



“We are cautiously optimistic regarding these results because, if confirmed, we may have identified a contributing factor to the innate immune issues associated with ME/CFS and a molecular target for potential treatment strategies.” – Lombardi et al.
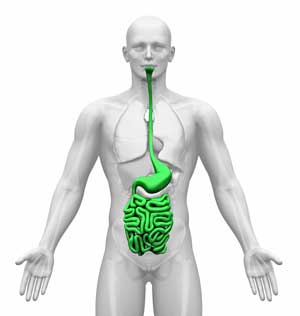
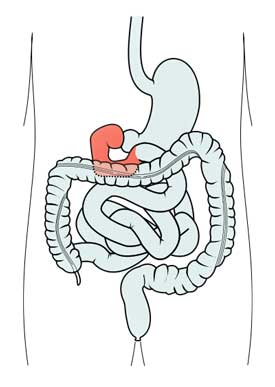
Over the past couple of years the Whittemore-Peterson Institute has been quietly digging into the human gut. They found that the duodenum – the first section of the small intestine – of many people with ME/CFS is inflamed and has become infiltrated with lymphocytes (disease-fighting white blood cells) – a clear sign that the immune system is on the attack.
Plasmacytoid dendritic cells in the duodenum of individuals diagnosed with myalgic encephalomyelitis are uniquely immunoreactive to antibodies to human endogenous retroviral proteins. De Meirleir KL, Khaiboullina SF, Frémont M, Hulstaert J, Rizvanov AA, Palotás A, Lombardi VC. In Vivo. 2013 Mar-Apr;27(2):177-87.
Further investigations indicated that innate immune cells called plasmacytoid dendritic cells (pDCs) were present in higher than normal levels. The first part of the immune system on the scene of a pathogen attack, the innate immune system initiates a kind of a broad inflammatory attack against pathogens. It’s the innate immune system that’s behind those flu-like symptoms you experience when you get a cold.
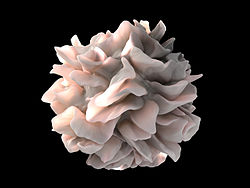
Dendritic Cells
Swarming the tissues in our body (skin, nose, lungs, stomach and intestines) that pathogens first come into contact with, dendritic cells (DCs) are an important part of our immune response. If they find an invader, they snatch a piece of it (an antigen) and then dash back to the lymph nodes where they present it to T-cells for inspection.
The type of DCs the WPI found in ME/CFS patients – plasmacytoid dendritic cells (pDC’s) – are a bit different, however. Much more than just messenger cells, pDCs are actually important virus-fighting cells that produce huge amounts of antiviral cytokines such as type-1 interferons (IFNs) when activated. Both the pDCs and the interferon they produce activate natural killer cells – which tend to be dysfunctional in ME/CFS. Usually quite rare, they typically make up just 0.4% of the lymphocytes in the blood.
Dysregulated pDC’s dendritic cells have been associated with the some autoimmune disorders.
HERVs
The WPI found higher than normal levels of pDCs in the duodenums of ME/CFS patients. When the WPI took a deeper look at the pDC’s they found them to be infected with endogenous retroviruses called human endogenous retroviruses or HERVs.
Endogenous retroviruses are ancient bits of retroviruses that have made their way into our DNA. Almost all are now benign bits of degraded DNA; a few retain enough of the original viral DNA to become partially active. Some HERVs able to initiate destructive immune responses have been associated with autoimmune disorders such as Sjogren’s Syndrome, multiple sclerosis, and lupus.
Autoimmunity and the Gut
What do autoimmune disorders have to do with the gut? There’s a good chance the gut may play an important role in the development of some autoimmune disorders. The idea goes something like this: first, leaky gut linings allow massive amounts of gut material to leak into the bloodstream. That gut material then triggers a a massive immune response that overwhelms the immune system’s ability to keep itself in check and it begins mistakenly attacking the body – resulting in autoimmunity. Several studies have associated celiac disease – which can impair gut linings – with an increased risk of autoimmune disorders.
pDCs also play an important role in triggering B-cells to produce immunoglobulins to commensal (non-harmful) gut bacteria. Damaged pDC cells could result in bacterial overgrowth, intestinal dysbiosis, and other gut issues. The symptoms associated with all of these problems, of course, are common in both ME/CFS and FM.
