Simmaron’s mice, culture, and human studies suggested that impaired autophagy in ME/CFS had resulted in increased levels of oxidative stress and inflammation.
- The Phase I rapamycin trial assessed the ability of rapamycin (in varying doses) to improve symptoms and autophagy functioning (using a factor called beclin-1).
- Gunnar Gottschalk, the primary investigator, reported that with most of the results in, a significant percentage of patients were considered responders. He stated that the responders “were seeing a significant number of changes in key symptoms, including reductions in PEM, improved energy, and reduced brain fog.
- Rapamycin has also produced a significant improvement in autophagy activity, which was associated with a significant improvement in the multidimensional fatigue inventory (MFI), suggesting that improvements in autophagy resulted in improvements in fatigue.
- While others benefited, it appears people with viral disease onset (EBV, CMV, HHV-6) or SARs-CoV-2 (coronavirus) responded best, and Simmaron will focus on this subset in the Phase II trial.
- With the good results from the Phase I trial, Simmaron is moving forward with a more extensive Phase II trial, which will increase the dosage by about a third (see blog for details). The trial is open to the patients of the physicians participating in it.
- The new trial will also include an antibody test developed by Simmaron in conjunction with Thermo Scientific to provide a more accurate assessment of the mTORC1 pathway being targeted.
- The researchers are seeking FDA approval by developing a diagnostic panel using two factors ( pATG13, BECLIN-1) that predict who will benefit from the drug.
- Simmaron is looking for more funding to complete the phase II trial.
- Note that a PolyBio-funded long COVID rapamycin trial, which assesses different factors, is also underway.
Right now, it appears people with viral disease onset (EBV, CMV, HHV-6) or SARs-CoV-2 (coronavirus) are responding best, and Simmaron will focus on this subset in the Phase II trial. They see responses in people with other subsets (autoantibodies, MCAS, etc.) but not as frequently.
They expect a preprint of the Phase I trial results to be published within a few months.
Phase II Trial
With the good results from the Phase I trial, Simmaron is using its recent strategic partnership with AgelessRx to move forward with a more extensive Phase II trial. (With about half the funding needed for the trial in hand, they have started enrolling patients and are attempting to raise the remaining funds. The trial is open by invitation only to the patients of the physicians participating in it.)
The Phase II trial will be different in a number of ways. For one, instead of using Rapamycin from other sources, they are switching to a compounded generic (Dr. Reddy’s®) from AgelessRx to ensure everyone gets the drug with the same bioavailability. They will also use two standardized dose regimens which will increase the total dose of the drug by about a third.
Dose – two options:
- start with 5mg – increase by 5mg/week for 3 weeks = 20 mg at end.
- start with 5 mg – increase by 2.5 mg over 6 weeks = 20 mg by end.
The trial will also include new questionnaires (the DePaul Symptom Questionnaire and a functional capacity questionnaire called Funcap).
hCG F <50, insulin, and Quant sirolimus plasma will be added to the lab tests. Plus, DNA samples or cheek swabs will be saved for future CYP43A/Cytochrome P450 genetic analysis (fast vs slow metabolizers).
More significantly, a new antibody that Simmaron developed with Thermo Scientific for pATG13 will be included. This antibody will provide the most accurate assessment of the mTORC1 pathway the team has found to be upregulated in ME/CFS.
They will also utilize mobile phlebotomy to gather blood samples.
FDA Approval the Goal
Simmaron has already found 130 age and gender-matched healthy controls over the past year, but they are not including a placebo arm in this trial because they’re seeking FDA approval for the drug in ME/CFS in another way.
They aim to develop a diagnostic panel using pATG13 and BECLIN-1 to predict who responds to Rapamycin. They’re using a “high-complexity lab” called Coppe Labs (Konstance Knox, PhD), which specializes in the development of FDA diagnostic panels.
