

Geoff’s Narrations
The GIST
The Blog
The Gist is at the upper right

Researchers have been targeting something they don’t know the identify of. They’ve had hits and misses.
It seems soooo enticing! Multiple findings suggest that something in the blood is causing or contributing to ME/CFS, FM and/or long COVID. The easiest answer to that would seem to be to just find a way to wash it out of the blood – and that’s what several groups are trying to do.
It would help, though, if we knew what the mystery substance is. Unfortunately, Ron Davis has said that finding it is not “a trivial matter” (translation – not easy to do) and that has not been achieved yet.
Identifying it would be extremely helpful, though, as it would probably provide a biomarker, and result in a more targeted and effective treatment approach via monoclonal antibodies, inhibitors, T-cell therapy, antigen-specific immunotherapy and others.
As it is now, researchers are left with taking a broad-brush approach and are trying to cleanse the blood of all -as Dr. Kaufman put it in an Unraveled episode, “all the garbage that’s built up in it” in hopes of catching “it”.
THE GIST
- Multiple findings suggest that something in the blood is causing or contributing to ME/CFS, FM and/or long COVID. The easiest answer to that would seem to be to just find a way to wash it out of the blood – and that’s what several groups are trying to do.
- Knowing what the mystery substance is would help a lot, as it would probably provide a biomarker and result in a more targeted and effective treatment approach via monoclonal antibodies, inhibitors, T-cell therapy, etc.
- As it is now, researchers are left with taking a broad-brush approach and are trying to cleanse the blood of all manner of possibly pathogenic substances in hopes of catching “it” which brings us to the recent therapeutic plasma exchange (TPE) trial.
- The plasma in the blood is targeted because it carries the antibodies, immune complexes, cytokines, and other pathogenic substances.
- In a placebo-controlled, randomized, etc., long COVID trial, about two-thirds of the participants plasma was removed and then replaced with albumin repeatedly over several weeks.
- Unfortunately, the plasma removal did not produce any results; neither the participants’ symptoms nor their biological factors changed.
- While the authors noted that other forms of apheresis (plasma exchange) exist, they proposed that the pathogenic portion of the long COVID patients’ illness either occurred very early or was occurring in the tissues, which the TPE could not reach.
- This study does not mean the “something in the blood idea” is dead. In fact, we’re more at the beginning of the plasmapheresis story than the end. The form of apheresis used in this study is just one of the four that might be helpful.
- A study assessing a different form of apheresis called INUSpheresis, which filters pathogenic factors out of the blood (instead of removing and replacing the plasma), had much better results. Many pathogenic factors were reduced and the substantial patient improvement occurred.
- The knock on the study was that it was not randomized, placebo-controlled, etc., and the authors only reported the findings of those who improved. Still, the study suggested that the right kind of apheresis might work well in long COVID.
- Apheresis is not the be-all and end-all. In most autoimmune diseases, apheresis is considered a last-resort treatment—to be used only when other treatments fail. For the time being, we’re kind of stuck with broad blood cleansing efforts in ME/CFS and long COVID, but knowing what the mystery factor in the blood is could give us access to all sorts of more effective, targeted treatments.
- Coming up next: the immunoadsorption saga in Germany
The Therapeutic Plasma Exchange (TPE) Long-COVID Trial
The latest effort hailing out of Spain, “Plasma exchange therapy for the post COVID-19 condition: a phase II, double-blind, placebo-controlled, randomized trial“, involved a trial of 50 people who were either given six sessions of therapeutic plasma exchange (TPE) or a sham plasma exchange, and then followed for 90 days.
Plasmapheresis simply refers to the separation of the plasma from the blood. The more or less colorless plasma contains proteins, immune factors (antibodies, cytokines, complement factors, acute phase proteins (CRP)), electrolytes, nutrients (glucose, amino acids, fatty acids, and vitamins), hormones, waste products, clotting factors and enzymes.
In therapeutic plasma exchange (TPE), however, large amounts of the patients’ plasma are removed and then replaced with a substitute fluid, such as donor plasma, albumin or other solutions.
Different types of plasma exchange are possible. The therapeutic plasma exchange used in this study is most commonly used to treat autoimmune, neurological, and blood disorders.
The Big Bust

The TPE trial failed on all counts: neither symptoms or biological factors improved.
Functional status, symptomology, quality of life, and cognitive scores did not improve in individuals who received TPE compared to placebo, overall or in any subdimension evaluated.
