



“Sometimes I think I’m so overwhelmed that the little, simple things that other people do or say really set me off emotionally, which we know only makes the pain worse.“ Person with Fibromyalgia
One of the most puzzling aspects of my Chronic Fatigue Syndrome/Fibromyaglia has been my body’s unusual sensitivity to stress. Oddly, it’s not the big stressful events, it’s the smaller, day to day stuff that’s been so confronting. Every day, it seems, is a roller coaster ride of symptoms that often flares up with the slightest stressor.
A review article suggests that with regards to the body; infection, physical or psychological stress – it’s all the same.
Depression, ME/CFS and FM
We know that while depression is at times found in Chronic Fatigue Syndrome and Fibromyalgia the disorders are very different. The search for answers, the tendency to overdo in ME/CFS and FM, the different type of HPA axis activity, and, perhaps most strikingly, the inability of depression or anxiety to influence ME/CFS and FM study results, indicate the diseases have large differences.

ME/CFS and Fibromyalgia are NOT depression; but that doesn’t mean an inflammatory milieu doesn’t underlie all three.
A recent study finding that neither stress nor mood disorders increased an adolescent’s chances of coming down with ME/CFS after an infection indicates chronic fatigue syndrome is not a behavioral disorder. Studies assessing how much mood disorders contribute to study findings in ME/CFS rarely find that they affect them at all.
This doesn’t mean that some aspects of depression may not be relevant. Rates of depression appear to be fairly high in both disorders, stressful events can trigger any of the three disorders, and stress exacerbates each. Maes has suggested that depression and ME/CFS as well as other disorders share a common inflammatory basis.
It’s that inflammatory basis of these disorders that drives the discussion in this review article.
From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Slavich GM, Irwin MR. Psychol Bull. 2014 Jan 13. [Epub ahead of print]
Inflammation, Depression, Sickness Behavior and Micheal Maes
The close similarity between the symptoms of ‘sickness behavior’ and those found in depression has suggested to researchers that depression could be immune mediated; i.e. is caused by cytokines, and, indeed, hundreds of animal studies show cytokines cause hypersomnia, fatigue, anorexia, cognitive problems, reduced motor responses, and reduced social and exploratory behavior.
Essentially these studies indicate that increased inflammation produces results in animals that are very similar to what major depressive disorder looks like in humans. In the early 1990’s Michael Maes – now doing a great deal of research on ME/CFS – was one of the first to put forth this theory. (Several Maes papers propose similar inflammatory processes underlie ME/CFS and FM and depression. )
Stress – A Major Inflammation Trigger
A early clue in the inflammation/stress connection is the fact that inflammatory disorders such as rheumatoid arthritis, chronic pain, cardiovascular diseases, diabetes, and obesity appear to be particularly associated with depression. This suggests that inflammation may both set the stage for a variety of disorders and increase the risk of mood changes at the same time.
Infection and physical injury have long been known to trigger inflammation, but recent work suggests that ‘stress’, including psychological stress, can invoke inflammation in the body as well.
A Turned-on Anticipatory Immune Response Spells Trouble
“We have proposed that the primary innate immune system response to contemporary social stressors involves up-regulation of proinflammatory immune response genes, which combat bacteria and other extracellular pathogens, and a reciprocal down-regulation of antiviral immune response genes, which target intracellular pathogens such as viruses” (Irwin & Cole, 2011).
Intriguingly, the inflammation is initiated by the immune system arm that has been most implicated in ME/CFS: the innate immune system that immediately reacts to pathogens.
These researchers assert, though, that the immune system doesn’t need a pathogen to get into gear, it simply needs the possibility of a pathogen or an injury or simply a dangerous situation. In the cave man times the anticipatory immune system might get triggered by a dangerous animal; in modern times it can get triggered by any situation that’s perceived as a potential threat. (Such as simply the thought of a walk around the block for a person with ME/CFS or FM?)
When triggered, the anticipatory immune response mobilizes cells of the innate immune response to start moving to sites of potential injury or infection (i.e., to the mucosal areas such as the gut, mouth and nose where pathogens first contact the body).
Continual triggering of the anticipatory immune response via infection or other stressors up-regulates immune factors that fight bacteria and extracellular pathogens (Th2 response), and down-regulates immune factors that fight viruses (Th1); i.e., it reduces our ability to fight viruses.
(This ‘increased Th2/decreased Th1 response has been proposed many times in Chronic Fatigue Syndrome.)