(If I have it right, if the clinical trials go as hoped, people with high pATG13 (blocks the MTORC1 pathway, which upregulates autophagy) and low BECLIN-1 levels (indicating low autophagy) will probably fit this profile). Ultimately, general practitioners will be able to order these labs to determine which patients should try the drug.
If Simmaron is successful, this will be the first time that patients who are likely to benefit from a treatment have been biologically identified. That’s a big deal in a heterogeneous disease in which patients and practitioners often engage in lengthy searches for something that helps.
Simmaron is looking for more funding to complete the Phase II trial.
Right now, Gunnar Gottschalk and Avik Roy are working on an application for a rare ROI NIH ME/CFS grant. Getting one would be a stunning success for the Simmaron Research team, but they’ve laid the groundwork well. They started with mouse studies, moved into humans, and validated their findings in numerous ways.
PolyBio Long Rapamycin Trial Also Underway
A model of Rapamycin latching onto mTORC1 and preventing it from activating.
Long COVID has its Rapamycin trial. The $800,000 trial at David Putrino’s new CoRE clinic, funded by the PolyBio Foundation, is determining how Rapamycin may affect immune and hormonal functioning and symptoms. It’s possible that Rapamycin, for instance, could be helpful with the T-cell exhaustion that’s shown up in both ME/CFS and long COVID. Dr. Putrino, the clinical lead, stated, “We are hopeful that this will be a first step in validating a cheap, safe, and effective long COVID treatment.”
With two Rapamycin trials underway, we should learn a lot about what Rapamycin does in these diseases and what kinds of patients it may help.






I need to consistently isolate and rest to manage this disease..
Would I need to isolate more if I took Rapamycin?
i’ll take the trade as long as I can exert myself a little without getting sick.
Search this fb page, thus treatment works very well, Multi-dosing naltrexone for myalgic encephalomyelitis ME cfs
Vanessa, I’ve tried Naltrexone more times than I can count. I do not tolerate it well–even at low doses. IT has NOT WORKED for me. ( I’ve responded to your comments about this before)
I’m glad it has worked for you. Anyone that has had success fighting this thing, I am truly thrilled.
However, this a an article about a Rapamycin trial—for crying out loud!
That’s a shame, I was hoping it might help you.
I’m doing Primal trust too, it is Fabulous.
It can be a tricky drug to get right, I gave up twice, anyways all the best!
Chiming in: i have great succes on nicotine patches (5days on, 3 days off). Clears up brain fogg. Made me able to finish my phd.
Thank you. Sorry you had to respond like this but maybe she’ll get the message. We can hope.
I have helped so many people, All from my heart, that are now living wonderful lives! That is my only drive.
I’d bet you have a CYP2D6 mutation like me, or take other drugs affected by or modulating it with LDN – hence the similarly bad results. Rapamycin requires activity at CYP3A4 so it is processed differently and may have differing results.
What are your credentials that you are recommending drugs with negative side-effects to people with ME/CFS?
We’re all patients here and we should take that into account when we’re deciding to try a proposed treatment or not.
I will start Tomorrow the 6 mg weekly dose of Rapamycin.
I have Long Covid
Symptoms are Fatigue Muscular/ Joint Pain and internal tremors.
How long I shall be on Rapamycin 6 mg to see improvement?
Thanks
Thanks for this article, Cort.
In a recent YouTube video, Kaufman said that about 50% of people in the trial are responding, and that this is a pretty good outcome.
Will be interesting to see the final published results, including how much those responders improve by.
Right. I would not expect miracles but finding something that consistently helps one group of patients would be great.
Hi Matthias, can you please provide a link to the YouTube video? I searched for it but could not find it.
I’d like to share it with my Dr and others here might also
Hi,
Not sure this is the “right” video, but this is from 2 months ago, Dr. Kaufman on Rapamycin:
https://www.youtube.com/watch?v=LRXHPmuDPnc
Even the thought of something that could help is uplifting -:)
Yep that’s it
Thanks!
