

The Lights are ploughing new ground – not just in ME/CFS – but in how post-exertional malaise and fatigue occurs after exercise in humans (and, one must say mice.). In this third blog in a series of blogs exploring PEM and ME/CFS. we take advantage of a chapter in a book they wrote that explains how they’re going about explaining PEM in ME/CFS.
PEM Series
- A “Fatigue” Disorder No More? – What Multiple Sclerosis Taught Us About Fatigue and Chronic Fatigue Syndrome
- A Chronic Fatigue Syndrome Brain on Exercise – Not a Pretty Sight
A Unique Sensory System For Pain
It’s clear that a unique sensory system is present that is dedicated to alerting the brain that pain signals are present. When something induces pain, damage tissues, or threaten tissue damage specialized receptors call nociceptors pick up that information and relay it to the central nervous system.
That information is relayed via a specific transmission line that includes the dorsal horn of the spinal cord, the spinothalamic tract and the portions of the thalamus, insular cortex and cingulate gyri.

A “transmission line” that sends pain signals from the body to the brain has been found… Does one exist for fatigue?
The discovery of that pathway lead to the creation of modulating substances such as opioids that can reduce pain by acting on portions of that pathway.
The Lights believe a similar neural pathway exists for fatigue. Their goal is to elucidate that pathway from its molecular origins in the muscles to the brain in order to identify areas researchers can tweak in order to turn off unrelenting symptoms of fatigue. Their goal is to do fatigue what has been done for acute pain. (Chronic pain is more complicated).
Let’s see what they found in a overview of a chapter on fatigue they authored: Myalgia and Fatigue: Translation from Mouse Sensory Neurons to Fibromyalgia and Chronic Fatigue Syndromes. Alan R. Light, Charles J. Vierck, and Kathleen C. Light. Translational Pain Research: From Mouse to Man. Boca Raton, FL: CRC Press; 2010. Chapter 11. Frontiers in Neuroscience.
A Unique Sensory System for Fatigue?
“We further propose that this elementary sensation is transduced, conducted, and perceived within a unique sensory system with properties analogous to other sensory modalities such as pain. We call it the “sensation of muscle fatigue.”
Specific receptors for the sensation of muscle fatigue have not been identified. Some research suggests fatigue specific pathways in the spinal cord are present and some areas of the brain appear to regulate fatigue. These included brain regions that have been highlighted in chronic fatigue syndrome and fibromyalgia: the prefrontal cortex, insular cortex, and the anterior and/or posterior cingulate gyrus.
Like pain, the Lights report that fatigue is a protective phenomenom. Fatigue is a sensation believed to be induced by the central nervous system to protect from a detrimental reduction of energy stores.
Sensory fatigue, for instance, reduces commands from the motor cortex to activate the muscles. [Some Japanese researchers hypothesize that reduced commands from the motor cortex are causing fatigue in ME/CFS.] Sensory fatigue also triggers sympathetic reflexes to increase energy levels in the muscles by increasing blood flows to them and decreasing blood flow to non-working muscles.
Muscle Metabolites to the Fore
“We suggest that there is a simpler sensation of fatigue that is triggered by inputs from specific receptors that are sensitive to metabolites produced by muscle contraction.”
It makes sense that fatigue sensations begin with the muscles. When the muscles are exhausted or in danger of being damaged there has to be some way to a) pick up that information and b) relay it to the central nervous system.
The Light’s believe that the detection of “muscle metabolites”: substances such as ATP, lactic acid, pH, protons and others that are produced when muscles are under stress must begin the process.
Researchers demonstrated more than 70 years ago that metabolites produced during muscle contractions could evoke feelings of tiredness and ultimately pain. Blood pressure increases were also associated with tiredness or heaviness (cement legs feeling?). [That’s interesting given Newton’s findings suggesting problems with blood pressure variability are present in ME/CFS.] These early researchers also demonstrated that these fatigue sensations were transmitted via nerves in the muscles.
Later research indicated signals from these nerves activate the sympathetic nervous system via something called the sympathetic exercise pressor reflex. Again they found a sympathetic nervous system connection – those sensory nerves also appear to affect vasodilation of the blood vessels in the muscles. It is by opening and closing the blood vessels that the working muscles are provided with the energy they need to work.
Community Effort
The Lights goal was to uncover the molecular changes at the muscle levels that were sparking the sensations of fatigue and pain after exercise. They found metabolite receptors required a combination of metabolic by-products to function. Increased levels of Lactic acid and changes in ATP, for instance, were required for ASICS receptors to detect small changes in blood pH levels. Metabolic acidosis (high proton levels only) will not trigger the nerve signals needed to produce fatigue.
