



Many people with Fibromyalgia and/or Chronic Fatigue Syndrome combine prescription drugs with supplements, but this is one of the rare studies that combines both to maximize the benefits of each. The genesis of this trial, however, stretches back to the HIV/AIDS days when Dr. John Kaiser in the San Francisco Bay area was supporting HIV/AIDS patients’ health with supplements.
The HIV drugs that came out saved lives, but they also hit the mitochondria hard. When that happened he tweaked his immune formula and saw the toxicity problems — the neuropathy, pain, inflammation, and fatigue they were experiencing — reverse. A double-blinded study bore out his clinical findings: the HIV/AIDS patients’ immune systems showed improvement on the supplements.
People with Chronic Fatigue Syndrome, however, were a harder case. They showed signs of mitochondrial and immune dysfunction, but only a small number responded to his mitochondrial/immune formula. If they didn’t improve after a couple of months Dr. Kaiser would add what he called ‘a touch’ of methylphenidate to ‘catalyze’ the mitochondrial boosting elements in his formula — and often it worked. The drug/supplement combination produced far superior results to taking either the drug or the supplements by themselves.
He suspected that the supplements provided more energy by enhancing mitochondrial functioning, and helped overall by supporting the immune, nervous, and endocrine systems. With the energy production system in better shape, the low-dose stimulant actually had something to stimulate. Instead of depleting those systems, it engaged them. The patients had more energy without the payback they might see otherwise.
Ritalin (methylphenidate)
Ritalin is a drug that’s been around for over sixty years. (Check out a History of Ritalin here ) Ritalin increases extracellular levels of dopamine by blocking the transporters which remove dopamine from the system. Higher levels of dopamine at the nerve ending results in increased dopamine signaling.
This translates into increased activation of the reward and pleasure centers of the brain, reduced ‘hyperactivity’, increased concentration and reduced stimulus overload. Some people report increased feelings of well-being as well.
Successful Proof of Concept Trial
A proof of concept trial suggested he was on the right track. Over seventy percent of Chronic Fatigue Syndrome patients experienced at least a 25% reduction in their fatigue (CIS) scores, and this reduction was sustained over a three month period. A 34% reduction in brain-fog/concentration problems and a significant reduction in visual analog scores (a pain measurement) were also seen.
If you’ve tried methylphenidate (Ritalin) by itself without success, don’t think this means it won’t work for you. While a 2006 trial found that Ritalin (10-20 mg/day) reduced fatigue and improved concentration in only 17% and 22% of ME/CFS patients, the success rate may be far higher when this stimulant is combined with the appropriate supplement support.
From Rituximab to Valtrex to the Lightning Process, some people with ME/CFS always seem to respond in dramatic fashion, and this was true here as well. He mentioned one 33 year old woman who had been disabled and unable to work for seven years. She suffered from headaches, insomnia, and exercise intolerance. She could not get to an ME/CFS specialist for treatment. Six weeks into the trial she took on a full-time job and has not only continued to work, but since then has had a child, dropped the treatment — and was fine. He stated that several people who had been unable to work are now able to work.
The Current Trial
The real test, of course, is the placebo-controlled, double-blinded trial now underway. The trial is being run at four ME/CFS experts centers at Palo Alto, CA (Dr. Montoya – Stanford University), Manhattan, NY (Enlander? Levine?),Salt Lake City, UT (Dr. Bateman), and Fort Lauderdale, FL (Dr. Klimas). Dr. Montoya is interested enough in this study that he’s banking patients’ blood in order to perform sophisticated mitochondrial and immune tests, including change in oxygen consumption, if the trial is successful.
Showing improved health and improved mitochondrial functioning would be a huge win in several ways. It would validate that there are mitochondrial problems possibly present, provide a new and relatively inexpensive treatment option, and help to legitimize the disorder.
Nobody is saying it’s going to cure ME/CFS, but it could provide help and insights into what’s going on. ME/CFS physicians and experts have clearly embraced this work. The doctor thanked Drs. Podel, Bateman, Klimas, Natelson, Levine, and Montoya as well as Suzanne Vernon for their support during the two years it took to design the trial.