Nor did any of a wide variety of biological factors (hematocrit, CRP, ALT, GGT, ferritin, creatinine, triglycerides, LDH, INR, bilirubin, aspartate aminotransferase, antimitochondrial antibodies, anti-smooth muscle antibodies, anti-parietal cell antibodies, anti-liver-kidney antibodies (LKM), anti-nuclear antibodies). Even though the authors dug deep to check out clotting, none of the five tests (prothrombin time, fibrinogen, D-dimer, International normalized ratio (INR) and platelet count) were impacted.
The study was a complete wipeout. Six sessions of TPE over three weeks changed nothing. It was as if the patients were replacing their plasma with their own plasma. The “something in the blood” hypothesis seemed to be shot… Or was it?
Is Apheresis (and the “Something in the Blood” Hypothesis) a Bust?
While the authors noted that other forms of apheresis exist, they suggested that the main problem was probably that their TPE was simply applied too late (2 years post-onset). They believe the “key pathogenic events” in long COVID-19 occurred very early or were occurring in the tissues—not the bloodstream. For TPE to work in long COVID-19, it has to be done early.
Of course, that doesn’t jive with the “something in the blood studies,” which have repeatedly found that something in the blood (not the tissues) is causing problems in laboratory animals or lab culture tests.
This study isn’t the end of the plasmapheresis story, either. In fact, we’re more at the beginning of the apheresis than the end. At least four kinds of apheresis could help in ME/CFS, FM, and long COVID. They include:
- Therapeutic plasma exchange – removes about 2/3rds of the plasma and replaces it with a substitute
- INUSpheresis – filters the plasma of autoantibodies, cytokines, etc. and then returns it to the body
- HELP Apheresis – uses high doses of heparin to clean up as many clotting factors as possible – see Patrick Usher’s story
- Immunoadsorption – targets and filters antibodies out of the blood.
INUSpheresis – a More Effective Kind of Plasmapheresis for ME/CFS and Long COVID?
Take the recent INUSpheresis long COVID study, which produced far better results using a different kind of apheresis.
Centrifuge vs Filtration
Centrifuge – The Spectra Optia® Apheresis System, that the TPE study used, employed a centrifuge to replace 63-78% of the substances in the plasma with albumin. (Above 78%, side effects kick in and returns diminish.) By doing 6 TPE sessions over three weeks, the researchers should have steadily lowered the amount of pathogenic substances in the long-COVID patients’ plasma. One would have thought that if something in the plasma contributed to their illness, the long-COVID patients would have seen some relief asymptotically and biologically, but neither happened.
Filtration—The INUSpheresis® group used a double-filtration approach that filters the plasma of immune complexes, autoantibodies, cytokines, viruses, and toxins and then returns it to the body. The protocol also includes a naturopathic infusion of acetyl glutathione (an antioxidant) and a vitamin preparation.
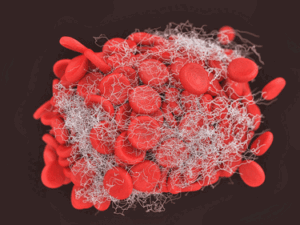
Among other things, the INUSpheresis trial reduced the number of clotting factors.
In contrast to the TPE study, the 27-person INUpheresis study found that markers of inflammation declined dramatically (sCRP, IL-1 beta and IL-6; 33%, 48%, and 64%) and so did, by one measure, oxidative stress (H202) (90%). Markers of coagulation (fibrinogen and homocysteine) dropped just as dramatically (70% and 64%) and cholesterol, triglycerides (TG), LDL, and HDL, fell below reference levels. Plus, other factors associated with coagulation (fibrin fibers, rouleaux structures (stacked red blood cells) disappeared.
The INUSpheresis study, on the other hand, was not placebo-controlled and only reported on patients who had improved (!). It was encouraging to see some patients improve (the authors said 70% did), but we need a good study to determine how many benefit.
Since both studies were designed to clear the plasma of pathogenic substances, it’s hard to know why one failed while the other did so much better.
In the end, we have two conflicting results. We have a small but rigorous study using a standard TPE procedure, which failed to move the needle in long COVID, and we have a different kind of apheresis that produced much better results in a less rigorous study.
We’re not nearly at the end of the plasmapheresis story. Dr. Ruhoy has said that she’s finding plasmapheresis helpful in some patients, and we still have at least two more types of plasmapheresis to get to: immunoadsorption and H.E.L.P.
Not the Final Answer Anyway…
Time will tell how the different forms of apheresis will perform in diseases like ME/CFS, FM, and long COVID-19, but even if they don’t do well, it’s not the end of the “something in the blood” story.