Fascia Play Key Role in Transmission of Pain
They noted the sensory nerve fibers containing these receptors were mostly in the fascia, not in the muscle tissue, making the fascia probably the most important tissue with regards to evoking muscle pain.
Moving Upwards
“The metabolites we found to synergistically activate a combination of molecular receptors suggests a unique detection system for the rapid use of energy stores, which can signal the body to protection against metabolic and traumatic injury.”
Next the Light Working Group started applying muscle metabolites in various combinations to see which ones, if any, triggered signals to pass up to the dorsal root ganglia found just outside the spine. These are nodes through which the sensory signals from the body pass.
Increasing ATP, protons, and lactate gradually caused muscle-innervating DRG neurons to respond. Further research suggested that the DRG neuron activation indeed produced muscle fatigue and heaviness sensations.
Further work indicated that the primary receptors that latch onto metabolites and send fatigue signals to the spinal cord include the ASIC receptor (likely ASIC3), a P2X receptor (likely both P2X5, and P2X4), and surprisingly, TRPV1.
Couch Potatoes Provide a Clue
More evidence for the central role these metabolites play in producing fatigue and pain after exercise came from a study of delayed-onset-muscle-soreness (DOMS). DOMS occurs when people who have not exercised, exercise intensely. They feel no increased pain for a day or so but after that experience muscle soreness.
The research group duplicated DOMS in mice by resting the poor mice and then causing them run until they collapsed (it took two hours!). Then sixteen hours later, after they sacrificed them they found increased mRNA for muscle metabolite detecting genes such as ASIC3, P2X5, P2X4, and/or TRPV1 in the dorsal root ganglia neurons that innervated their limbs. That suggested these substances can cause extended periods of pain and fatigue.
But how to test this in humans [without exercising them to exhaustion, sacrificing them and dissecting their neurons]? They put a healthy person on a bike, exercised him to exhaustion and then measured levels of mRNA for muscle metabolite detecting receptors as well as inflammation and sympathetic nervous system activity in his blood. They found significant increases in all of these and next stage of their work was on.
Landmark Study
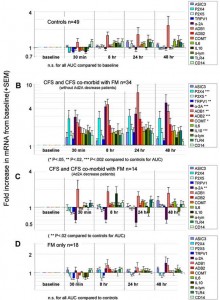
Next they devised an exercise protocol (25 minutes of whole-body moderate exercise) designed not cause fatigue or pain in healthy humans and then gave it to healthy controls, and people with chronic fatigue syndrome and/or fibromyalgia . They measured the mRNA levels of muscle metabolite sensing and other substances (ASIC3, P2X4, P2X5, TRPV1, adrenergic α2A, adrenergic β1β1 and ββ2, COMT, IL6, IL10, TNF-ctα, TLR4, and CD14) before and up to 48 hours after exercise.
From that came the most dramatic protrayal of post-exertional malaise ever seen in ME/CFS. No changes in mRNA levels occurred in the healthy controls, but large shifts in mRNA production occurred in the people with ME/CFS. That was expected but how quickly they occurred and how massive they were astonished the research team.
Even moderate exercise produced changes that were far in excess of what they’d seen in healthy people or even the couch potatoes who’d been exercised to exhaustion. It appeared that the system that detects changes in muscle metabolites had gone on very high alert, indeed, in the Chronic Fatigue Syndrome.
Not Just Chronic Fatigue Syndrome?
These metabolite detectors may also play a role in several disorders associated with ME/CFS. Fibromyalgia is an obvious possibility, but studies have linked some of these same detectors/receptors (ASIC receptors and TRPV1) with inflammation and colon sensitivity – which suggests they may be in play in IBS.
Causes of Receptor Upregulation
But what is causing this mysterious receptor upregulation? The paper listed three main possibilities.
Viral Infection/Stress/Immune Up Regulation of Nerve Growth Factor – Nerve growth factor is one possibility. The Lights suggested that any event –such as a viral infection which increases cytokine levels – could increase NGF levels and ultimately sensory muscle fatigue and pain.
Mitochondrial problems that blunt aerobic capacity and increase glycolysis could increase muscle metabolite levels, muscle fatigue and pain and ultimatley increased receptor levels.
Sympathetic Nervous System- The sympathetic nervous system always seems to pop up in chronic fatigue syndrome. During exercise the SNS moves blood from the non-working muscles to the working muscles in order to provide energy and remove muscle metabolites. Adrenergic β2 receptors found on the smooth muscles in the blood vessels and adrenergic α receptors found in the non-working muscles play key roles in this process.