The trial includes taking the nutrient supplements and 5-10 mgs of Ritalin twice a day, a significantly smaller dose, Dr. Kaiser said than was typically prescribed. It requires four visits to the site over a period of 12 weeks. Volunteers will receive a $150 stipend to help with the costs of the visits. If you are a volunteer and you complete the trial, you’ll get an additional three months of the supplement free.
Key Need – Patients!
Somehow, patient recruitment is the most expensive part of any clinical trial, and with the debility and sensitivity to medications present in ME/CFS I’ll bet it’s worse in recruiting for ME/CFS. He noted that less than 10% of patients ever take part in a clinical trial, and 30% of clinical trials never succeed in recruiting anyone.
That brings to mind a Sildenafil ME/CFS trial begun maybe ten years by a researcher at UCLA. The trial, which was designed to increase blood flow to the brain (the big brain… not the little one typically associated with Sildenafil) made sense, but I’ve heard nothing of it since.
This trial has widespread support from ME/CFS experts. Let’s get it enrolled!
Synergy Webinar On Wednesday
“The Synergy Trial-Pursuing an FDA-Approvable Treatment for CFS” is a free one hour webinar that will be conducted by Jon Kaiser, M.D., Medical Director of K-PAX Pharmaceuticals on Wednesday, May 21st at 4pm EST/1pm PST.
This webinar will:
- Describe the treatment being studied by the Synergy Trial.
- Describe how the goal of this treatment is to healthfully improve mitochondrial energy production across all cell lines.
- Describe previous study data utilizing this intervention.
- Review key enrollment criteria for participating in the Synergy Trial.
Participants will also be able to take part in a live Q & A session with Dr. Jon Kaiser. The Synergy Trial is testing a hybrid therapy of an available medication (low-dose methylphenidate) combined with a potent CFS Nutrient Formula as a treatment for CFS/ME. Enrollment is ongoing at 4 research sites across the United States.
To register click: https://attendee.gotowebinar.com/regist … 6332231938


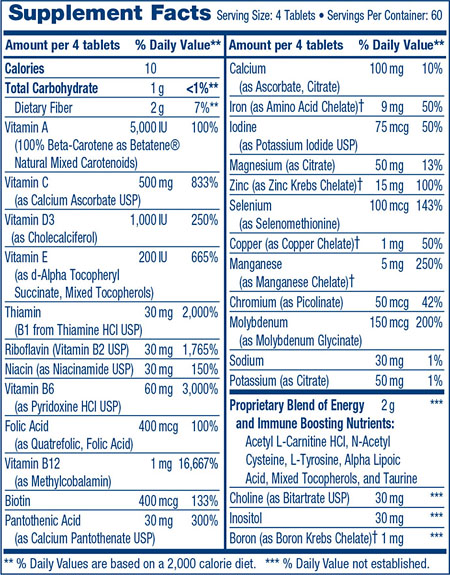






I was started on Adderall for “energy” by my psychiatrist – I was up to 40 to 60mg daily for 2 years. I was all for it, trying to maintain my career.
The effects were devastating (and I was on the appropriate array of mitochondrial support supplements). It wasted all of the precious reserve I had left from my once athletic physique and lifestyle. It wrecked my brain. Trying to wean off was the biggest nightmare I have ever faced (at least 4 months of withdrawal, after a gradual taper) along with a 30 lb weight gain.
Long term stimulant use causes permanent changes (aka damage) in the dopamine system of the brain. It overrides a symptom, but it does not effect a cure to the mitochondrial damage that has been done, and in my opinion, contributes to further damage to both the mitochondria and the adrenal axis.
I can certainly sympathize with your desire to use it – I would have done the same thing. Hopefully, this trial which features lower doses and a supplement that the author thinks supports the body’s ability to use Ritalin will be much different. I think he agrees with you that the ME/CFS body is just not set up to handle these drugs – hence the nutritional supplementation. No argument at all about the dangers of high dose stimulants…Thanks for sharing your experience.