Digging Deeper!
The WPI found pDCs with HERVs; then, in rather impressive fashion, they set out to determine why they were there.
First, they created a laboratory model of pDCs producing HERVs. When they analyzed those pDCs they found they were producing high levels of certain proteins. Then they looked to see if those proteins were present in the guts of ME/CFS patients with pDCs, and they were.

Gene polymorphism results suggested a weakness in the innate immune response could be present in ME/CFS
That means that HERVs they found were indeed active – a key finding. Whether or not they’ve causing gut and other issues is another question, but the potential is definitely there for these infected pDCs to produce bacterial overgrowth (believed common in both chronic fatigue syndrome and fibromyalgia), altered bacterial flora, and other gut issues.
Obviously quite intrigued by that finding the WPI then looked to see if a genetic weakness could explain the presence of the HERVs in the ME/CFS patients. A gene polymorphism study identified a set of gene polymorphisms (alterations) more commonly found in ME/CFS patients with gastrointestinal (GI) issues. An in-depth analysis of genes associated with the innate immune response found significant differences in these patients as well. The findings are preliminary, but they could be describing a patient population that’s genetically at risk for innate immune system derived issues (pDCs) in the gut – and perhaps autoimmune issues as well.
They’re attempting to confirm the polymorphism findings in a larger group of ME/CFS patients as well as in an additional group of ME/CFS patients from three different continents.
Making Progress – Under the Radar
The WPI is no longer in the news much. Instead they’re moving cautiously and methodically to explore a an intriguing finding. If it all works out, they may have a molecular target they could conceivably use to shut off the HERV production and perhaps help resolve gut issues and even autoimmune tendencies and immune activation in ME/CFS.
Gut Work on the Clinical Side
“There are multiple steps in my treatment protocol that appear to be working well for a majority of my patients, but we still have much more to learn before we can successfully treat all of those who are affected,” – Dr. De Meirleir.
Dr. De Meirlier’s statement that his treatment protocols are working well for most of his patients was borne out by a recent Norwegian ME/CFS survey that identified Dr. De Meirleir as a physician with good success rates (2/3 improved; 1/10 declined). Now, as the Medical Director of the WPI, Dr. De Meirleir may have been the first ME/CFS physician to focus heavily on the gut.
At a WPI lecture Dr. De Meirleir stated he generally begins healing the guts of his patients, a process that involves identifying dietary issues (including the removal of dairy, gluten, and fructose) and used fecal analysis to determine if a pulsed program of antibiotics, probiotics, and prebiotics and digestive enzymes will have a positive effect.
Dr. De Meirleir ended his lecture suggesting ME/CFS fits in a continuum of autoimmune diseases that includes lupus, RA, type 1 diabetes, and relapsing/remitting MS, all of which involve a dysregulation of the 2’-5’OA synthetase (RNase L pathway and Th1/Th2 immunity.
An Aside: the Gut – A Complicated Place
Since the gut with its millions of bacteria is one of the most complicated parts of the body, it’s no surprise that the treatment picture is rather complicated.
The fact that Audrey, for instance, had responded pretty well to dietary restrictions, then didn’t respond to antibiotics/probiotics, and then responded very well to fermented foods simply demonstrates how individualized gut treatment plans can be (and really, how they must be to be effective).
We saw Esther find many ME/CFS symptoms get better or resolve with Xifaxin – an antibiotic focused on the gut. We covered a study suggesting that some herbal preparations were as effective as antibiotics. Dr. Rowe reports that ME/CFS patients with undiagnosed milk allergies will probably benefit little from other potentially beneficial treatments until milk is removed from their diet.
We’ve covered gluten-free diets, FODMAPS, and low glycemic and Mediterranean diets. We still have Paleo, anti-inflammatory diets and anti-histamine diets to go.
The gut is a complicated place – it’s going to take quite a while to fit the right combinations of probiotics, prebiotics, antibiotics, fecal transplants, herbs, foods, etc. to help a particular patient. That kind of complication makes the WPI’s gut studies all the more interesting. If infected immune cells in the gut are behind all this mess – and we’re a long way from proving that – it makes the process of fixing the problem all the simpler.
Stay tuned. The last study the WPI published was in 2013. We should know much more about how this research is stacking up this year.