But how did that response get locked in?
Out of Whack Stress Response = Out of Whack Immune System
The key to this chronic immune imbalance, the authors believe, is a stress response that causes an inflammatory response to get triggered quickly and easily.
HPA Axis and Autonomic Nervous Systems
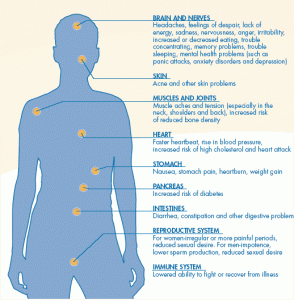
Do the stress response systems in ME/CFS and FM set up for an ongoing activation of their ‘anticipatory immune response’?
Activation of the HPA axis usually reduces inflammation, but under conditions of constant stress, a phenomenon called ‘glucocorticoid resistance’ may occur. Glucocortoid resistance may or may not be associated with ME/CFS or FM, but low cortisol, which has similar effects, is.
Either way, the HPA axis becomes a contributor, not a hindrance, to inflammation, and in fact, HPA axis associated inflammation is believed to contribute to a variety of disorders including cardiovascular diseases, rheumatoid arthritis and other autoimmune disorders, asthma, PTSD, and generalized anxiety.
The sustained sympathetic nervous system response seen in the heart rate variability results in both ME/CFS and Fibromyalgia, also results in a Th2 dominant immune response.
The immune findings in Chronic Fatigue Syndrome then, may reflect a stress response that gets turned on quickly and easily.
A Locked in Stress – Inflammatory Response
Greater Risk in ME/CFS and FM?
Given the issues many ME/CFS and FM patients face getting locked into a hair-trigger inflammatory response might not be too surprising.
It turns out that stressful major life events (such as chronic illness, death of family member, divorce, or loss of one’s job) are the single greatest predictor for depression, and major life events that result in ‘interpersonal loss’ or ‘social rejection‘ are particularly powerful. People who experience a major life event that results in the severing of ties with a person are particularly at risk.
Many people with chronic fatigue syndrome and fibromyalgia, of course, experience losing friends, spouses, and even other family members post-ME/CFS. Rejection by the medical profession is another sort of ‘interpersonal loss’ that often occurs.

The big emotional stressors that modify health – rejection and isolation – are there in spades in FM and ME/CFS
With regards to psychological stressors, conflict, threat, isolation and rejection, all of which are greatly increased in ME/CFS, appear to be particularly effective at producing inflammation.
The weakened social ties may be particularly disturbing given that having stronger social ties can reduce the risk of mortality by up to 50%, a result that is only matched by quitting smoking. Socially isolated individuals are 2 to 5 times more likely to have increased levels of the inflammatory factor, CRP, than people who are not socially isolated.
(If this scenario is correct then many people with Fibromyalgia and Chronic Fatigue Syndrome are not only battling an inflammatory response that’s on a hair trigger, but the lack of acceptance and understanding both in the medical and public arenas (“But you look so healthy!”) means they also face personal issues that may increase the inflammatory component of their illness. Several studies have suggested that emotional distress triggers physical symptoms in ME/CFS.)
Inflammatory Disorders Show Up Again
It’s not surprising that each of these losses increases the patient’s risk of depression. What is surprising is how these types of losses affect some illnesses. Studies indicate that these types of stressors markedly exacerbate disorders such as asthma, cardiovascular disease, rheumatoid arthritis, and some cancers. What do these disorders have in common? They all have a strong inflammatory component.
This suggests that stress, whether it’s from an infection or is psychological enhances inflammation.
Inflammation Itself Causes Stress
It’s a bit worse than that. Studies indicating inflammatory states are associated with increased stress suggest that just being in an inflammatory state is emotionally stressful. Increased levels of IL-1B, for instance, are associated with increased feelings of stress and more difficulty maintaining a positive outlook, while Il-6 is associated with increased anxiety, depression, and anger,
Studies indicate that both early and later life stress is associated with increased levels of inflammatory cytokines (Il-6) and inflammatory markers (c-reactive protein aka CRP). Negative social interactions in daily life are also associated with increased IL-6 levels.
The same brain regions (insula and anterior cingulate cortex) appear to be engaged whether one is fighting off a bacterial infection or is exposed to stress. Importantly, these regions of the brain play a large role in determining how much pain we experience.