My compliments to Cort Johnson for accurately describing the proposed mechanism and developmental history of this experimental treatment. I totally sympathize with the CFS sufferers who hesitate to embrace the use of stimulants. I avoided prescribing them for many years for the same reasons that have been mentioned here. However, based on what is now almost 5 years of experience with this treatment, I can say with confidence that the key is to provide the stimulant at a low enough dosage in combination with substantial mitochondrial support. The nutrient formula we are using has been refined and tested over the past 10 years. It is manufactured to the highest possible standard. Our preliminary data is very encouraging and, should this trial provide positive results, we are absolutely committed to do whatever it takes to continue down the path toward FDA approval. However, unless we are able to reach our goal of enrolling 125 patients by The end of the summer, even a positive treatment effect may not reach the level of statistical significance the FDA requires. I encourage everyone who is interested in learning more about this promising treatment to attend today’s webcast (registration details above). It will also be recorded and posted on YouTube for future viewing. Please forward this information to anyone you think may be interested. My best wishes to everyone dealing with this condition and my deepest thanks to the patients and researchers participating in the Synergy Trial. Jon Kaiser, MD
How do I sign up for the NY trial?
The first thing to be useful in 24 years was vitamin B1 – of which I take 2g+ a day in a pattern which helps me write. (Check my blog and search on B1 to get those posts).
I may respond to the cocktail approach.
I have taken stimulants – a number of years ago for a 10 month period with no major side-effects (except that the change was small, and not worth the extra doctor’s visits required), so I know I could probably tolerate the Ritalin.
I live in Hamilton, NJ, close enough to try NY.
I haven’t done research studies because of the effort required, but this one MIGHT possibly work enough to be worth it.
Alicia
Just figured out how to reply to the surveyors.
I am a perfect candidate – no other drugs except Celebrex, no antidepressants, etc. – and quite non-functional.
But too old by 5 years.
Sheesh.
Sometimes you just can’t win for trying! If the trial holds up, though – all the parts should be readily available.
I talked to the KPax people, who told me the non-prescription version works very well for many people – don’t need the Ritalin part.
I started this morning.
Will report if it works better than the B1 I set aside for this test.
Interesting that B1 levels in the supplement are quite high – maybe this would be good for you.
Dr. Kaiser, unfortunately I just discovered this article and thus missed the webinar. I have suffered for many years with this illness and now I am bedridden at least 4 days a week. I am unable to work or care for my two children. I am desperate at this point. I am a 42 yo single mom and I feel like my life is over. I am extremely interested in your efforts to help ME/CFS sufferers. How can I join the study? I reside in Redlands, CA.
I would call that phone number listed. You have to be able to get to San Fran 4 times I think. It would be best to be doing it as part of the trial but you’re seeing a doctor you might interest him in this combo as well. Good luck!
For those who missed the live broadcast or would like to watch again, the webcast for “The Synergy Trial-Pursuing an FDA Approvable Treatment for CFS” is now available by clicking the link below:
https://www.youtube.com/watch?v=Q_yFlocDRoQ&feature=youtu.be
For more information or to see if you qualify to participate in the Synergy Trial, please call: 1-855-318-HOPE (4673)
Dr. Kaiser:
I would like to enroll but, have been on Cymbalta for nerve pain. I am currently weaning off but, may not be off in time to enroll. I would really like to participate but, guess I am excluded?
Thanks,
AB
Cort,
Sorry to report that, after a whole week of being unable to write a coherent word or get anything done, I stopped using the KPax non-prescription formulation – and I’m going back to my B1.
I’ll blog about it and getting back on B1 later this week – I literally haven’t been able to write. It was a horrid feeling. Maybe it would have worked for me if I’d tried it for longer. I intended to – but just couldn’t take the utter lack of energy – it felt like my brain didn’t kick on for the whole week.
I’ll watch for the trial and the report of the results to see if I could have done something different – but I’m swearing off trying anything for now.
On the positive side, it confirms that the B1 IS working for me – but I’d hoped to do better than 20%, which is about what I get with B1.
Alicia
That’s too bad, sorry to hear of your bad experience with Adderall. Methylphenidate is a different substance with different mechanisms of action than Adderall, however — I think methylphenidate actually is known to protect the brain from amphetamine toxicity, and that the two are often given together for just that reason — so perhaps that will be the differentiating factor in these trials.
A lower than normal dose would also seem to be a critical factor – even the 10-20mg bid of Ritalin mentioned here sounds high to me. For the more seriously impaired, I’m wondering if even a milligram or two a day might be enough. Perhaps that would allow a more gradual recovery of the mitochondria and allow them to repair more before upping the dose.
Adderall stays in your system longer than ritalin does maybe you should of went with ritalin like in this post
If one of the sites were anywhere near me, I would volunteer right now.