Chronic Fatigue Syndrome and Fibromyalgia Microbiome – Work Under Way
Multiple rejections to Dr. Lipkin’s microbiome grant application notwithstanding, the gut in ME/CFS is getting some attention. Dr. Chia, of course, has presented his findings on enteroviral gut infections in ME/CFS. Besides his and the WPI’s work several gut studies are underway or have been completed recently.
Some promising gut work is underway but needless to say much more is needed to understand the guts effects on ME/CFS and FM
We await the publication of the Solve ME/CFS Initiative’s study of gut flora changes after exercise. Rebecca Hansen’s large Microbiome and Inflammation (an NIH-funded study) examining bacterial composition in both the gut and blood as well as endotoxin and lactoferrin levels is due to finish up this year.
Dr. Pridgen’s antiviral study grew out of a belief that herpes virus infections in the gut play a key role in fibromyalgia. The study included a pathological screen of herpes viruses in gut tissues and will be published this year. The Lipkin/Chronic Fatigue Initiative/Microbe Discovery project is identifying viruses, bacteria, and fungi in fecal samples as well as cytokines in the blood, taken from 100 ME/CFS patients and 100 carefully matched controls.
Finally, Fluge and Mella are using endoscopy, biopsys and ultrasound to assess gut functioning in ME/CFS patients receiving Rituxiimab.







Well, I for one don’t place much faith in the WPI following the previous debacle.
Having said that, I have had a lot of gut issues over the course of my CFS. But that might be the end product of other processes.
I still think the problem is in the CNS / brain.
As people have said before, though, I don’t really care what the cause is, just want to find it and treat it!
Really keen to see the results of the Fluge/Mella study.
Bacteria in SIB0 create toxins which are then sent to the brain so the gut can be causing brain problems.
As well you know 🙂
I agree Mattias. WPIs entire line of past retrovirus research was questionable at best. Now we are just supposed to accept more of it?
I definitely understand wariness – particularly in the pathogen field! The WPI is, however, under a different research director – Vincent Lombardi – who apparently never signed on to the XMRV finding. The lab also passed muster from an NIH committee that checked it out after XMRV…
I think they’re in good shape – but I do cross my fingers with any pathogen finding. Validation is always needed…
I agree. I think the cause is a dysfunction in the autonomic nervous system in the brain. It affects everything!
Any information on how to rebalance gut flora in the meantime though is helpful, its difficult to know what will set off a reaction anymore.
Joanna, my thinking was similar a few months ago (just gathering from what you wrote here). The thing is the enteric nervous system AKA “second brain” lies within the gut. There is a serotonin-gut connection also…that can be Googled. But, balanced good gut flora and a healthy gut bacterial flora are essential to brain and nervous system functioning. The bacteria in the gut actually helps synthesize and manufacture nutrients and neurotransmitters. There is a connection I never understood until recently. So, don’t underestimate the gut : )
Is there any connection between this phenomenon and what happens with the cm virus? . Seeing as it causes so many issues for the immune system when active and inert.
Everybody i know with ME have problems with there gut. But the question still remains chicken or egg. Even if it it secondary treatment is very importent.
I don’t have any gut problems. I never have. But I have severe chronic fatigue syndrome and Fibromyalgia. I’m sure a healthy gut is vital to recovery, but I think that it’s repair will not help some of us. I suspect the onset of my CFS and Fibro occurred due to mercury inhalation and disturbed GABA/Glutamate function (due to post-accute withdrawal of Valium). I also have three serious sleep disorders which result in very poor sleep. All these have wreaked havoc on HPA axis regulation. Gut dysbiosis, for me, may be a bi-product of those assaults, but not the cause. But, I could be wrong!!
Since a gluten free diet made me so wel for years that I could go to a gym and exercise I’m keen to see research on the gut. It isn’t gut or brin, the gut affects the rest of the body. For me brain fog disappeared
I don’t believe it is a viral infection in the gut (that the body can’t rid itself of) causing ME/CFS symptoms. The problems are caused by a hyper-aroused immune response, first recognized by the body’s second brain (the gut), that signals the big brain in the head to launch an attack against anything and everything it deems foreign (viruses, bacterias, chemicals etc). ME/CFS is an autoimmune disorder.
I think abnormal activity of the autonomic nervoussystem can be the cause of everything. For me the central question is: what causes this abnormal acitvity? I agree on autoimmunity.