ME/CFS and FM Interlude (Not From Review Article)
All of these cytokines have been associated with ME/CFS. Il-6 appeared to help set the stage for entry into ME/CFS after infectious mononucleosis. Emotional distress in ME/CFS was most strongly associated with IL-6 levels in another. IL-6 was most associated with symptoms in another.
“Neural systems involved in processing physical pain (e.g., resulting from intense cold or heat) also play a role in processing social pain (e.g., resulting from social evaluation or rejection).”
It also appears that the parts of the brain that process physical pain also play a role in processing ‘social pain’. This, of course, brings up the question whether the over-active pain circuits in FM that increase pain in response to physical stressors such as exercise and even the touch of clothing also translate into greater physical pain in response to difficult social situations/negative thoughts?
Neuro-inflammatory Sensitization
The authors believe this integrated neuro-immune system primes an individual for further inflammation and further stress in a process called ‘neuro-inflammatory sensitization’, .
In the short term chronic stress activates the inflammatory response and parts of the brain that causes increased pain, social anxiety, hypervigilance and the anticipation of adversity. In the medium term this evolves into disrupted sleep, chronic pain, social withdrawal and a depressed mood, and finally, if it goes on for several years, to increased susceptibility to infections and inflammatory diseases, accelerated aging and increased mortality.
Reducing Inflammation – Reducing Stress / Reducing Stress – Reducing Inflammation
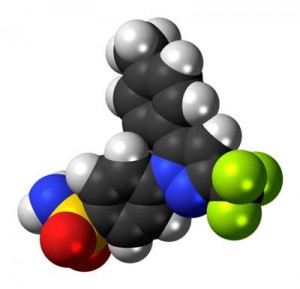
The anti-inflammatory Celecoxib proved surprisingly effective in treating some people with depression
If the authors are right then anti-inflammatories should be able to reduce stress, fatigue, pain and mood problems, and, in fact, it’s become clear that some antidepressants reduce pro-inflammatory cytokine levels. Celexicob’s strong performance in an antidepressant trial suggested how large a role the immune system may play in producing depression. (Celexicob plus an antidepressant had 50% greater results than the antidepressant alone. Pridgen’s use of Celexicob as an antiviral in his Fibromyalgia trial suggests another use for this anti-inflammatory.)
About 50% of depressed patients were classified as responders in an Etanercept (TNF-a suppressor) trial. Finally, in the much same way as we’ll see in the Antoni study below, a subset of patients (depressed patients with high levels of an inflammatory marker (CRP)) responded well to another TNF-a inhibitor, inflammable Infliximab. Infliximab improved anxiety, depression, rates of suicidal thoughts, fatigue, etc., in this heretofore treatment resistant group.
(The Gut Approach – Levels of C-reactive protein (CRP), tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) in ME/CFS patients dropped significantly with six weeks of bifidobacterium infantis 35624 supplementation. The study did not, unfortunately track symptoms changes, but it did suggest the inflammatory milieu and hence the depression, etc. in ME/CFS could be driven by the gut.)
Targeting Subgroups That Benefit From Stress Management
“It appears that IL-6 may be a plausible cytokine “biomarker” for designating a neuroimmune dysfunction subgroup of CFS patients who are especially sensitive to emotional distress responses as they relate to symptom exacerbation. ” Antoni et. al
Like any other ‘treatment modality’ stress management techniques are only effective for some. Antoni et. al propose immune factors can determine which ME/CFS patients benefit most from stress management techniques such as relaxation, reappraising stressful situations, and having good interpersonal skills. They found these techniques were most effective in patients at the “tipping point” of neuroimmune dysfunction (those with high IL-6 levels) and did not make a difference in those with normal IL-6 levels.
Antoni et. al. point out some studies suggest that whatever gains occur from CBT do not derive from modifying one’s illness perceptions but from portions of the program that reduce emotional distress. CBT’s effectiveness may be limited, in fact, by it’s core focus on modifying ‘illness beliefs’.
A better approach might be to modify, at least in some patients, the emotional distress and the inflammation it promotes.
Conclusion
Physical and emotional stress may very well trigger the same physiological reaction in the body. With ME/CFS and FM patients displaying a kind of stress response that promotes inflammation and it’s symptoms (fatigue, pain, mood, cognitive problems), and dealing with inflammation both from a physiological and an emotional standpoint might be a good idea. Eventually immune tests may be able to target people most likely to benefit from mind/body therapies.








Interesting that you/they mention bifidobacterium infantis 35624, AKA the commercial probiotic Align. Every time I stop my Align for more than three days or so, my depression gets much worse.