Hey Cort – thanks for reporting on this hot subject. Did it mention in these preliminary findings if the rapamicyn helped improved sleep and if it helped reduced neuroinflammation, not just body inflammation? I know some aspects cross the brain blood barrier and repair it but was interested to see how effective it is in cooling the central nervous system down.
Preliminary findings were that rapamycin improved sleep but no brain scans were done – so no info on neuroinflammation. Nice point – it has “limited ability” to cross the blood-brain barrier
I’d like to share a couple of interesting links I found while researching in response to this article. It turns out that a compound called Withaferin-A from Ashwagandha (Withania somnifera) may work similarly to Rapamycin: https://pmc.ncbi.nlm.nih.gov/articles/PMC5723685/#:~:text=One%20of%20the%20most%20significant,to%20both%20metformin%20and%20rapamycin.
Also there appears to be an online source of Rapamycin that ships to Canada:
https://rapashop.net/?gad_source=1&gbraid=0AAAAA-nW35F_D264EmS-nLgseDGvZ1l3C&gclid=CjwKCAiAzPy8BhBoEiwAbnM9O3DhPffF0kSsRXGaYR7z3iQPUU-RDMMwiem4UybWxwABrtqKqqRi7BoCG8gQAvD_BwE
Any opinions on this?
I tried Rapamycin for 8 months, taking 6mg weekly. All it did was raise my glucose and lipids, two side effects that seem fairly common. Good to know it’s helping some though. Many folks in the Rapamycin group got theirs from India, mine came from China via the UK. If you do try it, get your lipids and glucose tested 2 to 3 months after starting. A lot of folks start taking metformin and statins and had it helped me at all I would have been willing to add those two.
My son’s lipids went up as well. And no appreciable improvement in symptoms.
I am somewhat skeptical on rapamycin but keep an open mind.
I think ME/CFS could be 2-3 distinct illnesses, or at least 2-3 illnesses with some shared biochemical factors but also significant differences. So I do think it’s quite possible that one drug might work quite well for 40-50% of people but not others.
But we will see
Yes, I’ve read that. Makes sense.
Absolutely. I wish studies would focus on which subsets of patients are helped by which Interventions and what would be a potential biomarker (eg pat with high lipids/ antibodies/ SFN profit from the studied treatment)
Otherwise wie are stuck trying things that might make us worse..and it also takes a lot of time
I also believe, especially after becoming social within the ME community, that what gets diagnosed as ME is in fact several different things. The problem with ME being treated as a disposal diagnoses for complex patients is that it confounds the research significantly. It’s to the extent that I know people diagnosed with ME who clearly do not have any direct relationship between physical exertion and symptoms. They deserve help and support for whatever it is they do suffer from, but as long as we all get lumped in together the people with clearly identifiable ME are in a much less hopeful situation.
Totally agree, there are a subset of people that were on tetracycline, me being one of them.
My nephrologist verbally told me that the tetracycline damaged my kidney tubules.The disease is called aquired franconi syndrome.the kidneys loose too much phosphate,bicarbonate.there are two types of franconi disease,aquired from tetracycline and a few other drugs,and hereditary which is way worse to have so don’t confuse the two.theres a couple youtube videos on fanconi syndrome.the disease can be caused by degraded tetracycline.for me, I was on tetracycline for way to many years back in 75′.it also causes heavy metal accumulation in the body
Yes, I also agree. At this point, ME is just a designation for a group of symptoms. Until we have biomarkers to discern between one illness and others, there is no way to know what interventions help with what particular illness. It’s like treating “runny nose” the same way, without knowing if the cause is a bacterial infection, a viral infection, allergies, or some irritant in the air.
Yes, I’ve been taking rapamycin 6 mg weekly for three months. No change
I’ve been on it over a year and it’s given me some life back, was a miracle for me as I was extremely severe. Currently on 6mg once weekly, no known side effects, lipids and a1c fine.
Hi Andrea, I’m so happy for you. When did you start to see improvement?
