

Two medical practitioners/researchers, Dr. Bested and Alan Logan, with a long history of involvement in chronic fatigue syndrome (ME/CFS), have written a series of three review papers suggesting gut flora and gut issues impact the fatigue, pain and mood issues present in ME/CFS and fibromyalgia. In this blog we complete the review of those papers.
-
Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part II – contemporary contextual research
-
Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part III – convergence toward clinical trials
How important is the gut in ME/CFS/FM? Clinicians specializing in ME/CFS appear to think its very important – at least in a significant subset of patients. A recent Jason survey that combined frequency and severity symptom scores to get a single all encompassing symptom score found that gastrointestinal symptoms were in the second tier of symptoms; not as prominent as unrefreshing sleep or post-exertional malaise but occurring with a similar degree of frequency and severity to concentration problems and muscle pain, and more important than symptoms such as tender lymph nodes, dizziness and fever and chills.
Now for the overview of the review papers.
From Bottom Up to Top Down
By 1930, the authors indicated that the idea that gut issues could cause depression, fatigue and other problems was mostly dead, replaced by the notion that depression and anxiety were the source. Not for another seventy years, until Logan’s proposal in 2003 and 2005 that chronic fatigue syndrome and depression could be effected by gut flora and other gut issues was the idea of gut manipulation broached.
Inflammation
The authors propose gut caused inflammation may be play a key role in some fatigue, pain and mood disorders. The idea that inflammation may play an important role in these disorders is not new; the authors are simply proposing that the gut could be a prime source of that inflammation.
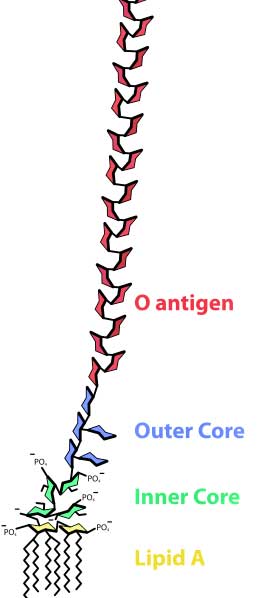
Some studies suggest levels of bacterial endotoxins called lipopolysaccharides are present in ME/CFS
Studies involving the administration of small amounts of bacterial endotoxins, many of which can be formed in the gut, paved the way for the modern recognition that leakage of materials out of the gut into the bloodstream could have significant effects. Very low levels of toxins produced by Staphylococcus, for instance, have been shown to cause acute anxiety, depression, cognitive problems and increased pain.
Some toxin leakage across the intestinal walls may always be present, and healthy blood typically contains small amounts of bacterial endotoxins (lipopolysaccharides – LPS), but higher endotoxin levels are associated with increased cardiovascular risk and the development of diabetes. (Several cardiovascular risk factors are increased in ME/CFS as well). These endotoxins can trigger elevations of cytokines that are associated with ‘excitotoxic neuronal overstimulation’ which sounds very much like the glutamate imbalance Marco has been proposing in his neural inflammation blogs.
LPS-induced inflammation has been shown to increase the activity of a tryptophan-degrading enzyme that has been associated with depressive symptoms and indirectly, anxiety. LPS, in fact, doesn’t need to elevate cytokines to make an impact on the brain; simply by damaging the blood-brain barrier LPS have been shown to allow all manner of toxins and pathogens entry to the brain. (Dr. Mady Hornig suggested the blood-brain barrier may be impacted in ME/CFS). In fact, LPS can inhibit the process of removing neurotoxins from the brain and can potentiate future cytokine releases in reaction to stress.
Several studies by Maes suggest increased endotoxin levels are present in chronic fatigue syndrome and treatment protocols designed to reduce them can be helpful. Maes has also found increased endotoxin levels in depression and believes gut derived inflammation plays a role in both disorders. He describes a complete remission of chronic fatigue syndrome using antioxdants and a ‘leaky gut diet’ in a young girl with high levels of IgM antibodies against enterobacterial LPS in this case report.
Gut Leakage
Given that LPS are common in the gut, it’s perhaps not surprising that increased intestinal permeability is associated with fatiguing, pain producing and sometimes mood altering illnesses such as ME/CFS, fibromyalgia, complex regional pain syndrome, insulin resistance, autism and obesity.
Remarkably, and this is something we’re going to focus on later, studies have found that exercise can induce a small amount of gut dysfunction even in healthy people. Not surprisingly, given the predominance of females in most of these disorders, women are more prone to experience increased gut permeability after stress than men.