While therapeutic apheresis is commonly used in Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy (CIDP), and myasthenia gravis, it is not considered the standard of care for most autoimmune illnesses. A recent review stated that, with the advent of more effective treatments, TPE is now indicated only in severe cases of lupus and rheumatoid arthritis and “catastrophic cases of antiphospholipid syndrome” where other treatments have failed.
Finding the mystery element in the blood could open the door to far more effective treatments.
Conclusion

With no hard and fast answers we turn to immunoadsoprtion – which has been getting quite a run in Germany.
Efforts to cleanse the blood of pathogenic substances are proceeding in ME/CFS and long COVID. Two recent studies indicate (surprise, surprise) that this is anything but a simple process, as several different forms of blood cleansing exist. A recent placebo-controlled, randomized, etc., therapeutic plasma exchange (TPE) study, which removed the plasma and substituted it with something else, did not affect symptoms or biology in long COVID.
The (non-placebo controlled, non-randomized, etc.) INUSphersis trial, which used a filtration process, markedly reduced inflammatory, clotting, etc., findings and improved symptoms. Some doctors have also reported success with plasmapheresis.
- Coming up – a different kind of blood cleansing in “The Immunoadsorption Saga in Germany”






Never been convinced by this
The INUSpheresis® group used a double-filtration approach that filters the plasma of immune complexes, autoantibodies, cytokines, viruses, and toxins. Did the other studies filter for toxins?
Sounds expensive
I imagine it is, although it also seems kind of simple.
From what I can tell with TPE you remove the blood, centrifuge the plasma out – add some albumin back in – and send it back in.
With the other, I guess you remove the plasma, send it through a filter, and then send it back in.
£3,000 per session in London
(How) did it work for you?
The TPE study didn’t actually filter anything at all – it simply removed about 2/3rds of the plasma each session and replaced it. So it removed everything but always left some behind as well.
INUSpheresis cost a lot but worked for me – for maybe a week. That is, I was symptom-free despite performing many more activities than usual. But, whether due to exposure to pathogens on the flight home from Germany or to the physical stress of a dental appointment, I soon relapsed. I am convinced that I could live a normal-ish life if I had access to affordable apheresis (I doubt it has to be INUSpheresis) on a regular basis.
Very interesting! So getting rid of something in the blood helped temporarily! How many sessions did you have?
Whatever was in there just popped right back I guess. Still it’s encouraging.
I think the key is going to be to find what in the blood is causing the problem. Once that happens they should be able to target. Unfortunately, it’s not easy to do that but it has been done in other diseases.
The next blog is on immunoadsorption – which goes after antibodies – and has had some interesting results in some patients.
I had two sessions, with a day of rest between. I think that apheresis is just a treatment – not a cure – because whatever produces the deleterious agent in the blood is upstream. However, extrapolating from other experiences, I imagine that apheresis might induce a lengthy remission under the right circumstances.
By the way, if anyone decides to try INUSpheresis, they should shop around. The fees and add-on requirements differ wildly.
I think maybe the upstream producer of deleterious agents may be pathogenic bacteria in the gut. I have had no luck pursuing that path, though.
Everything went downhill for me after two back-to-back food poisonings in 1979. I was only 27 and still on my monthly cycle and I could tell my blood had changed. When I got back to the U.S., I spent two years going to doctors trying to figure out what had happened but they couldn’t find anything.
One thing we know – if there is a mystery substance in our blood that’s causing problems we haven’t found it yet – or if we have (I’m thinking those beta adrenergic antibodies) we’re not clear about it.
I was diagnosed with Antiphospholipid Syndrome (APS) in late 2022 and was informed that, in addition to being an autoimmune condition, SH2B2 is also involved, similar to its role in insulin signaling and other cellular pathways. Additionally, Adenosine 5′-Phosphosulfate (APS) and proteins like β2-glycoprotein I (β2GPI) play a role in the disease.
Plasmapheresis is typically considered only in severe thrombotic events—when APS leads to life-threatening blood clots that do not respond to anticoagulants.
I assume that’s the HELP version of plasmapheresis – which contains lots of heparin – and is designed to break up those clots. That’s the kind of plasmapheresis that the South African researchers – whose name escapes me – are interested in.
Hi Cort,
Yes, Dr. Beate Jaeger was the first to offer HELP apheresis in Germany. Prof. Resia Pretorius from South Africa visited Germany in 2021 to conduct research. https://drbeatejaeger.com/apheresis/
Two years ago, Dr. Kiprov, who has worked in the field of Therapeutic Apheresis for more than 30 years and has published extensively on the subject, Start at 1:43:00 in the conference video. https://www.youtube.com/watch?v=y3ycGhlabxU&t=6181s He mentioned at 2:06:56 in a video that APS (antiphospholipid syndrome) can lead to catastrophic APS in some cases.