Rochlitz says that toxic porphyria or genetic porphyria can cause it.
I have been reading Rochlitz’ books lately. I’m not certain about the porphyria. I think mast cell disease is more of a problem than we’ve realized. But this could easily be caused or exacerbated by toxins from bad gut bacteria.
Certainly I believe there are gut problems with many CFS/ME. Ken Lassesen has been very helpful on that subject here on Health Rising. I just received a DNA test of my gut bacteria from uBiome. Honestly, it is the first test I’ve had of anything that was wildly abnormal. Figuring out what to do about it is another matter. But at the genus level, some of my bacteria are wildly out of whack. Almost no acidophilus, for example.
ANS problems could cause reduced gut motility, bloating and pain….I wonder if reduced gut motility could result in changed gut flora?
Yes, gut dysmotility can result in altered gut flora.
Well, I’ve had “just” pain for several years.
After almost a decade I almost got rid of it by taking green smoothies (gut connection).
Then after a while feeling awesome I started having horrible bloating and stomach pains. Ever since (that was 3 months ago) everything went downhill. Now I have horrible fatigue, brainfog, a lot more pain, headaches and the list goes….
And I have no idea what happened but it was something on my stomach…
Isn’t that something….
If there are any micropunctures present in the gut from GMO foods (which everyone now eats whether they know it or not, thanks to Monsatan), and then green smoothies are ingested which are almost always extremely high in oxalate crystals that get absorbed because of the damaged gut, a much worse result can happen. Huge amounts of oxalate crystals can build up all over the body and brain, depending on one’s genetics, and block methylation DNA pathways and lead to all kinds of painful and confusing down-the-stream diseases, the least of which is kidney stones. And almost no doctors are aware of this except some nephrologists or DNA/methylation trained practitioners. Yahoo groups: Try Low Oxalate Diet = resource and networking for this situation
So interesting Becca! Thanks for passing that on…Who knew???
Hey Rebbeca that’s a nice information
I did consider the oxalate connection for a bit, but wasn’t aware of the true mechanism.
Can you give me more details?
I used to be able to eat everything. Ever since that I started reatcting to the most inofensive foods like bananas, than oranges and one time I almost died after a beet juice (made with ONE beet).
Strangely I did got better from my stomach with a bunch of supplements (or time…). If I don’t eat fruits I can stop having pain and bloating but the other symptoms just get worse. I did test negative for fructose malabsorption btw and I seem to tolerate well dates and some other fruits in small quantity. But if I stick with fish/chicken, vegetables and potatoes I feel that it is a better option.
Do you have OI or POTS as a subsymptom of CFS? If so it might explain the beet juice, NHS around 2009 discovered rapid blood pressure drop from beetroot via nitrates. My mother in law has some heart issues and chronically low BP – I made beetroot soup for us all to have in 2010 and within a couple of hours she was hospitalised – she is fine but she and her daughter (my wife) have this reaction to the juice in relatively small quantities too. We assumed my wife immune to effects because she was fine after the soup, she was pregnant at the time, soon after the pregnancy I made her a beet and apple juice and she almost passed out, had to lie on floor with feet up for an hour.
“For me the central question is: what causes this abnormal activity?”
You were probably born with innately superior immunity, that has gone haywire due to a constant bombardment of foreign entities; the genes for up-regulation of the immune system (autoimmunity), and many, many triggers (chemicals, viruses, stress, estrogen/serotonin dominance etc).
i wish i could take part in one of these trials – im based in the UK and firmly believe in this as most of my M.E/cfs symptoms increase dramatically after food – is there a way i could be involved ? After eating food i feel like Im going to die , often within minutes and not only that I cant walk or have difficulty breathing and fall asleep too even in very inappropriate places ! I have found that cutting out yeast, gluten and sugar has helped alot but it hasnt still solved all the answers- I do still have symptoms and extreme fatigue, swollen glands etc
Have you checked POTS. Easy to do the heart rate test at home.
I do believe in the gut as the main cause, but there are still other contributing factors. The herpes virus is definitely involved in my experience. We will see. I still don’t think a treatment/cure will be available to me in my lifetime. I’m 67 today, and have had this since I was 34. Hormones are definitely another factor. I got very sick after pregnancy. I’ll be watching, hopeful, not holding my breath.