If these receptors are not working right reduced blood flows could cause muscle metabolites to build up causing an early onset of fatigue and pain.
An up regulation of these receptors could reflect a system that’s desperately trying (and failing) to move the blood along, At its worst, it’s possible that the metabolite buildup in the muscles is never completely washed away, even after rest – leaving the muscles in a state of constant distress.
This could result in a constant barrage of signals asking the sympathetic nervous system receptors on the blood vessels to increase blood flows. But if wolf is cried too many times in the nervous system it stops listening. Thgat could lead to a more or less permanent state of fatigue and muscle pain as the SNS stops responding to signals to increase blood flows.
It’s also possible that the adrenergic receptors are simply sending the wrong message to the blood vessels. Whatever the case, research done after this paper in 2010 has highlighted the role the adrenergic receptors – and thus the sympathetic nervous system – plays in producing muscle fatigue in ME/CFS.
Sympathetic Nervous System “Burnout”
The sympathetic nervous system “burnout” model suggested that the adrenergic receptors that open and close the blood vessels may have become burned out by an unrelenting barrage of signals.
The idea that those receptors simply are not responding any more seemed to take a blow when the Lights found that low doses of propranolol, an adrenergic antagonist designed to shut down those very receptors worked in Fibromyalgia patients. Not only did their symptoms improve, but their sympathetic nervous systems resumed normal functioning.
Back to the mice they went to try and figure out what happened. Animal models suggest the upregulation of some sympathetic nervous system receptors (a1 and a2) found on the sensory nerves (not the blood vessels) contributes to neuropathic pain and inflammation. (Demonstrating remarkable complexity, those same receptors on the spinal cord appear to inhibit pain.) There was little evidence, however, that beta adrenergic receptors were found on sensory nerves.
Initiating inflammation in mice and then “harvesting” them indicated that beta adrenergic receptor levels on the sensory nerves increased greatly in response to inflammation and remained elevated for as long as seven days. The fact that the beta adrenergic receptors were the only receptors still increased in the dorsal root ganglia neurons strongly suggested they played a key role in producing long term pain after inflammation.
The low dose propranolol was probably not affecting blood flows in the FM patients, but it probably was turning off the adrenergic receptors on the sensory nerves that were sending pain signals to the brain; i.e. they felt much better.
Levine Steps In
Levine at the University of San Francisco stepped in next with a series of experiments. He showed that stress in combination with HPA axis and sympathetic nervous system activation was able to flip a switch in the adrenergic signaling pathway that resulted in increased pain sensitivity (hyperalgesia). [Both HPA axis and sympathetic nervous system problems are present in ME/CFS and FM] This suggests the adrenergic receptors may increase sensitivity to inflammation.
[At least three researchers have proposed that ME/CFS and FM patients systems are over responding to inflammation: Miller’s work suggests dopamine reduction sensitizes the response to inflammation in ME/CFS; Younger proposes an over-sensitization to leptin may be present; and now Levine’s work suggests HPA axis and SNS activation in concert may trigger an over sensitization reaction involving adrenergic receptors. Any of these could translate the relatively mild inflammatory state found in many people with ME/CFS into increased levels of fatigue and pain]Mechanism Found
Now the Light’s had a mechanism that could explain both the proponolol results and the long-term pain after exercise. ME/CFS patients were essentially getting hit on both ends; at the same time an ongoing sympathetic nervous system response was possibly shutting down blood flows to their muscles, it was also upregulating the adrenergic receptors on the sensory nerves increasing pain and fatigue.
By 2010, the date this chapter was written, the Lights and associated researchers had already achieved remarkable insights into the molecular basis of fatigue and pain after exercise. Next up we look at their work from 2010 onwards.
PEM Series
- A “Fatigue” Disorder No More? – What Multiple Sclerosis Taught Us About Fatigue and Chronic Fatigue Syndrome
- A Chronic Fatigue Syndrome Brain on Exercise – Not a Pretty Sight











Thank you, Cort. You make it possible to keep up with these things and to have hope. Is it premature to experiment with propranolol to stave off crashes? Worse than the pain, at least for me, is the extraordinary coma-like and unsteady state I go into with PEM. Wondering if propranolol could lessen the severity or otherwise have a positive effect. (You’re awesome — so appreciative of all your work.)
I really wouldn’t know about propranolol – it certainly did help but I’m no doctor. Note that it was low dose propranolol. I think its worth a try myself.