Additionally, a few other practitioners offer INUSpherese for Long Covid, chronic infections (e.g., Lyme disease), autoimmune diseases, and preventive detoxification.
https://lcacommunity.org/h-e-l-p-apheresis-faq
There are a few others offering INUSpherese for Long Covid, chronic infections (e.g. Lyme disease), autoimmune diseases and preventive detoxification.
https://lcacommunity.org/h-e-l-p-apheresis-faq
These are the average cost:
Cyprus: 9.500 Euros
Germany: 13.000 Euros
Switzerland: 15.000 Euros
South Germany (Bavaria -Bad Aibling): 18.000 Euros
Also, the Cleveland Clinic offers “Plasmapheresis and Plasma Exchange”
https://my.clevelandclinic.org/health/treatments/24197-plasmapheresis-plasma-exchange
While we are touching on work being done in Germany, I thought this research highlighted in the link below sounds interesting.
I have long been a skeptic of a viral factor perpetuating (as opposed to triggering) ME/CFS, but have softened my view and am more open minded on it now. This group will be looking at the role of EBV.
Speaking of viruses, it’s great that Polybio is trialling Truvada. I do wonder though why it hasn’t been trialled so far. Are there any positive off-label experiences with it?
https://www.bihealth.org/en/notices/ebv-may-trigger-autoreactivity-and-cause-me-cfs-bih-led-cure-me-research-project-awarded-federal-funding
Danke für den Hinweis auf das neue Forschungsprojekt der Charité / TU München, Matthias. Ich hatte davon noch nichts mitbekommen. In den letzten Jahren war mein Eindruck, dass die führenden Forscherinnen und Forscher in Deutschland die Idee der Herpesreaktivierungen als Treiber von ME/CFS nicht weiterverfolgen würden.
1.8 Mio sind nicht 18 Millionen – aber ich bin sicher, dass wir spätestens 2027 mehr wissen werden über ME/CFS.
Plus: Wenn die Charité und die TU München zu ME/CFS und Herpesviren forschen, dann hat das sicher eine Art Signalwirkung und wir werden vielleicht auch vermehrt virologische Projekte sehen. Das ist ja alles von der Immunologie her, was jetzt hier passiert.
I think we need to remember here that “autoimmunity as a driver” of ME/CFS is but a hypothesis. We know that autoimmunity is present in many cases of ME/CFS but it could be a bystander rather than a driver (e.g. reflecting chronic inflammatory processes). There is some evidence that certain auto-antibodies may correlate with clinical symptoms, but the link is far from strong or rigorously established through replication…
We also know that the hallmark of ME/CFS (PEM) is probably not mediated through autoimmunity as autoantibodies have a very long half life and take a long time to be produced…
We also have no single treatment directed against autoimmune processes that was definitely shown to work in ME/CFS… (BC007 failed, Rituximab failed, immune adsorption failed, plasmapheresis failed…)
This does not mean that we should not further explore this venue but it should caution against monomanic explanations of ME/CFS (here: “it´s an autoimmune disorder”).
Well and as always: I may be wrong, time will tell.
Thoughtful post as always Herbert.
I am interested in your thoughts on neuroimmune mechanisms. For me, an inflammatory state in the brain and CNS provides the best explanation for ME/CFS, and it has been the most convincing explanation for me for more than 15 years. This was reinforced for me last year with research showing IL6 in the brain sends signals to muscles in the periphery and readily exhausts them.
A key question is what perpetuates this centrally mediated state. Reactivated viruses or something else?
And the Nath-led study alluded to these things
Hi Matthias,
IL-6 (Interleukin-6) plays a significant role in viral infections, the immune system (including autoimmune diseases like lupus), and muscle disorders such as dysferlinopathies. Dysferlinopathies are a type of muscular dystrophy caused by mutations in the DYSF gene, affecting muscle membrane repair.
I believe Professor Spuler and/or Helena Escobar could provide you with more insight on this topic.
https://www.mdc-berlin.de/news/press/developing-crispr-therapy-muscular-dystrophy
Best, Sieglinde
Link with previous rapamycin blog:
https://pmc.ncbi.nlm.nih.gov/articles/PMC10731051/
“In dysferlinopathy, the autophagy program has been found to be activated for degradation of mutant dysferlin aggregates (15). Inhibition of mutant dysferlin aggregate formation in the ER by rapamycin, an mTOR inhibitor, has been reported in a cellular model (63).”