Donna,
I am 69 and I ditto everything you wrote . With the exception of our 2 yr. Age difference, we have had much the same experience.
It’s a long time to have lived with this condition.
All the best to you ,
Patty
Interesting…… I was diagnosed with gastritis and duodenitis around 11 yrs ago, as my health was beginning to go downhill. Despite my best efforts with diet and supplements I still have problems with this and it is one of my worst symptoms.
I have always/always/always had problems with my gut. I also have always/always/always been extremely tired, sluggish, etc…. I remember being a child and just want to sleep instead of play. I am now 30 years old diagnosed with depression, anxiety, endometriosis, fibromayalgia, CFS, and MPS. I really wish I was a test subject here for the Dr.’s Fibromaylgia/CFS & gut studies. I believe that my problem stems from my gut. I really would like to see the findings on this problem, as now everything has gotten worse for me.
A lot of work is being done on the microbiome now – outside of ME/CFS and some inside of it – we’re going to learn an awful lot in the next couple of years.
I’m in the UK too and feel slightly envious when I consider the differences between the British and the American medical services. I know that we have a health service which is free and for which I’m very grateful, but I wonder, if we had a health insurance based system, would we have more British companies/specialists carrying out the ground breaking work that is carried out in the States ?
Careful what you wish for. Only the very rich get anything like appropriate medical care in America anymore–and that is because they pay very high prices out of their own money. Most of us pay outrageous prices for our insurance and any doctor that take the insurance won’t listen to anything or investigate anything or think out of the box in any way. You’re in, your 5 minutes are up, and you are out. Only people needing lifesaving surgery and the like get any real help from docs here. It’s a horrible and non-healing system. Everyone I know who also has a chronic medical condition has basically given up and doesn’t go to doctors unless absolutely necessary because we all feel they cause massive stress (the whole system does, including the expensive insurance companies trying to get away with not paying your bills and causing you endless stressful phone calls to correct their unjust mistakes and refusals, as well as the cheap, untrained staff, and the doctors who are hurrying so much they hurt you more than help–often literally), and, above all, we all feel they do not help us or heal us in any way. An American doctor has just written a book stating most of what I have just said. She definitely stated the doctors are in a hurry and don’t listen at all.
The grass isn’t any greener over here. Sadly.
Cort, Brain fog very severe today…too much so to compose an intelligent comment. But I just had to write and say thank you for all you are doing on our, ME patients, behalf…bless you!
Thanks Nancy:)
I have positive echovirus antibody tests (which surprised me). Unfortunately, I couldn’t tolerate Equilibrant.
The findings about gut pathology by WPI may explain why many people with CFS and FMS have Small Intestinal Bacterial Overgrowth (SIBO) and Histamine Intolerance. Although I am not convinced that gut problems are the sole underlining cause of CFS, they certainly contribute to the symptoms. I don’t know anyone with CFS or FMS that does not have gut problems or that hasn’t tested positive to SIBO breath test that shows bacterial overgrowth in the small intestine. Histamine Intolerance is caused by low levels of Diamine Oxidase (DAO) the enzyme that breaks down histamine found in foods. DAO is produced in the lining of the small intestine so it makes sense if there is inflammation there, that could lead to lower levels of DAO. I manage my gut issues by taking repeated rounds of Rifaximin & Neomicin (about one 3 week course every couple of months) to keep the levels of bacteria in the small intestine under control; and I consume a diet of foods low in histamine and sugars and take DAO supplements 15 minutes prior to each meal. This keeps me pretty healthy and symptom free most of the time but its not a “cure”. Prior to this program I tried a gluten/dairy free diet for a year with no benefits and worked with a scientist doing cutting edge research on probiotics with not benefits. I’m still looking the perfect recipe to heal my gut completely. Its good to know researchers are working on this.
I have never had gut problems except when I was in the phase of trying multiple supplements or new miracle cure, fad food or diet on the market to try to get well. Then my gut started acting up and my physician told me to stop all supplements etc. etc. because it was harming me. So, I did and the gut issues gradually went away. So, the question might be, “Is it really our gut or is it us trying to cure ourselves and inadvertently causing gut issues?” I’m finding more and more people with ME/CFS whose gut issues started after trying various supplements and “miracle” cures.
