

“Key transducers of nociception and pain”
Much of the activity in nerve cells is regulated by very small channels that regulate the flows of ions such as calcium, sodium and magnesium in or out of the cells.
Given the central nervous system problems in ME/CFS and FM and the clear role ion channels in producing increased sensitivity to pain and stimuli, they would seem to be an obvious target for researchersl This study, though – from the NCNED Australian group – is the first to concentrate on them in ME/CFS.
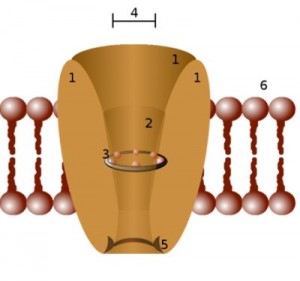
Ion channels control the flow of ions into a cell. The ion channels examined in this study mostly affect nerves.
The ion channels examined in this study – the TRP channels – were recently called the “key transducers of nociception and pain”. (Neurons associated with the vagus nerve also contain many TRP ion channels.)
These channels trigger cells to respond to changes in their environment caused by pathogens, oxidative stress, chemicals, toxins and pH. These ion channels can be activated by numerous inflammatory by-products including toxins, cytokines and irritants.
Given the wide range of substances these ion channels react to having them bug out on you could lead to a lot of problems. Some researchers think the nervous system in ME/CFS and FM patients is over-reacting to stimuli. If it is it could start here.
Super sensitive ion channels could send pain and sensory signals coursing along the nerves to the brain at the slightest provocation. Translated into pain and other stimuli this barrage of sensory signals could hamper your ability to focus and think.
This study didn’t actually test ion channels; they looked for single nucleotide polymorphisms (SNP’s) in the genes governing how these ion channels function. SNP’s are very small changes in the genes that can alter how proteins and ultimately cells function. Some SNP’s produce no change (are benign), while others alter gene transcription in fundamental ways that produce physiological changes in how nerve and other cells function. For instance a SNP could cause an ion channel to open a bit more quickly in response to some stimulus thus telling the nerves to send pain or other signals more quickly to the brain.
Many SNP’s have been associated with diseases.
The Study
Immunology and Immunogenetics Insights 2015:7 1 Examination of Single Nucleotide Polymorphisms (SNPs) in Transient Receptor Potential (TRP) Ion Channels in Chronic Fatigue Syndrome Patients Sonya M. Marshall-Gradisnik1,2, Peter Smith2, Ekua W. Brenu1,2, Bernd Nilius3, Sandra B. Ramos1,2 and Donald R. Staines. 1. School of Medical Science, 2. The National Centre for Neuroimmunology
The Australian group examined 240 SNP’s in 21 genes governing TRP ion channel functioning (over ten per gene on average) in 115 people with chronic fatigue syndrome and 90 healthy controls.
Results
“These are primitive genes that are involved in many cellular signals in the brain, gut, cardiovascular and immune systems, as well as in the mediation of pain.”
In studies like this you hope to have some SNP’s show up but what you really hope to see are SNP’s concentrated in a couple of genes. Finding SNP’s concentrated in a couple of genes would suggest those genes are bad shape; bad enough shape to possibly contribute to disease.
That’s exactly what this study found. It found 13 small gene alterations nine of which were found in one of the 21 genes tested (TRPM3) and four of which were found in two other genes TRPC4/ TRPAI).
The results were close to being even more pinpoint. A fourteenth polymorphism that just barely missed significance (p<.051) was also associated with TRPM3 and two more polymorphisms for TRPA1 and TRPC4 (p<.065 / p< .068) were on the edge as well.
This is the second rather shockingly specific finding in the last year. The Stanford MRI study that found that almost all of the ME/CFS patients had alterations in a very, very small part of the brain (which none of the controls did) was the other. We rarely see such pinpoint results in ME/CFS.
The Genes
Two of the suspect ion channels are “thermosensory channels” (thermoTRP’s) that are activated by changes in temperature – putting a spotlight possibly on inflammation and thermoregulation. Studies indicate that the appearance of these channels in the sensory neurons is closely associated with the appearance of pain. Because they integrate several signaling pathways drug development to block them from functioning is being actively pursued.
TRPC4
The TRPC genes play important roles in memory, attention, sensory acuity, emotion, pain, and motor control in the amygdala, entorhinal cortex, hippocampus, and prefrontal cortex. The specific TRPC gene highlighted in this study – TRPC4 – can affect intestinal functioning and smooth muscle contraction.