Thank you, Anon.
Unfortunately, I’m all too familiar with the DYSF gene, as I have several confirmed markers.
If you like, see the markers in RED: https://swaresearch.blogspot.com/2025/01/dysferlin-protein-key-roles-locations.html
@Matthias, thank you – my own thoughts how neuroimmune mechanisms may be caught in a perpetuating loop her:
https://www.kinder-verstehen.de/wp-content/uploads/Mini-Hypothesis-ME.pdf – and yes, I think viral reactivation plays a central role. Best, Herbert
Thanks Herbert. I think the parallels with TBI are very interesting.
Did you see this study that links interleukin 6 in the brain/CNS with muscle weakness? As if the interleukin 6 in the brain is sending a remote signal to the muscles
https://meassociation.org.uk/2024/07/neuroscience-infection-brain-inflammation-triggers-muscle-weakness/
Thanks Matthias, yes I have seen this – here is another summary: https://theconversation.com/brain-inflammation-may-be-the-reason-behind-muscle-fatigue-after-infection-and-injury-239817 – these brain-periphery connections are central imho, best H
There’s still some learning here, Cort. Centrifuges separate particles based on density, while filters work on particle size. The contrast of the results tells us something about the characteristic(s) of the blood borne entity that is implicated in these invisible illnesses. Hopefully Ron Davis and others take note of this.
Dr Isaac Eliaz in Santa Rosa, Ca offers aphaeresis treatments. I have not tried them myself, but here is the link to his clinic.
https://www.amitabhaclinic.com/for-the-patient/therapeutic-apheresis/
I research far and wide to find treatments that may help with my symptoms of ME/CFS and Long Covid. One of the best resources I have found is My Lady of Lyme https://www.ladyoflyme.com/ . Even though the blogger has lyme disease, there are many overlaps with my symptoms. This blog is where I found out about the natural blood thinner bulouke and an excellent chewable disgestive enzyme. Most recently, I have started drinking aloe juice every day as recommended on the site for digestive problems. I am only into week two, but it has been like a miracle. It coats your digestive tract. A friend said that aloe juice is widely used in the Puerto Rico for stomach problems. I drink it straight but if you don’t like the taste you can mix it with some juice.
Interesting that you used Bulouke Betty. I have had permanent improvements to cognitive function using a combination of lumbrokinase, serrapeptase and nattokinase. My intention was to target possible microclotting and fibrin plaques.
Hi Rosiebee,
How my of the serrapeptase and nattokinase do you use? Do you get these products from Amazon?
Sorry for the late reply Betty, I seemed to have missed your question somehow. I purchase these products from The Finchley Clinic UK.
I started with just one product at the lowest dose and increased very slowly. Then I added the next product in the same way (at the same time), and then the third. I ended up tolerating surprisingly high dises: Nattokinase 4,000FU; Serrapeptase 1 million IU; Lumbrokinase 400,000FU. All delayed release.
I stayed at these doses for several months, until I could see no obvious further improvements. And tapered off again.
The only thing we can conclude is that what is filtered out in terms of substances is not the thing that is in our blood that causes the disease. There are multiple methods to remove substances etc.. If these fail we at least know what is not the cause. So the ‘something’ that is in our blood and causes the disease remains a mystery….
Negative studies also provide important information
I’m wondering if something as simple as a leaky gut leading to inflammation and a person’s immune system to go BIZZERK…or
something as simple as SIBO which would mean toxins being absorbed by the small intestines,where toxins are not supposed to be. Ive always read that the small intestines are a very sterile environment
tested positive twice to D-LACTATE ACIDOSIS then negative on the NHS test.
The first private blood test was high 480UL range is 120-246UL, the second one was 279UL.
I still think that the Toxoplasmosis Ghondi parasite could also go undetected in regular testing
I just got my blood results after giving 15 tubes of blood through three sticks in my hand. My veins are awful and the blood stops flowing after a while. Oh well, enough whining.
I have consistently high homocysteine and low CO2 levels along with elevation in various tests of EBV. I am not sure if EBV is a cause of my illness or a marker for immune deficiency.
I just wondered if anyone else has this profile.
The something in the blood appears to me to be a good focus of research. However, I do think that research shouldn’t just be looking for “1” thing. It’s just as possible that there are a number of items like, auto antibodies, cytokines corrupted immunoglobulins creating the problem. I wish there was a way to speed this all up.
Good point! It may very well be a bunch of things.