This is wonderfully encouraging. At last there’s an investigation of something which could help us.
I’m not denigrating all those fantastic people working on basic research – far from it, but we desperately need an effective treatment.
Thank you Cort.
Hey, it’s not a cure but if it could help out – what a boon that would be. I’ve wanted to try Ritalin for years actually; it’s effects of improving concentration, reducing hyperactivity (wired but tired) and helping with calmness would be right up my alley. There are some indications it can help with endurance as well.
This is interesting. My experience with Ritalin was disastrous, I’m
bedbound and there was no energy
just extra being wired so made more tired, no thanks.
The supplements look very minor though to expect much?
That was my question about the supplements. Is that really enough? I guess we’ll find out.
This is the sort of trial we patients need and it’s great. I suppose the wider point is that we know Me/cfs is an area of ‘hanging threads’- that is research that’s done, is promising but no one is able to take it further/pick up on it due to lack of funding and wider political will/interest. Whilst this trial is great how can we ensure this won’t leave just another ‘hanging thread’;something relegated to a a few pages in some scientific journal? In addition there doesn’t seem to be the desire or even hostility to getting this sort of potential treatment more widely investigated.
Also forgive my ignorance but is it possible to watch the webinar without having your image out there?
Zippy, while you must give an accurate email address in order to register for the Synergy Trial webinar tomorrow, you do not need to give your real name nor will your image be seen. Please feel free to register at: https://attendee.gotowebinar.com/register/1627842716332231938
The webcast for “The Synergy Trial-Pursuing an FDA Approvable Treatment for CFS” is now available by clicking the link below: https://www.youtube.com/watch?v=Q_yFlocDRoQ&feature=youtu.be
This research study looks promising to me and gives me some hope that these researchers are on the cusp of finding a universal treatment for the debilatating fatigue and brain fog that a lot of us experience. Years ago, maybe 5, I tried various different stimulants and finally found one that turned out to be a miracle drug for me! A lot of the other stimulants had way too many side effects (like ritalin)and left me feeling worse off, wired, and unfunctioning. Then I tried Vyvanse (Lisdexamfetamine) and it mildly, but effectively, eased me out of the constant brain fog I had been deaLing with for so long. There were also very minimal side effects, if any at all. I believe Vyvanse was created with a low-abuse potential to ward off people becoming addicted to it. There are several different doses of the med and I currently take a pretty high dose of 70mg first thing in the a.m. then again around 2p.m. This dose gets me through the day without feeling drugged like all the other stimulants made me feel. I would encourage anyone with brain fog issues to try this medication if they have the opportunity to do so and to see how it could improve their life. My insurance does cover it, but with a higher copay. There is also a rebate card to use to decrease the cost. Unfortunately I can’t use it because I take 2 capsules a day, or 60 a month, and the rebate card only covers the normal month supply of 30 capsules.
Sent from my Verizon Wireless 4G LTE smartphone
Good news. I hope more doctors learn about this drug. A recent trial in ME/CFS was successful…Check out a blog on that here – http://www.cortjohnson.org/blog/2012/12/28/26/
Very interesting. I did not know that methylphenidate (Ritalin) actually activates the mitochondrial respiratory chain. However, the following two studies clearly demonstrates that methylphenidate does boost mitochondrial function:
http://www.ncbi.nlm.nih.gov/pubmed/17188451
http://www.ncbi.nlm.nih.gov/pubmed/20635140
These studies found that mitochondrial complexes II and IV were increased by methylphenidate, but complexes I and III were not boosted (in fact methylphenidate decreased complex I activity in cerebellum and prefrontal cortex).
I have myself tried taken cocktails of supplements that boost mitochondrial complexes, and support mitochondrial function. Such supplements include:
Pyrroloquinoline quinone — stimulates complex I.
Gingko biloba — stimulates complexes I and III.
Melatonin — stimulates complexes I and IV.
Methylene blue — stimulates complex IV.
Deprenyl — stimulates complex IV.
Bezafibrate — stimulates complex IV.
So now we can add to this list:
Methylphenidate (Ritalin) — stimulates complexes II and IV.