I have heard so soo many stories of people who had IBS onset before or during ME/CFS hitting like a train and many stories of people whose stomachs churned or their appetite totally left for weeks or months after CFS onset and they could only eat certain things or things that sounded good. This is true for me (IBS-C as a kid which phased out and hit again at 19 w/ a vengeance), and ME/CFS hitting like a train May 14, 2006…appetite totally left. I had to just eat what I could face, didn’t have much desire for food–something completely out of the ordinary for me. Then, Aug of this year after a 80-90% recovery I got hit again w/ ME/CFS re-onset and sure enough my appetite totally left…just like the 1st time when I got sick at 14. It took about 2.5 months to really start to come back. I lost about 6 lbs…just like as a teen. And now I have just motility issues, tight muscles, and many other common ME/CFS symptoms. This time I had recurring staph aureus throat infections and at least 2 rounds of Cipro and then liquid Amox-Clav to swish and swallow b/c may have partly been coming back b/c bacteria left in braces. All the antibiotics screwed up my stomach…acidy, couldn’t eat much at once, then hungry shortly after or it felt like it b/c my stomach burned. Totally un-did months of “just because” probiotics for maintaining health. Then shortly after resolution of the throat infections and stopping antibiotics I was hit w/ a 99.5-99.7 fever, horrible body aches…shoulders, inflamed jaw that popped around, terrible pain into my high neck, ankle pain, then awful brain fog, and all the nervous system and ME/CFS symptoms came rushing back! I was an hr from home at my apt near school and couldn’t drive home. My mom had to come pick me up and there went last Fall semester. I am finally improving…many supplements, LDN, Florinef & Atenolol for POTS, DGL and pancreatic enzymes for low stomach acid etc etc later. Oh, and Laura Hillenbrand’s whole illness kicked off w/ her gut..in can be read in her New Yorker essay, also at least a couple people in The Optimum Health Clinic’s (UK) documentary of patients experiences. It’s on their Youtube Channel. Then of course IBS is an acknowledged co-mormidity to ME/CFS. There’s something there and it really might be HUGE!
my me/cfs began with gut symptoms.(tahoe-march 1982) i went from 106lbs to 75 lbs in less than two months. so i have always felt that there is a strong gut connection! it has taken me many years to gain my weight back and though my digestion is better it still has a way to go. my biggest issue now is fatigue and pain.
I have had fibro most of my life. Three years ago I had my gall balder removed because of a precancerous cyst. Life went downhill real fast afterwards. Constant diarrhea, heart attack, barrets ,Gerd,etc. I lost 25 pounds in a short time and was hospitalized several times for dehydration . Constant sinus and ear infections caused me to take way too many antibiotics which resulted in cdiff. After four hospital admissions I had a fecal transplant and am now taking VSL probiotics. Tried florastor for 6 months. I also have SIBO so am trying to stay on a diet but am still losing weight. It has been two months since the fecal transplant and my gut is slowly getting better but the fatigue is terrible. I have said for years that there was a connection between CFIDS and my gut but that is just one connection. I am not giving up hope, which I could say the same for some of my doctors. No pun intended but always go with you gut, it doesn’t lie to you.
Don’t know if anyone will see this, but just in case, I thought I may be able to help somebody. I don’t have IBS symptoms regularly, but my wife does, and she has been greatly helped by using an oral suspension of Ketotifen. US docs can prescribe it, but it’s not commercially available here, so she imports it from Canada. It’s my understanding it’s legal. She’s convinced it’s underutilized in the US. It relates to gut permeability problems.
I hesitate to add even more potential complication potential to this issue, but I have been following both the gut/health forums and the herbicide/pesticide forums for some time, and a major question has been troubling me. Since the herbicides that contain glyphosate as active ingredient had been thought to be safe for humans ‘because human tissue does not use the shikamate pathway and only plants do’ (the chemical industry position for many years), it has recently been pointed out that many gut bacteria DO utilize the shikamate pathway that is attacked by glyphosate. Since the EPA has recently, at the request of industry, increased the allowable redidue of glyphosate in foods (because GMO use requires increased glyphosate to overcome resistent weeds, and they have found that crop harvest is easier if glyphosate defoliates the crop just prior to harvest) there was increased uptake into humans. Does this glyphosate survive in active state to transit the gut environment long enough to gte to the gut biome to alter the gut biome assemblage of bacteria? Does this happen to decrease beneficial bacteria and increase harmful gut ecology? If so, has enough research been done yet to justify the EPA increase in allowable contaminant levels in humans? It would be very interesting for epidemiology to be adequately done to clarify any possible overlaps of gut health problems with geographic areas of higher glyphosate exposures either directly or through food residues. Apparently there is a distinct lack of good research to adequately demonstrate safety. But, perhaps the glyphosate is no longre active by the time it transits through the gut enough to alter the shikamate pathway… but it still troubles me.