There’s a lot to learn about the gut!
Good review (of the review) Cort.
I feel models such as this are getting close to providing a complete model for conditions such as ME/CFS : predisposing factors/microglial priming, a triggering inflammatory stressor, immune activation, HPA axis dysruption, central microglial activation – and all feeding back to maintain a chronic inflammatory state.
I found this recent review (again taking depression as the starting point) sets it out very clearly and also (coincidentally) throws diabetes/metabolic syndrome into the mix and intriguingly offers another explanation for the apparent gender bias that may begin in the gut (LPS translocation) :
Mind and body: how the health of the body impacts on neuropsychiatry
“Finally, potential sex-differences have been suggested when assessing the effects of LPS on cytokine gene expression. Indeed, females had increased hippocampal levels of IL-6 of TNF-α with respect to males after repeated administration of LPS (Tonelli et al., 2008).”
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3866391/
Thanks. This field is getting really big and I noticed as I was going through this review that several other large reviews have been done. The quote below is from the first part of the paper.
Notice how they start with ‘strong’ and notice how problems with the HPA axis appear to be ‘baked into’ psychiatric diagnoses as they stand right now. If you have a stress response disorder you, almost by definition, have a psychiatric disorder. That doesn’t seem quite right. (Shouldn’t they be endocrinological disorders?). Notice also how they immediately tied inflammation into these types of disorders.
T
“Strong and continually developing evidence now suggests a link between disorders which involve Hypothalamic-Pituitary-Adrenal axis (HPA) dysregulation and the risk of developing psychiatric disease. For instance, adverse or excessive responses to stressful experiences are built into the diagnostic criteria for several psychiatric disorders, including depression and anxiety disorders. Interestingly, peripheral disorders such as metabolic disorders and cardiovascular diseases are also associated with HPA changes. Furthermore, many other systemic disorders associated with a higher incidence of psychiatric disease involve a significant inflammatory component.”
The stress response do not react normal on stimuli. But what is the cause for this abnormal reaction? If you have addison disease this repons is abnormal to.
The paper I linked to mentions Addison’s and Cushing’s as opposite examples of diseases causing HPA dysfunction.
Why the abnormal response in us? Polymorphisms impacting on HPA reactivity or inflammatory factors such as TNFa (linked to sickness behaviour in primary biliary cirrhosis) or CRP, gender, ‘life stressors’, peripheral inflammatory conditions such as rheumatoid arthritis of diabetes, pathogens etc. No reason to suspect that the same mix applies in all cases (in fact highly unlikely) but it all results in the same state.
I’d favour a ‘proof of concept’ trial with something like minocycline and if it shows promise – work back from there.
Yes, that’s the big question isn’t it? I believe Broderick believes that an unresolved infection may be keeping ME/CFS patients systems stuck in an suboptimal state.
Thank you so much for this review! This explains my CFS to a ‘T’. It explains my father’s and son’s as well. I hold out hope the treatments described here may also help me. I am desperately eager to hear more!
I can agree ongoing inflammation is probably a major factor in “CFS”. However, I think this discussion is incomplete without acknowledging/exploring environmental toxins as a driver of ongoing inflammation. I put much more weight on my Chronic Inflammatory Response (CIRS) dx than my “CFS” one. Shoemaker has at least made an attempt to show causation. I still see no evidence of how to define “stress” and what is “too much stress”. Furthermore, such discussion allows an end to further investigation in to other causes of inflammatory “stress”, such as autoimmune conditions like Celiac, Sjogrens, Lupus, etc.
This seems to be the CDC’s position: ” emotional stress” is a big player in this condition and until proven otherwise (and we’ll let someone else do that) CBT and other mind body techniques are the best way to address it.
My experience with chemical sensitivities suggests you’re right. While the review focuses on demonstrating that negative emotions can trigger inflammation, it also concludes that inflammation (from any source, from infection, from the gut, from toxins) has both negative physical and emotional consequences. An interesting paper came out demonstrating that the same three cytokines in metabolic syndrome (IL-1B, TNF-a and IL-6) are associated with depressed mood, etc. They conclude that a low level inflammatory condition can have all sorts of effects.
I have had CFS/ME for 20 years with no pain. Then in December my oldest friend died suddenly from cancer (and I had lung cancer last summer even though I’m a non-smoker). I had a work deadline, had to pull Christmas together for our family, and was grieving my friend. My stress was overwhelming, and felt like a ball in my lower abdomen, for a week.