TRPAI/TRPVI
TRPA1 regulates the activity of sensory neurons. Nerve fibers containing TRPA1 densely innervate the skin, airways and gastrointestinal tract. Pro-inflammatory and pain producing agents such as bradykinin, histamine, prostaglandins, and trypsin can all activate TRPA1. Once activated TRPA1 prompts the nerves to produce more pain and more inflammation. A super sensitized gene like TRPAI could go far to explain the pain problems in ME/CFS. The TRPA1 ion channel is a key player in the production of headaches and migraines.
TRPAI also interacts with the rather notorious TRPVI gene that has been implicated in migraine and other pain conditions. The two are so closely connected that it’s possible that a balky TRPAI ion channel could affect TRPV1 channels. Increased levels of TRPV1 receptors popped up in ME/CFS patients after exercise in one of the Light’s studies.
The TRPV1 channel is widely distributed in neuronal as well as non-neuronal tissues. In the peripheral nervous system, TRPV1 is highly expressed in a hangout place for herpesviruses – the dorsal root (DRG) ganglia which are implicated in pain sensitization. TRPV1 is over-expressed in several chronic pain conditions such as rheumatoid arthritis, osteoarthritis, bone cancer-induced pain and several neuropathies.
TRMP3
The lion’s share of polymorphisms were found in the TRPM3 gene. The most recently described and least well known TRP gene, TRMP3 was uncovered when it was found that the steroid pregnenolone sulfate activated it. Pregnenolone is the precursor to the mineralcorticoids, glucocorticoids, androgens and estrogens. It triggers the TRMP3 ion channels in the brain cells to release glutamate – an excitatory neurotransmitter.
TRPM3 ion channels have been implicated in inflammatory pain syndromes as well as rheumatoid arthritis, and the secretion of pro- inflammatory cytokines.
This channel’s ability to regulate insulin/glucose intake could impact metabolic functioning. Another intriguing function of this channel is to induce contractions of the smooth muscles than line the blood vessels and other areas. TRPM3 is not that well known yet, but it’s clear it plays an important role in detecting noxious stimuli in healthy and inflamed tissue and research is growing.
Wrapup
Such specific findings are both unusual and gratifying in ME/CFS. They suggest the researchers are on the right track. While the findings in this paper need to be replicated, for now, at least, there’s no muddiness – no need to strain at putting together some scenario that might work. There’s just the rather eye-popping finding that nine (almost ten) of the 13 gene alterations found significantly more often in ME/CFS patients in Australia were concentrated in just one of twenty-one genes examined.
It’s a bit unfortunate those genetic alterations occurred in one of the more recently described and less well known ion channels (TRPM3) but research into this ion channel is growing. (A recent study uncovered a substance able to open TRPM3 ion channels much more quickly than pregnenolone. That substance will be used to better understand TRPM3 ion channels. The finding indicated that TRPM3 activation contributes to neurogenic inflammation.)
What could be happening with these ion channels and ME/CFS? A tendency for them to genetically be set on a hair trigger could perhaps explain the many and often overwhelming sensory sensations (pain, fatigue, problems with stimuli) found in the disorder.
Drug companies are reportedly eagerly pursuing drugs that alter the function on these ion channels.







Was it the problem with the genes that cause the illness or did the illness cause the problems with the genes
The genes would come first I believe. I believe polymorphisms are variations in the genes we are borne with. They would be one of the legs of problem – a genetic predisposition plus an environmental insult, say.
If the genes came first, how were these ion channel governing genes ‘genetically set on a hair trigger’ in the first place?
I am thinking of a possible epigenetic development ‘triggering’ these SN Polymorphisms.
I’ll hypothesise: My great-grandparent, grandparent or even parent had normal (without TRP altering SNPs) was exposed in some way to pathogens, chemicals or toxins – perhaps through occupational exposure before the effect of these on human health were understood. Could this produce SNPs which in turn set the gene governing the ion channels on a hair trigger in the next and subsequent generations?
Once the trigger was there, could the switch be tripped so that my grandmother, my mother and/or me were born with the switch flipped i.e. one or more TRPs altered genes are present in me causing my disease?
I have no idea if this is a new hypothesis or if all I am doing is reading the evidence and arriving at an obvious conclusion. I can tell you it has taken me 3 hours to write this comment.
??????????
(Me thinks I am full of TuRPs – or something that would only need one letter altered by 180 degrees.) Thinking so hard makes me crazy.)
Seriously, if anybody can make sense of what I’ve written – do you think this could be happening?