Thank you so much, me too. Tried so many things which made me worse. I improved immediately. I sure hope they can get FDA approval, but am fearful of what’s going on with all federal agencies that it won’t happen at least for 4 years. It can be difficult and expensive for folks to get ahold of privately.
Hi Andrea
Would it be possible to ask in which areas it has helped. Is it just fatigue or also pain and sleep? Thanks Doug
Hello! For me it tackled the severe PEM which kept me bedridden. I still have a lot of pain and feel lousy, but am able to do so very much more without PEM. It’s weird, but amazing regardless.
Also in the uk chronic fatigue with pain gets diagnosed alot as FMR fibromyalgia but the symptoms of brain fog post exercise malaise ect are the same! Has anyone heard it being used for FMR? many thanks Alison
Reminds me of the subset of people who start an antifungal and feel immediately better then go back to baseline a few months later (myself included). Antifungals including Rapamycin are tough when it comes to using the CYP3A4 enzyme. 107+ drug interactions (including statins) is a lot. But I’m sure we all can agree it’s worth it if it helps.
I think when we are writing about certain success stories with Pharmaceuticals It’s always important to include the side effects within the story. I have looked at this drug and number of times and also noted a range anecdotal side effects throughout subreddits. This makes me quite cautious.
Yes. Because we are all desperate for treatment and hope. But we need to take the risks into account.
Ana, I appreciate your response. I have long covid and am highly sensitive to Pharmaceuticals. I’ve tried a number of them and they’ve all made me feel worse. I’m trying to be cautious.
I am so sorry! I have ME and SFN and same… LDN have me major anxiety…a bunch of other drugs gave me hallucinations, aseptic meningitis or cognitive slowing…
Its all so complicated…
I had serious issues with LDN – I also have severe reduction of function in the CYP2D6 enzyme complex, which appears to be related. 2D6 causes major issues processing other drugs, you end up drowning in them eventually as they build up faster than the enzyme can metabolize them.
GeneSight or other drug metabolism testing can tell you a lot about what you should be cautious about or use reduced dosing for if you want to try it at all.
Zap, did they resolve eventually? I feel like I am in a downward spiral mentally even though I discontinued.
Deowning in drugs is what I often felt.
Thank you for the info!
Unfortunately I am in Germany so I have to look for testing.
Yes, thankfully after stopping the medication and getting orders reinstated for my IVIg everything returned to my “normal”.
You may be able to find another genetics outfit that can do the testing. I had some original results from doing 23andMe genetic testing – it can be run through a service called Genetic Genie which will show Methylation and Detox genetic profiles.
From my research, Rapamycin is metabolized by CYP3A4 – anyone that has issues with or takes drugs that modulate that enzyme may have mixed results.
I think a lot of the variability comes down to the fact that mutations in detox profiles are not accounted for when trialing medications.
Is Rapamycin a current drug approved for other illnesses?
The article mentions dosing up to 20mg, however all other sources that I came across mentioned 6mg/wk. does anyone have any information on this? Thank you
IN GENERAL, the compounded rapamycin has inferior absorption to the one that you’ll buy from a regular pharmacy. This is the reason for the very different doses
Maybe Cort can highlight this fact as taking normal rapamycin at the compounded doses could enhance any negative effects.
Thank you. I have gotten it from a regular pharmacy.
@Cort, would you please be able to shed some light on this ?
Thank you
Guess who’s back Cort? The crying fool 🙂 I am pleased that Peterson is involved in this investigation. I think that for this medication it is important to select or select the right patient for whom it does or does not work. In addition, the duration of the disease should also be taken into account as Lipkin found that the immune system is overactive for the first 3 years and then underactive. If I remember correctly. I’m curious about the outcome. Many more studies should be done, including with bezondiazepines, for example. This substance acts on GABA, a neurotransmitter that has a dampening effect in the brain. These types of studies should not have to cost that much money in the first place.
Very interesting Gijs.
From my own experience, I can say that when I was first undermined by ME, not knowing what was happening to me, the main symptom accompanying the fatigue was fever. Yes, my immune system was overactive.