Boosting mitochondrial function might also lead to viral clearance in the case of coxsackievirus B infection (a virus strongly linked to ME/CFS): in a study of murine coxsackievirus B3 myocarditis, complete viral clearance was associated with increased complex I and III activity, whereas lingering chronic infection was associated with decreased complex I and III activity. The study is here:
http://www.ncbi.nlm.nih.gov/pubmed/21968812
The study authors concluded that “the regulation of energy metabolism appears crucial for an effective virus elimination and may be of prognostic and therapeutic significance.”
So boosting mitochondrial function may not only improve symptoms, but may conceivably have an antiviral effect as well.
I also posted a thread on this topic here:
http://forums.phoenixrising.me/index.php?threads/boosting-mitochondria-increases-energy-leads-to-viral-clearance.13464/
Finally, note that the effects of stimulants like methylphenidate can diminish after some weeks due to a tolerance build-up. However, high doses of magnesium (preferably transdermal magnesium) taken just before methylphenidate can help prevent this development of tolerance. Magnesium can be used to help prevent tolerance build up of many drugs, including street drugs like heroin. Magnesium is an NMDA agonist, and it is this NMDA agonism that helps mitigate tolerance. Other NMDA agonists such as dextromethorphan also help mitigate this tolerance build-up.
Wow…thanks for all the info.:)
Will this cause problems for someone already having Heart Palpitations or dizziness??
Carole
Interested to see the results of this one. I’d also love to see a modafinil trial as it too supports the mitochondria.
South American natives chew coca leaves to keep alert. These small doses sound pretty similar in that they could give sufferers a bit of a boost during the day, hopefully with fewer side effects than caffeine.
I’ve been on Adderall since I was diagnosed by Dr. Lapp in 1998. (I’m no longer his patient.) I’ve recently had to go to 60mg/day. Without it I’ve no doubt I’d have been bedridden far more often. I take the extended release version. I’ve been able to handle the side effects so far, but I must admit, reading you all warn of adverse effects of long term use has me concerned. Maybe I should try switching to Ritalin? Any thoughts?
Put me in the group surprised that methyphenidate is part of any long term ME strategy. Our college health center handed them out like water for studying in the 70’s. I only took 10 mg a few times to pull all nighters but I just can’t imagine taking it indefinitely or in any higher dose. “Wired and tired” was the description we used to use also. I supposed if you’re brain has been rewired differently by ME it’s a consideration.
If there’s a study in Boston, which is 45 min from me, I’ll be there if they want me. I have family who can drive me if it’s a bad day. I’ve been part of other studies in the past, notably the intrauterine cryotherapy process to treat menorrhagia at Brigham and Women’s.
I’m exceptionally med sensitive, so that might leave me out, even 10 mg sounds like a lot to me.
Nina – 54 yrs old, diagnosed PVFS 1994, now disabled and on SSDI as of 2013.
Hi Nina,
I also live about 45 minutes from Boston. Would love to hear more about the studies you’ve been involved with at B and W. And how you found out about them. Like you, I would jump at the chance!
This makes me feel somewhat hopeful. Unfortunately I am vacillating between bed bound & house bound & the nearest center is 7 hr drive. Feels out of my reach right now. The $150 stipend is to help w/ cost of the visits? Will volunteers be charged for their visits to the clinics or is this for travel expense?
Never mind, the webinar cleared it up: visits are free, after the initial visit/screening those that make it through will receive $50 for each of the 3 remaining visits as reimbursement on expenses for being involved. Plus a free 3 months of treatment following the trial.
Wish they could add a Midwest site, an area that is extremely lacking in ME expertise.
Cort, do you know of any website that documents the location of ME/CFS patients on a map? I could see this as a useful tool for future researchers to determine locations collecting samples. We need to get researchers to think outside of the box in regards to our patient population being able to contribute. Maybe HealClick?
I’m working on it as well. It’s 75% complete but we ran into trouble with the developers and ran out of money. A “I’m interested in Clinical Trials’ question is part of it. Researchers should be able to click on the map and see everybody who is interested. We’re about $750 away from finishing a very comprehensive mapping tool for ME/CFS.
This will be a great tool!