Christine,
My maternal line is also Scottish descent – and there is a history of illness in the line going back to my great-grandfather, my grandmother, my Mom, me, my son : scoliosis ( some severe/some mild) neurological issues, asthma, rheumatoid arthritis, mirgraine. My grandmother lost 4 out of 7 children to complications of childhood viral illness. A 5th almost died.
My brother was seriously ill with measles virus as a child. My son ( 1985 – age 4) got lab-documented Epstein-Barr virus and developed multiple neurological symptoms, which took years to mostly resolve.
So, it seems in my case as if there is some definite genetic predispostion. But also, it seems as if a new type of EBV may have passed through the population beginning in the late 1970s. The Incline Village story is very interesting – “Raggedy Ann Town” in Hippocrates, July/August 1987. ( sorry no link) Plus, Dr. Anthony Komaroff ( Harvard) has stated that he saw a few cases
in the 1970s, but the big surge began in the 1980s.
Interesting that in the 1960s/1970s EBV was being looked at as a potential bioweapon, and as a way to deliver genetic material to alter the DNA of various cells. I can give more details on this, but trying to limit.
Epigenetics are about expressions of genes, not about single nucleotide polymorphisms. SNPs are fully inherited unless they mutated early in life. Epigenetic factors can of course be inherited as well.
Thanks Merida
I would be happy for you to elaborate on EBV as a potential bioweapon.
My ancestry is generations of coal miners going back to at least 18th century on my Dad’s side and agricultural workers on my Mum’s side. On both sides there is likely to be exposure to toxins, hunger, stress of poor working conditions etc. My Mum definitely had CFS/ME type illness.
I really ‘buy’ the epigenetics theory of changes to genetic makeup both during lifetime and inherited by future generations.
Christine
Sorry Cort, this is in the wrong place, I meant this reply for Merida but can’t see how to move it up.
Christine
I am not a scientist, but I was first drawn to epigenetics via a documentary about an isolated village (I think in Scandanavia) where there was an unusually high prevalence of Type 2 Diabetes. It was shown that direct ancestors of this population had died during famine. At that time there was little scientific validation of epigenetic.
If any part of the process is altered could that not alter gene expression?
As I said I am no scientist but I don’t think there is enough evidence to disprove the above hypothesis – is there?
Last time I spoke with one of Dr. Jose Montoya’s assistants, I proclaimed that he (Montoya) most likely believed that Herpes Viruses, specifically, perform some sort of alterations to our immune system. Unfortunately, I was right. Dr. Montoya does believe that Herpes Viruses do perform some sort of alteration to the immune system, and not the other way around…
Mark Davis’s recent study found that CMV infection causes tremendous changes to the immune system even when the person is healthy..
Thanks Cort,very interesting.
Thanks Cort, you explain this complex paper so well.
Great to see another pinpoint study. 🙂
Thanks – yes, nice to see the findings congregate in one spot 🙂
Thanks Cort for laying this out. If one takes an epigenetic approach, though, could it be that a body damaged by ME/CFS that ‘turns on’ or ‘turns off’ the genes in question?
It could turn on or off these genes for sure but I don’t think it can change the genes themselves. I think these are variations of genes that we are borne with. Whether they are being expressed in another matter. Epigenetic changes could cause these genes to be expressed more or less or not have any effect.
I imagine that if this study is validated they’ll look at the epigenetics involved. (???)
Well, I should have read more than the first comment!
considering that several genetic conditions seperate to CFS can generate the disease state (fulfilling all the clinical criteria ever devised) – Ehlers Danlos Syndrome for example, I’d guess epigenetic changes . theres also the people with gene problems causing methylation issues – quite a sizable subset it seems .
I agree that more and more overlaps between CFS and other disorders are being id’d which is terribly interesting. I have ehlers danlos syndrome and also one of the MTHFR mutations (affecting methylation), and later in life I began to develop disabling CFS symptoms in addition to a plethora of other problems. (chronic pain, dysautonomia symptoms, etc). My personal theory at this point is that CFS will eventually be found to be a collection of similar but different dysautonomia syndromes, with different triggers and causes. As I keep reading and watching the efforts to solve the puzzle, more and more connections/overlaps are being suggested (mast cell disorders, mitochondrial disorders–both of which are commonly suggested as being factors affecting people with Ehlers Danlos Syndrome). And at this point I most definitely do believe that a person’s specific SNPs and genes plus certain epigenetic factors (as yet unidentified for sure) will be found to be the key.
Cort, any idea which drug companies are pursuing ion channel altering drugs. Would be interesting to know if there are clinical trials planned or in progress.