Robert sent this very interesting comment in but it didn’t go through – so here it is –
In A NUT SHELL. Chronology not perfect.
HAD CFS FOR 18 YEARS. sTARTED AFTER PESTIICIDE exposure 2 years in found the reason I was bed ridden was mold in my house. Recovered substantially after moving out but not enough to be functional and work and what not. – http://s185.photobucket.com/user/antares41_41/media/mold/floor.jpg.ht ml?o=51
Over the years I progressively got worse until I was pretty much bed ridden “again”. My capacity was “necessary” household chores a little computer and some tv was all I was capable of. Tried to will my way out of it and I would have PEM the next day so severe if I tried to stand I would get weak similar to being sea sick. Very dizzy. Followed by a couple of days of funk where I did practically nothing.
Didn’t really have another breakthrough for another 12 years when I discovered gluten and dairy a large part of my not being able to get out of bed, & severe bloating, and a feeling like I was being poisoned. Largely went away. Still had the fatigue and the PEM and racing heart and low bp after eating.
I suspect the high mineral content of the water in T or c, nm was responsible for this and when I switched to distilled the high HR and Low bp went away. And I gained “some” stamina. A few months later started doing about 3 3oz dan-actives a day and the first three days I was like cured. But slowly regressed back to basic chores computer “some tv”, night sweats, sleeping disorder.
Than I discovered that eating just two meals a day separated by 6 hours no snacks last meal 6 to 7 hours before bed almost completely eliminated my sleeping disorder. I now sleep 8 virtually uninterrupted hours! So my schedule is first meal at 11am and last at 5 or 6pm. Nothing but water after this NO CARBS! Do do a couple of coffee’s and an occasional gatoraid but never after 5pm.
Bed at 12am and up when I feel like getting up which is between 8 and 9:30 depending on how stressful the prior day was. I am a stickler with the 12am bedtime weather I’m so tired I can’t keep my eye’s open or not tired at all cause I suspect the consistency with my feeding times and bed time are key to my uninterrupted sleep.
I can’t empathize enough how important Getting all the required sleep cycles Rem, non rem, deep rem is! Helps immensely. Still have the PEM still confined to the light activity but no “long” naps during the day (most the time).
What got me to do this long drawn out comment is back in august I took a ten day course of Xifaxin. Didn’t help with my symptoms at all. Did “seem” to give me some issues with anxiety. Very disappointing. I’d had high hopes for this.
Roughly 3 months ago did a 10 day course of cephalexin for an abscessed tooth stomach shut down. All my food had to be regurgitated. Waited 3 days tried again and couldn’t believe the relief I got from fatigue, brain fog, PEM, and fibro like symptoms!
Got my dad (retired MD) to give me a scrip for amoxicillian. Did that ten days so roughly 20 day course of two different abx and 2 1/2 months after a lot of the relief I got from the above symptoms have lasted.
I should note I quit at 20 days cause the symptoms were starting to come back. I suspect the conventional abx worked where the xifaxen failed because the infection bleeds out into areas surrounding the intestinal tract for instance mesentery tissue.
http://en.wikipedia.org/wiki/Mesentery
Also before this have never done abx since onset of cfs some 18 years ago. Did do some about a year or two before onset.
Interesting.
Robert later added this:
Opps! Looks like the link I supplied to the picture of what was under the tile on my kitchen floor didn’t work! The house had other problems as well like fuzzy black mold inside the wall right under where the A/C drip pan in the attic had overflowed more than once over the years.
http://i185.photobucket.com/albums/x74/antares41_41/mold/floor.jpg
I added the floor pic cause I can’t help thinking the reactivity many of us have to mold as well as gi symptoms are two gigantic clues to how we can diagnose and treat and probably most important prevent CFS.