Small Intestine Bacterial Overgrowth (SIBO)
Small intestinal bacterial overgrowth (SIBO) is just what it says – higher than normal levels of bacteria in the small intestine. Low gastric (stomach) acid levels are found in depression and increase the risk for small intestinal bacterial overgrowth (SIBO). SIBO has been found in ME/CFS, FM, IBS, insulin resistance and a disease called erosive esophagitis (reflux disease). The authors suggested that depressed gastric reflux patients respond poorly to traditional remedies because their gastric reflux is driven by bacterial overgrowth in the intestines.
Register for the CFIDS Associations Gut Microbiome Webinar featuring the results from a pilot study comparing the complete gut flora before and after exercise. Register here
SIBO, which may very well be common in ME/CFS/FM, can interfere with the absorption of proteins, fats, carbohydrates and nutrients, can produce toxic elements and can increase gut leakage. Small wonder, then that gut researchers assert SIBO is associated with fatigue and mood changes along the with diarrhea and bloating. But the authors noted that the body-mind connection goes both ways; stress has been found to encourage the overgrowth of bad bacteria and increase intestinal wall permeability as well.
(A small 2008 study found of SIBO was present in about 30% of people with fibromyalgia and a 2004 study found 20% prevalence and noted that SIBO was associated with increased pain. )
The Modern Diet and the Gut
Diet may have a crucial impact on the gut. Modern diets with their low variety, low levels of fermented foods, high proportions of fat and sugar, and low fiber may be particularly hard on the gut. Studies have found greater bacterial diversity (the more diverse the bacteria in your gut the better) and higher counts of good bacteria in rural Japanese populations eating traditional diets featuring fermented foods than in urbanites. African children living in traditional subsistence cultures also have more good gut bacteria and a greater diversity of bacteria than Western European children. The authors propose that our modern diets create ‘inflammatory microbiomes’ in our gut flora.
One interesting study using a dye found that high saturated fat diets allowed the dye entry into the hippocampus of the brain (!), suggesting that this type of diet both increased gut leakage and reduced blood-barrier permeability. Rodents fed Western type diets have been shown to spring leaks in their blood-brain barriers as well. Several studies finding reduced depression and anxiety levels in people switching to the Mediterranean diet with its high proportion of vegetables, olive oil/nuts and fiber, and low intake of red meat and processed foods, demonstrate the power of diet to influence mood and well-being. (Mediterranean-type diets are also associated with reduced cardiovascular risk, which, of course, is associated with inflammation.) Studies indicate that high fiber, low saturated fat diets can reduce endotoxin levels in the gut in as little as one month.
- Dig Deeper: The Best Diet For ME/CFS/FM
Fermented foods (sauerkraut, kimchee, fermented pickles, kombucha, miso, etc.) contain Lactobacillus strains that can prevent intestinal permeability, increase antioxidant levels, and reduce stress-induced LPS levels. L. plantarum, for instance can reduce the pain of IBS and reduce oxidative stress levels in smokers. Providing L. plantarum to animals increased their gut diversity and had anti-obesity effects later in life.
The Missing Link? D-Lactic Acid
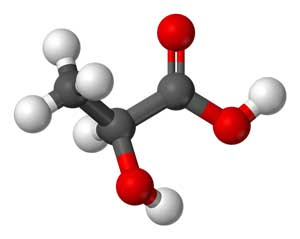
The overproduction of D-lactic acid could play a key role in gut derived fatigue and other central nervous system issues
A gut study found significantly higher levels of enterococcus and streptoccocus species in the fecal samples of chronic fatigue syndrome patients vs controls. Interestingly, upon exposure to glucose, these bacteria produce a form of lactic acid called D-lactic acid that is more difficult to break down and more prone to leak through intestinal membranes. D-lactic acid also appears to be able to move through the blood-brain barrier and interfere with neuronal energy supply. Animals with carbohydrate intolerance and high levels of D-lactic acid exhibit anxiety, aggression and poor memory. All these problems occur at D-lactic acid levels previously thought to be innocuous.
Given its ability to penetrate the blood-brain barrier, the authors suggest D-lactic acid may be the missing link in correlation between gut issues and fatigue, cognitive and mood problems. They propose that SIBO and increased intestinal permeability in a variety of disorders (including ME/CFS/FM) boost D lactic acid levels in the bloodstream and subsequently in the brain.