But, those bouts of fever lasted more than 3 years. More like 20. I still fight fatigue, but at least, I don’t have to endure that unexplained fever any more.
Good point about the phase of ME CFS
I think the longer you’re sick and the more severe,
The overactive immune system gets exhausted
(Possibly the immune cell mitochondria are exhausted too)
And becomes under active as you say.
I’ve noticed myself the 1-2x times I had a small remission. My immune system got active again.
I can identify with the idea of an “exhausted immune system” perhaps becoming under-active after all that effort. I began bed-bound for a year, unable to even comb my hair. I slowly began to function but had to pace very carefully. My immune system had attacked my lungs as sarcoidosis and then moved on to my eyes as sarcoid uveitis…. all this manifesting an overactive immune system. My eye doctor prescribed Methotrexate, ONE 2.6mg every Thursday for years, which is practically none, but I always start as low as possible. (Children are started on 6 pills once a week) I’m an intermediate metabolizer and have had anaphylaxis 4 times, so I avoid pharmaceuticals.
Interestingly, my eyes settled down, then the lungs settled down. I still had an energy gas tank that had to be refilled constantly by pacing, but at least I was functioning, just carefully and very slowly.
Then the pandemic arrived. Of course, methotrexate lowers your immune system, so I became a germophobe, sequestered alone in my home. I developed systems of sanitizing food deliveries in garage fridge before coming into the house. Had minimal human interaction, always masked and gloved.
The only vaccine I attempted was the J&J because it used a cold virus which didn’t frighten me as much. I only had one, because even that sent me to the dreaded ER (full of sick people) with blood pressure of 193/99. I had stopped taking the methotrexate 2 weeks before, trying to make my immune system stronger for the vaccine.
Well, needless to say, the ER people had no clue what ME/CFS/FM was and no time to read the info I always carry with me.
So I told them I wanted to go home because I thought I could fix it. They were thrilled to get rid of me, so I went home, promptly took my one 2.6mg methotrexate and my BP went down in record time.
I stayed on it for 3 more years because I was afraid to stop. … until one month ago. I am 80 yrs old and still live in my home. I have an amazing lifetime LongTermCare insurance policy that paid for caregivers 3xweek on and off for a couple of years. But…
They don’t believe in ME because they can’t see it on my blood labs. They’ve worn me out with queries, nonpayments and appeal processes. I have supplied enough professional information to teach a class on it, but to no avail. It’s not healthy for me to keep trying to justify my need for in-home help. Every time they cancel me for a while, I have a bad fall. This last one almost sliced through my leg.
The brain fog continues, my hair keeps falling out, and my sensitivities to light and smells got a bit worse, so I decided maybe my immune system wanted to be left alone for a while, so I frighteningly stopped taking my methotrexate 4 weeks ago.
There’s been very little change except I tire a bit more easily, my brain fog marches on, and more hair is noticeably falling out more. I’m attributing some of this, including the arthritis bones in my toes and hands, to just being 80.
The hardest thing about ME for me is being a mere fraction of my former self in terms of capability, losing friends and relations who get bored that nothing’s changed. Even doctors give up on me, so I don’t bother them much any more, and I stopped trying to explain it a long time ago. It’s too complex for most people’s attention span. I have to agree.
It’s annoying and debilitating, but I’m in peaceful, clean surroundings with my photos and familiar things surrounding me, clear, uncluttered paths to walk from room to room, a front porch with flowers to sit and get fresh air, my mail is delivered to my porch because I can’t walk to the curb. My groceries are delivered by nearby stores. The garbage men get off the truck and come get the bins from my garage, empty them and bring them back. There’s a wonderful dog next door who lets me know if anyone is in my yard or driveway. A cleaning lady comes and a caregiver once weekly as well. I have one friend who texts every morning at 8:30am to make sure I woke up. The fire dept has all my health info and emergency contacts on my refrigerator door. They also have the code to a lockbox with a key to get in my house. All my important papers are in a fireproof briefcase near the front door. I stream my local church service, watch tv and rest a lot in between. My hair is still falling out and my hearing is awful, but I’m 80, so I finally stopped grieving that I can’t snow ski or travel, swim or play tennis. It’s not ALL because of the ME/CFS/FM etc.