The Canadian diagnostic list for cfs does not include gut problems. I am a classic case of cfs rapid onset with low grade fevers, enlarged lymph nodes, extreme fatigue. I got sick in 1995 and NEVER had any gut problems until about a year ago when I took Align probiotic thinking it would help me lose weight. All it did was cause stomach problems which I NEVER HAD BEFORE IN MY LIFE – well, ,maybe when I was pregnant.
As to WPI doing research – have any of you read “Plague” by Heckenlively and Mikovits? I am about 2/3 though and taking notes. I will post the significant details on my blog when I finish the book. I do not intend to write about the Whittemores – I live in Nevada, and it makes me sick to my stomach, no pun intended, to even think about them. Forget about what they did to Mikovits. I think Harvey is in jail right now or has been for awhile. I will summarize the carefully documented research on possible retroviruses infecting humans.
Interesting about the CCC!
Thanks, Paula Carnes! You beat me to it. I was scanning the comments to see if anyone mentioned reading _Plague_ by J. Mikovits and Heckenlively. I also made notes of pages on a post it, and when I can, I plan to write up my own “highlights.” I am with you. I am NOT in Nevada; I am disgusted by all I’ve learned reading the book. (I also have been astounded by the large number of significant errors in the text, until it occurred to me that perhaps they were rushing to print before anything “happened’ to it… ?)
so. I am angry to read that they are now “moving cautiously and methodically”… I read that a bit differently. ahem. It’s a dirty business. (I almost bought that test, back then.)
Don’t know if anyone will see this, but just in case, I thought I may be able to help somebody. I don’t have IBS symptoms regularly, but my wife does, and she has been greatly helped by using an oral suspension of Ketotifen. US docs can prescribe it, but it’s not commercially available here, so she imports it from Canada. It’s my understanding it’s legal. She’s convinced it’s underutilized in the US. It relates to gut permeability problems.
Thanks for passing that on – its a new one for me for the gut…
Fascinating reading, affirming much of what I have learned/discovered over the past 30 years!
Has anyone done any studies on the possible chronic infestation of the gut, leading onto chronic toxicity in CFS,ME,FM patients?
would be very interested if a link were to be found. 🙂
I first gave up milk with instant results of no more Irritable Bowel Syndrome. Then I gave up gluten, wheat, rye, oats, ,il let, etc., which meant no more horribly itchy blisters on my fingers and no more stomach cramps or bloating.
I also developed a serious B-12 deficiency after a year of hit and miss veganism. Trips to the library led to smells of burgers which I then ate and sometimes in the morning only eggs and bacon would do. I got information about B-12 deficiency which said it takes 2 years to develop a B-12 deficiency so I was barely eligible for such a serious B-12 deficiency. I have always felt the deficiency was related to my case of CFS, which I now refer to as Orthostatic Intolerance because understanding my body cannot withstand the upright position, sitting or standing, leads to less suffering for me.
This research suggests the gut is key. I am grateful for all the research that lead to this research and am grateful for a study mirroring my own severe symptoms.
So this research makes a lot of sense to me. I know it may take years of follow-up research and more research to explain why this could cause so many seemingly unrelated symptoms, but this is the first research I have read about in a long time that interests me.
Very interesting – have you looked into MTHFR mutation? I’m not really up on it but I think it could make it difficult for you to absorb B-12….the Open Medicine Foundation is sponsoring a study to see how common it is in ME/CFS and the effects it may be having.
First, let me give thanks for all the wonderful research so many people have done over the years. This info as well as the comments are so helpful. Cort, et al. I do think we shall make strides and inroads over the upcoming years. We are all connected somehow, and we must explore our similarities and differences. I do test positively for a number of tick borne pathogens and have had lots of GI issues prior to any antibiotic usage, I might add. My family could also be considered to have ME/CFIDS/CFS as well. Many with Borrelia turn up with autoantibodies and one set is to the gastric parietal cells which produce intrinsic factor enabling us to absorb B 12. Rifaximin and Neomycin were wonder meds for me as well. Borrelia and other pathogens are stored in gut mucosa and we know that lyme patients are immune compromised and we see reactivation of EBV and CMV etc. How all these puzzle pieuices of chronic illnesses fit together will be a phenomenal discovery. Ray, Shikimate pathway is in protozoans and esp Toxoplasma gondii. HIV seguing into AIDS seems to occur with people having these GI protozoans.
Oops! Meant to enquire about any records of chronic Parasitic infections ( above)