Gut Manipulation – Sometimes A Tricky Business
In short, the well-meaning lay advice to supplement with prebiotics , or the wrong types of probiotics, may have unintended consequences to sub-groups of patients within the modern day ‘entersothenia’ spectrum.
Like the immune system, the gut is a complex area and altering it can have unintended consequences. Different species of Lactobacillus have different effects, and some may be harmful. If D-lactic acid is an important player in gut-induced fatigue and mood changes, then probiotics should be selected for Lactobaccillus species that produce reduced D-Lactic acid levels and higher L-lactic and stay away from Lactobacillus species that do the opposite, such as L. delbruecki.
Too much prebiotic fiber, in the wrong person, could produce fermentation that produces higher D-lactic acid levels. They note that one popular prebiotic compound – beta glucans- was recently shown to increase not decrease intestinal permeability (woops). Increased intestinal propionate production due to prebiotics can result in locomotor slowing and induce a neuro-inflammatory response in the brain. People who have trouble with fiber or bran might be warned to stay away from these substances.
Gut Dysbiosis
Animal studies suggest that gut dysbiosis results in anxious, cognitively challenged states in animals and probably humans. Brain studies suggest that gut toxins activate the limbic system of the brain (including the fear center of the brain, the amygdala), probably via the vagus nerve, the center of parasympathetic nervous activity in the body. (Anxious behavior in rodents exposed to gut pathogens disappeared after their vagus nerve was cut.) Bifidobacterium longum prevented anxiety behavior in these animals. Lactobaccilus rhamnus induced reductions in anxiety behavior associated with changes in GABA levels in the brain, suggesting again that the brain is much closer to the gut than we think. The fact that vagus nerve excision extinguished those changes suggests the gut-vagus nerve-brain connection is a key one.
With their massive effects on gut flora, antimicrobials (antibiotics) have been shown to reduce anxiety behaviors in mice, and the authors suggested that the effectiveness of the antibiotic minocycline in depression may be due to its ability to rejigger gut composition. Minocycline and probiotics are more effective at reducing intestinal inflammation and permeability than either alone.
Probiotics
Animal studies have provided substantial evidence that probiotics can help reduce stress and enhance health.
Bifidobacterium infantis and lactis have been shown in rodent studies to reduce pain and even to reduce the level of pain nerve signaling from the body to the brain. B. lactis, moreover, reduced intestinal permeability and blood endotoxin levels. As L. farciminis ‘abolished’ hyperalgesia (hyper-sensitive pain states) in an animal model, it reduced limbic activation (amygdala, PVN) as well. L. paracasei‘s ability to prevent antibiotic-caused pain states without restoring Lactobacilli levels overall indicated that small changes in gut flora can have significant effects. Not only did L. farciminis reduce both gut permeability and endotoxin levels in one study, but it also reduced neuroinflammation
Bifidobacteria reduced cytokine levels and normalized brain hormone levels in an animal exposed to psychological stress. Forced exercise studies seeking to drive laboratory animals into a chronically fatigued state found that L. acidophilus reduced immobility and fatigue while reducing oxidative stress levels in the brain and TNF-a levels in the blood.
People with fibromyalgia should note that alterations in the normal gut microflora can increase the release of substance P, a key pain mediator in the brain. Even slight elevations of substance P in the blood are associated with increased anxiety, depression and aggression.
Research into cannabinoid effectiveness in pain relief is dismally low, but it’s clear that cannabinoid receptors affect anxiety and pain and can even limit endotoxin-produced blood-brain permeability. That’s noteworthy given preliminary findings that probiotics appear to affect cannabinoid receptor expression.
Human Trials
Probiotics
In what may be of special relevance to ME/CFS/FM, L. rhamnos us IMC 501 and L. paracasei IMC 502 reduced oxidative stress associated with intense physical activity. A psychological stress test found that women taking probiotics had reduced ’emotional arousal’ and reduced activity in their insula, a part of the brain implicated in ME/CFS/FM.
Conclusions
While researchers have isolated probiotic strains that appear to be able to lower endotoxin levels, reduce oxidative stress and inflammatory cytokines, improve ones resilience to stress, affect neurotransmitter levels, reduce gut permeability and lower toxin levels, the field is still in its infancy. For one thing, animal trials are the norm and cannot mimic the complexity found in humans. The bad news was that the authors projected that decades of work will be needed to determine how specific probiotic strains may interact with each other and the gut to affect health. The good news is they believe experimental evidence had been gathered to warrant extensive human trials.