People say I should be in a facility of some sort, but I feel like I’ve created my own “assisted living” place that has only foods I can eat, access to fresh air, nobody with hairspray or perfume on, blinds shut to keep the light down and I can have the tv volume at 60 to watch Wheel of Fortune and Jeopardy. Anywhere else I’d be stuck in a room.
My grandkids never knew me “when I could do things” but I can still give them advice and lots of hugs. Thank goodness for FaceTime.
I’m open to any advice or ideas regarding the methotrexate situation.
It’s such a strong, old chemo med. I like being off it, but jury’s still out.
Barb, that was a beautiful story of adaptation and acceptance. I wish you well.
What a brave attempt to live Life well and your wonderful attitude to such a debilitating ill Ed’s
Thank you Cort for this report.
As a Canadian, I’m pretty sure rapamycin will not be available to me, as treatment for ME, for many years, but it’s really encouraging to see this research progressing well.
That’s just horrible. You cannot ship to Canada from sites like alldaychemist.com? Sorry, I’m in the USA so naive on Canada’s drug laws. Their public health system seems to be really holding people back.
More like being held hostage.nobody ever gets treatment for any disease….lots of diognosis but hardly ever any treatment.when there is treatment, it’s usually a toxic drug that just masks the symptoms. TIME FOR DRASTIC CHANGE.
that’s why so many people turn to youtube.
These people go into medicine thinking they are going to help people,save lives but soon find out that’s not the case.Doctors hands on Canada are tied very tightly behind their backs.this all happened in the 90s. My immunologist was forced out just for telling kids parents to stop feed allergic kids dairy.Its all about pushing pharmaceuticals
$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
I have an immunologist in Australia who is prepared to try me on Rapamycin but is concerned that the proposed dose in the Phase 2 Trial is way too high (reported here as start with 5mg pw and increase to 20mg pw over 3-6 weeks.) Whereas the phase 1 trial was start with 1mg and increase to 6mg which is much closer to his comfort level. Would be great if anyone could help clarify please? Thanks!
Hi Martin
I watched the most recent podcast from Dr Kaufman where he discussed the trial .I couldn’t find the discussion on the higher doses. He mentioned starting Wk 1 on 1mg then increasing by 1 mg per week over 6 weeks.He also mentioned doing baseline blood tests for lipids,glucose an hbg a1c and testing again monthly. If these tests showed increased levels the dose was reduced to bring back to normal levels.
By the way I am in Australia and am trying to convince my GP to trial me on this but struggling to convince her. Could you let me know where your immunologist is based .Thankyou.
Thanks for the follow up Doug. I think I found the explanation on the different dosing is that the research team is switching to a compounded generic which has a lower bio-availability. So looks like the 1mg increasing to 6mg of the standard drug is the way to go. The immunologist I’m seeing is Dr James Yu at Belrose – https://www.northernbeachesimmunology.com.au He doesn’t have any particular interest or experience with ME/CFS but was very open to trying to help me. He’s happy to give me Rapamycin and monitor with regular bloods – and equally happy to try me on Nimodipine. I had blood tests last week and will see him again on Monday to review. Expect to walk out with a prescription for one or both of these drugs. (Why not try 2 at once after all? They don’t seem to be in conflict with each other.) Are you in Sydney? Would be happy to have a call and compare notes any time. You can get me on 0414 713 923. Good luck getting your GP to give it to you though!
Thank you for your contribution.
I am surprised (or am I not? ) that researchers never ever consider natural remedies to solve issues, but always try medications (pharma) that usually have side effects – damaging some part of the body.
I am taking Quercetin Lipo which also helps autophagy.