Having a Reflex Sympathetic Dystrophy(RSD)/ Complex Regional Pain Syndrome CRPS and FMS and CFS for 20+ years it’s understandable that every time I went to a support group for any of this diseases I was confused. My daughter has the ME and my granddaughter (her daughter) has all three plus POTS. I just went through four days of ketamine infusions and I can’t believe the difference in my energy levels. I keep telling myself to pace things but I feelvlike I just woke up after 20 years! Thanks for all your great information.
Congratulations. People wondering if there’s a genetic component need look no further than your family. 🙂
So, in four years do we have a ……………… treatment?! I’ll bet not. Is there ever? Do they even try to find any treatments or is it just a a theoretical experiment for these guys …..
Greg
Well there is propranolol.. but more importantly I believe they’re searching for receptors that can be turned off or manipulated to stop the pain and blood flow issues. The ANS is a crazily complex system, though.
First off I also want to commend you, Cort, for compiling all of this info. into a site that makes it easy to find. It would be more than challenging otherwise for people so ill and fatigued. And I echo Tee’s comments that your reports provide hope, which is so important and too often hard to find with the minimal attention this disorder has gotten through the years.
But after reading your article and then seeing the 2010 date pertaining to this study, I couldn’t help but despair some in the lack of anything tangible being achieved in all the time since then; not only in a dearth of any real treatment available but still a black hole existing in the medical establishment to even understand or acknowledge this serious illness. Why is it that even with good physical laboratory evidence, we can still expect our doctors to have no real understanding when we describe our symptoms? Just a rhetorical question but I am wondering to what degree this information gets used by people who can make a difference.
Thanks for bringing this article to light, Cort.
For some reason the doctors are the last to know -which is why ME/CFS experts that keep up on the latest information are so important.
I think we’re making good progress and that provides hope but we’re just not to the point were it’s showing up in patients lives much. My guess is that treatment possibiities will probably be one the latter things to come out of this research.
I am encouraged that the Lights have gotten the funding and are opening up new ground in our understanding of how PEM and fatigue after exercise happens. In the next blog on PEM I’ll bring us up to date as much as I understand what’s going on and try and get Allan or Kathleen to tell us what they see happening from here.
I was on propranolol for 3 years to stop seizures etc after exercise. It worked a treat for stopping the ‘fizzy’ muscles and thumping heart but I had to keep taking more and more to get the effect of blocking adrenaline. I seemed to be tired but not in a cfs way. After weaning myself off it I couldn’t believe how energetic I was. After having a heart rate of 46bpm for 3 years I felt like I was on speed! I could run again and remained symptom free for about 1 year but after a big biking competition I relapsed. I’ve battled to exercise ever since. I’m going to doctor tomorrow to ask to try it again. Have been iron deficient for 6 months so haven’t been able to go back on it yet. I’ve also found a few wines great if I’m not too bad as it must act as a vasodilator making me feel warm and not so twitchy.
Real interesting Julia. It sounds like it slowed down your system and then when you went off it your system rebounded. It definitely this drug can have a real impact.
Isn’t the yo-yo effect strange. …You get up to bike racing again and then push too hard and the whole thing collapses again….Besides being the puzzling disorder around ME/CFS is also one of the most fascinating.
Too bad they didn’t have you under the microscope as you were relapsing. I’ll bet we could have learned a lot.
Good luck on your next round.
This is really great written Cort!!! i like the ANS as key point for ME/CFS. ”ater research indicated signals from these nerves activate the sympathetic nervous system via something called the sympathetic exercise pressor reflex. Again they found a sympathetic nervous system connection – those sensory nerves also appear to affect vasodilation of the blood vessels in the muscles. It is by opening and closing the blood vessels that the working muscles are provided with the energy they need to work”. This is the problem! Interessting is that by POTS the found autoantibodies to adrenergic receptors!!J Am Heart Assoc. 2014 Feb 26;3(1):e000755. doi: 10.1161/JAHA.113.000755. Autoimmune basis for postural tachycardia syndrome.
Aha! I had forgotten about the autoantibodies to receptors study! (Otherwise it would have been in the blog.
Thanks!
Thank you Cort, I so appreciate your articles and that you are skilled in organizing important information in a concise format so we can understand it and not become overwhelmed and exhausted trying to figure out what it all means. You are a treasure. Great contribution.
Thanks 🙂 🙂 🙂
How would muscle fatigue fit in with cognitive fatigue?
Lyme Disease. I’ve been seriously I’ll from chronic neuro Lyme. Worst symptoms are CBS and nerve pain. I also have depression and Taking get mess for that.
Any talk about Lyme patients? It’s common to have ME/CFS and pain.