I can’t handle ANY stimulants anymore~even have to be careful with green tea and chocolate~more than 1 cup of tea or 1 square of chocolate in the AM is too much for me, and I drink my tea diluted…15 yrs ago or more I accidentally took a foster child’s ritalin instead of my thyroid med one day while packing to move, and it actually brought me up to feeling normal for once, as far as energy goes, BUT I have gotten MUCH worse since then and can barely tolerate 1/2 hr of physical effort…am short of breath in a few minutes and have to rest…Can’t handle ANY stress at all anymore~feels like my life is over…I found out over 10 yrs ago that cold meds, even some herbs, and the stimulants they use in weight-loss products like green tea, coco, guarana and etc. all cause my pulse to race, making me feel jittery and panicky…Found out a couple yrs ago I have a prolonged QT segment on my EKG so have a 2+ page list of meds I have to avoid~stupid Dr’s don’t always listen tho~gave me a scrip for a Z-pack (both ears infected + bronchitis) last week that kept me up during the night on the verge of a panic attack! Would’ve only taken him a couple min to look it up~I was so sick I trusted him! Makes me sooo angry! Then they gave me Prednisone for the swollen Eustatian tubes which greatly aggravated the depression, causing suicidal thots, crying spells, BiPolar II -like verbal hyperactivity (SO embarrassing/humiliating/allienating as everything comes bursting out and I become a total bitch at times!) That’s NOT the real me! AND it greatly INCREASED the aches and pains! I think it was killing the little that’s left of my adrenal glands. I was desperate to be able to hear for my son’s wedding Sat or I would have just suffered in silence, as I was almost completely deaf. This wk the ENT MD had to cut thru my eardrum to suction out all the gray mucous~he only did one ear, tho, hoping the other one will clear out on its own…The antibiotics DID get rid of the infection, at least (took amoxicillan for a week after the Z-pac disaster.) But then it upsets my stomach, of course…one positive~I’ve lost a few lbs ~no appetite. have to force myself to eat…Hope to keep losing now for my 40th HS reunion this summer (hoping I have the energy to make the trip.) I’m SO discouraged. I think the hyper-sensitivity (I’ve over-reacted to almost every med they’ve tried on me for the past few yrs!) is due to “leaky-gut” from what I’ve read. How do I mend that? I’ve gone grain-free (except for rice and oats). Thinking of getting the large-panel allergen screening this mo while the tests are “on sale” at my local AnyLab. Finally got insurance after not having any for sev yrs…but it’s not cheap. I live in SD and they’re sadly behind in most areas…haven’t found one single MD or chiro for that matter who knows ANYthing about ME in the 26 yrs I’ve had “chronic fatigue.” So thankful for the internet, as that’s where I’ve learned the most about this complex condition just in the past couple yrs. Thanx for being there!
Tough times MK! We’re going to have some gut blogs coming including one on healing the gut – stay tuned and hang in there.
By the way a couple sips of coffee send me buzzing (and then usually crashing later on…) – it’s SO WEIRD…..
Reading through comments interesting , as I never thought of this disease. I need to bring up to my doctors. The last two years I’ve been battling CFS matter of fact I fell asleep while typing this. And a second time I sleep way to much. Everytime they try a ssri or simular I end up in hospital with serotonin syndrome. I spend so much time in bed sleeping and so week now days I’ll try anything. Adderall just stopped working and I was at 90mg a daybwhnbjh
For those who missed the live broadcast or would like to watch again, the webcast for “The Synergy Trial-Pursuing an FDA Approvable Treatment for CFS” is now available by clicking the link below: https://www.youtube.com/watch?v=Q_yFlocDRoQ&feature=youtu.be For more information or to see if you qualify to participate in the Synergy Trial, please call: 1-855-318-HOPE (4673)
Is there not side effects of this drug taken over along period, I just read a post that a young man did and his autotopsy report stated he had heart failure due to the long time use of this drug, I have found a more effective way and natural and have sustained my energy levels for 6months now, going strong too. Any one that wants more info please contact me.
Hi Rosalyn,
I’m very interested in learning more about your more natural way of boosting energy. I’m afraid of taking something like Ritalin.
read your suggestion about fatique, and natural help. please, I am very interested and of great need.
OH this is a very old thread, wish i had seen it back then…I have CFS with underlying nacrolepsy without cataplexy, life is hard. I have been on Provigil for some years but now my insurance does not want to pay for it and is switching me to ritalin, hope it works. The Provigil does not work like it used to anymore so maybe I do need the change.
Does anyone know what the high dose of Magnesium that is recommended to reduce tolerance of the stimulates for cfs? thank you