Thanks.
Will
A couple are under way two of which involve pain…The skin one is actually not a clinical trial.
https://clinicaltrials.gov/ct2/results?term=TRPV&recr=Open&no_unk=Y
Recruiting TRPV Expression in Subjects With Sensitive Skin
Condition: Sensitive Skin
Sensitive skin syndrome is defined as the presence of burning, itching or any other unpleasant sensation on the skin, due to physical, chemical or psychological factors. It is frequently a self-diagnosed condition, and there are no accurate tests to recognize or quantify it because of the individual variations in perception and intensity of the related symptoms. The most accepted physiopathogenic theory is the presence of an altered barrier function of epidermis. Also, changes in the pH of the stratum corneum have been found to induce skin sensitivity through the activation of the transient potential receptor vanilloid (TRPV) neuronal receptors.
TRPV1 has been found in human keratinocytes, although its physiologic role in the skin is not yet established. Their presence in keratinocytes and cutaneous nervous fibers suggests a role in the sensitive function of the epidermis. Since this receptors can be activated by low pH (< 5.9), which is also important for the development of sensitive skin, we hypothesized that an increase in the expression of these receptors can be the responsible for the syndrome. 2 Recruiting Safety and Tolerability Study of SOR-C13 in Subjects With Advanced Cancers Commonly Known to Express the TRPV6 Channel 3 Recruiting Effect of Afferent Oropharyngeal Pharmacological and Electrical Stimulation on Swallow Response and on Activation of Human Cortex in Stroke Patients With Oropharyngeal Dysphagia Interventions: Behavioral: Dietary and oral hygiene recommendations; Dietary Supplement: oral TRPV1 agonist; Device: pharyngeal electrical stimulation; 5 Recruiting NEO6860, a TRPV1 Antagonist, First in Human Study Condition: Osteoarthritis, Knee
Thanks Cort for such a comprehensive summary of this research from here Down Under. As an Aussie, I’m really encouraged to see us ‘doing our bit’ to uncpvery another piece in the ME/CFS puzzle.
As for drugs to treat ion channels, my CFDS doctor says clonazepam works on the sodium channels, so it can help with things besides sleep. I’ve certainly found it useful & it seems to have minimal side effects. Will be interested to see whether the drug companies can come up with anything that betters it.
I meant “uncover” another piece in the puzzle. Stoopid auto-spelling!
I’m wondering if the body-wide fasciculations I have could be related to ion channelopathies. They are most horrendous and I’d sure like to find a way to stop them!
This interesting study brings up equally interesting questions.
Do the ion channels react to infections in addition to toxins, cytokines and irritants? Or maybe heightened cytokines from an infection produce a heightened effect in genetically-susceptible individuals?
Would patients be able to self-test for these genes through the currently available consumer genome tests? Patients are self-testing for other reasons, as it is. Or are these genes so new to the scientific world that self-tests would not be an option?
One can only hope that this study will be one of the lucky and rare ones to get replication and further study.
Yes ion channels can react to all of those…
Do the current DNA tests that are available today test for these specific gene variations yet?
I’ve recently had mine done and I don’t see any of the above mentioned listed. But then again my report only lists the genes that are of concern to me.
It is interesting that the people in my family experiencing CFS/ME/FM- type illness are also RhD neg. We posed this question at a support group meeting ( about 15 people) : ie How many had RhD neg. Well, 55% of the group had this blood type ! The highest per cent known in the world is about 35% in the Basques. There are also higher rates of RhD neg in the Celtic groups.
Also, the proteins which carry the Rh antigens are transmembrane proteins, whose structure suggest that they are ION CHANNELS. How about that !!
I was diagnosed with CFS/ME almost 30 years ago when it was thought to be a somatic symptom disorder. I knew then what many know now: it is a real and misery producing illness. I went from being in exceptionally good health to being almost completely disabled. I had to push on though as I had no other means of support. I started experiencing chronic migraines and major reproductive health problems. I am currently recovering from parathyroid cancer and the symptoms caused by calcium and magnesium imbalances sound so much like what you talk about in this great article. I also have a wicked case of PTSD and your comments on how ME can affect the amygdala and lower brain functions also rang true. This study validates so much of what I am experiencing in ways countless specialists couldn’t or wouldn’t diagnose. THANK YOU. I look forward to hearing more about your work.
The result in this study is very intriguing as it fits neatly with other research and the clinical picture. It is interesting to see that the at least twelve year old hypothesis that ME could be a result of a channelopathy may be true.