The following week I developed widespread pain for the first time. It is bilateral in both hands, upper arms and thighs. I am afraid that it will alway be with me, and it sucks!
I think the pain is from the severe emotional stress that I had that week when my friend died. Now my thymus is enlarged and I developed substantial pleural effusion as a complication of the lung surgery. The surgeon, pulmonologists and my internist all say it is from systemic inflammation, even though my CRP is 0.3.
I also have abnormal ANA with homogenous pattern. The rheumatologist is testing me for inflammation. Dr. Teitelbaum thinks I now have fibromyalgia. Oh joy! Stress is no joke. I could have avoided these effects by doing deep relaxation and guided imagery, massage, acupuncture and prayer, to destress. I blame myself for letting the stress get out of hand. If I had only known the effects, I would have addressed it aggressively to avoid this pain, which I still do not understand at all!
Hi, I have Sjogrens,Fibromyalgia,Peripheral Neuropathy,major depression,and cognitive problems are sneaking in….HELP! This article says it all.Now to find the right doc!
What a fantastic post! Thank you so much for sharing this.
I have struggled with managing stress most of my adult life. I suffered with depression and panic attacks for most of my adult life as well. So I find it very interesting that there has actually been some study on the relationship between stress and Fibromyalgia.
I was diagnosed with Fibromyalgia in 2011 though I suspect it existed far sooner than that. I can tell you sincerely that any higher stress or emotion (good or bad) *will* cause me physical pain. The worse the stressor, the worse the pain.
Noise is a huge stressor, or anyone having intense negative emotion near me (anger especially). I wear ear plugs 24/7 to tone down background noise as much as possible. I am also highly sensitive to chemicals via smell or if it is on my skin (must use natural products with no dye, alcohol or fragrance).
I also live in fear of being touched. I avoid crowds because I’m terrified of being jostled or bumped in to – it would cause a great deal of pain and make the terror worse. Social phobia x10 because of pain. Go figure.
So I have learned the hard way that I must keep calm and pay attention to the things that make stress, and therefor, pain worse.
I agree that stress triggers more inflammation. However, low energy levels and chronic pain make even small tasks stressful and cause psychological stress that – if well – one would cope with or ‘brush off’. When a person has energy he/she can cope much better with stressful situations of any kind. I personally feel that prolonged ME/CFS causes a downward spiral with patients coping mechanisms continually decreasing because of chronic inflammation, hormonal imbalances and an autonomic nervous system that is out of control. I am at the stage where even responding to this blog causes stress as I am tired after going to the hairdressers. Perhaps this is why there is such a difference between people who have just contracted ME and those who have been in a chronic state for several years – as I have. I believe that correcting hormonal and other imbalances would help get us out of this downward spiral but the endocrinologists and virologists don’t seem willing to help – we are lacking their support to bring about a higher degree of wellbeing, while we wait for a miraculous cure that may never come. Perhaps some of the treatments that work for lupus and other inflammatory diseases could also help. The NHS needs to wake up to the fact that most of us have multi-system imbalances that CAN be improved – if not cured.
I agree completely. It is the low energy that is causing the stress!
When my energy goes up – the problems with the little stuff all goes away. I really wonder if the problem is that in the low energy state the brain is in it just can’t figure out what to do…and it gets stuck on trying to track everything…It’s as if it doesn’t have the energy to filter out the little stuff and focus on the big picture.
Energy is the key to everything! I really think that. Dr. Lerner has said that as energy improves everything else improves.
Could you please tell me Cort who did the study about Celexicob and anti-depressants? (Or even if it’s possible to email it to me please?) I would like to know what doses of Celexicob they used
It was Pridgen and we don’t know, it turns out, the combination of drugs he used or the doses – BUT we should know within the month. 🙂
Great article! This definitely matches my experience. The only thing I would add is that I believe there is a genetic component that makes some of us more susceptible to stress response that ends up as ME/CFS/FBS and like some of the other chronic diseases. I’m wondering about the prevalence of the MTHFR mutation in ME/CFS/FBS. I saw a study where 200 people with chronic Lyme were tested. 198 had the mutation. Maybe it or other similar genes are the key. Once they are “unlocked” due to stressors we get the chronic symptoms.
Fully agree on that Lynn. A study is underway looking at the MTHFR mutation in ME/CFS. Should be interesting!
That’s incredible about Lyme!