Diet, drugs, stress and a complex gut flora all are part of a complicated gutsystem that needs more study
Much more data on the effects of specific strains is needed, although they cautioned that an ecologic approach to microbial manipulation is also needed. The gut is home to many types of bacteria and they warned that supplementation with a single or a couple strains of bacteria, no matter how beneficial they are, could ultimately diminish microbial diversity. A focus on a few commercially patented strains ignores the microbial richness that accrues from good dietary choices. They noted that fully 35% of lactic acid bacteria found in raw food survives the acid bath in the stomach. There is no substitute for good dietary practices, which apparently include a sufficient quantity of raw and fermented foods.
Probiotics are not a be-all and end-all. They are not going to replace antidepressants; nor did the authors envision them as first-line treatment for many conditions. They’re simply part of a complex mix of options for patients that need to be explored further. Diet, for instance, may play a large role in how effective probiotics may be. The authors did not anticipate that probiotics would significantly help a sedentary person with depression living on a fast food diet and taking pharmaceuticals that blasted the gut microbiome. The odds, they felt, of a single strain of probiotics providing ‘clinically meaningful and longlasting benefits’ was probably not high, and they cautioned against the unsubstantiated claims of probiotic businesses regarding anti-aging and anti-stress effects.
The authors closed the paper stating that probiotics are not a panacea, but the experimental evidence is encouraging and they deserve and hopefully will receive much more focus in the future.








Supplemental digestive enzymes will be a critical part of a gut repair protocol. Even more so than probiotics, I predict! Digestive enzymes with a meal and in between to digest pathogens. Liver cleanses will be important. There’s light at the end of the tunnel with this approach. RP
For what it is worth, half a year of home-made soya yogurt appears to have been helpful to me. I suspect it has given me more energy and the ability to combat stress better. I made it just like ordinary yogurt, with a soya yogurt starter.
Can’t hurt, might help.
Are you using a yogurt maker, Suella? I’m planning to get one…Any recommendations?
Agreed. 100%. The gut is the first to meet and greet, and metabolize food (including toxic substances now prevalent in the American diet) for cellular energy. I wish to FDA would pay closer attention to this than holding physicians and chronic pain patients hostage by promoting BiG pharma’s drive to raise their bottom line promoting the use of harmful antidepressants for everything from pain to depression that has not been diagnosed by a qualified psychiatrist. An atrocity when our government turns a blind eye to protecting those they are hired to serve. It is always about money. One must ask why autoimmune disorders are on the rise. We need unbiased research for our illnesses. TY Cort.
quote: “Like the immune system, the gut is a complex area and altering it can have unintended consequences. Different species of Lactobacillus have different effects, and some may be harmful. If D-lactic acid is an important player in gut-induced fatigue and mood changes, then probiotics should be selected for Lactobaccillus species that produce reduced D-Lactic acid levels and higher L-lactic and stay away from Lactobacillus species that do the opposite, such as L. delbruecki.”
Oh my. I did a fecal transplant which I believe made me worse. I was always a no growth for lactobacillus and ever since the transplant I am now a 3+ which perhaps is good on paper however judging from the quote above I could have done more damage than good if they were the wrong types. what do you think? Am I screwed?
My guess is no…I don’t know alot about fecal transplants but my guess is that they are screened for good/bad bacteria and yours was probably loaded with the good stuff (?)
Oh he was thoroughly screened and had an excellent guy profile. but really who knows what else people carry. Overall not pleased with my decision. But what’s done is done. I could have declined for no other reason other than this illness tends to wax and wane by nature….
Interesting stuff.
As someone with IBS since the start of my illness I can well appreciate (and it’s my subjective experience) that gut problems can precede mood, cognitive and ‘energy’ disturbances.
Also interesting about malabsorption. Back around 1990 I had full upper and lower GI tests (pre a ME/CFS diagnosis and attempting to treat the IBS in isolation) and all was fine except for fat malabsorption which, because it wasn’t due to any of the three standard reasons, was classed as ‘idiopathic’ and not reported back to my GP.
Unfortunately, as you clearly state Cort, there’s no clear cut remedy at the moment.
I’ve had gut issues since I got ME/CFS but not bad ones until lately. My gut, thought, was never in particularly good shape pre ME/CFS; its the one area that was out a bit in an otherwise very, very healthy person. As time has gone on they’ve gotten worse – lots of bloating and pain even as my stress has reduced a bit.
Timely post, Cort. Couldn’t agree more. My next blog is going to talk about my own experience with ignoring my gut for too long, how it almost killed me last December (emergency 5 hour surgery and a double colon resection) and the gross but revelational discovery as a result that I probably had parasites for years. To anyone who has had doctors act like those symptoms in your belly are innocuous, fire them immediately. When it is estimated that over 80% of the population today has some form of intestinal worms and parasites, my guess is 100% of CFS/M.E. patients have them. This is the World Health Organization’s biggest secret – they don’t have any new drugs to combat these antibiotic-resistant helmiths and they are overtaking North America in epidemic proportion. And guess what the latest drug to fight human cancer is? Ivermectin- an anthelmetic (de wormer) designed for animals.
If they’re using a de-wormer designed for animals it sounds like they’re really behind in this area.
My gut test years ago showed two parasites, entamoeba histolyca and giardia. The medication gave me really large erections (which I’d really been missing) but then the side effects scared me off. I wish I’d pushed on now.
I came down with Giardia on a backpacking trip a couple of years before I got sick. I recovered but that bug was still there apparently and I assume its still there now.
My guess is that a pretty good subset of people with ME/CFS had bowel problems that coincided with their onset.
Was just reading about ivermectin.. I think we gave it to our horses and cows .. more as preventative.. but why not humans to stop parasitic infection ie covid?
I believe inflammation and leaky gut is the root of CFS and the best treatment is the SCD diet.
Some times ago, I was diagnosed with crohn’s disease. I was feeling so tired all the time that even breathing was an effort. I have never been diagnosed with CFS but I had all the symptoms. It’s not funny when you’re 25 years old and need to go back home to sleep at 7pm – every days!
All the medicines prescribed by my doctor made me feel worse than ever – this is when I started to search on the internet if there was not some kind of natural treatment. This is how I found out about SCD – and it’s only after I started to follow this diet that my digestion got better and my fatigue progressively disappeared.
I still have leaky gut, I am sure of this because of some plaques that appear on my skin every time I eat nuts. But since I removed all the offending foods that are illegal on SCD, I have become a completely different person: I can go out on the evening then wake up very early and work all day long! I can go out with my kids and run after them.
I’m sure that anyone who has CFS has a leaky gut. It makes sense to only eat foods that are not going to release toxins in your blood stream and cause a lot of disturbing symptoms.
Excess fatigue is one of the symptoms reported for leaky gut: http://nutritiongang.com/leaky-gut-syndrome/
And SCD has helped more than one to get healed from CFS: http://www.scdiet.org/7archives/scd1/scd037.html
I know that SCD is not easy to follow, but it’s really worth it. I am so grateful that I have found about it, I hope that all of you suffering from CFS will give it a try. You don’t really live when you’re feeling excessively tired all the time!
Thanks so much Hakim for passing that on…For others SCD refers to ‘specific carbohydrate diet’. I have no question at all that this is a very important area for some people with ME/CFS. Thanks again and congratulations on getting better.
You’re welcome Cort and thank you for all your great articles! Concerning the meaning of SCD, there’s a typo in your reply above, you forgot one important letter on the last word which makes the meaning a little bit scary 😉
Whoops – thanks
Cort,
I’m reading an article on another blog that is linking CFS to leaky gut. It seems the author has a lot of experience (he says he has been treating this for about 20 years).
http://www.charismamag.com/life/health/17641-how-to-recognize-leaky-gut-syndrome
Maybe this can help.
Take care!
I am starting on the SCD next week in a hope to heal my gut faster and more permanent.
I must admit that reading the article it confused me immensely in which probiotic strains to avoid and which to favor. Also in view of the latest research you published on gut microbe and CFS I thought I better be carful what I take.
Curt I love your website but I struggle with understanding the summaries and practical conclusions I could follow like with the probiotic. ( sorry foggy brain)
Maud xx
This is a difficult area Maud. I confess I don’t know – except for L. reuteri – which strains to take either. If you click on the gut in the treatment categories – see righthand side of the page in the side bar or look for Ken Lassesens blogs they might help.
I recently talked to a nutritionist who works with ME/CFS about probiotic. She said to take them very slowly. I didn’t about six months ago and had bizarre symptoms (dizziness, heart fluttering). I never dreamed that probtiotics could have that effect. The good news is that if you take them slow you should be able to build up over time and they can be quite helpful.
Good luck!
Thank you very much Cort.
Xxx
I stumbled upon this article looking for something that might help my friend who has fibromyalgia. Does Ivermectin help?