The Invest in ME Conferences always provides talks on the cutting edge of chronic fatigue syndrome (ME/CFS) research and this year was no exception. This blog provides an overview of some of the talks. The information in the blog was taken from some good tweeting by Maija Haavisto and Phoenix Rising, the researchers abstracts and some other sources.
Maureen Hanson – The search for Biomarkers in M.E.
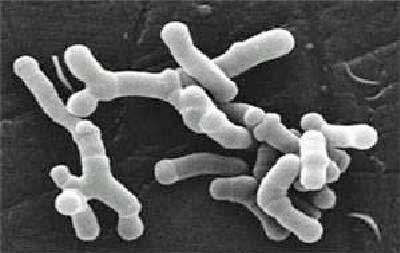
She found that bacteria belonging to bacterial families that produce butyrate, an anti-inflammatory (Ruminococcaceae, Bifidobacteriaceae), were significantly lower in ME. The healthy controls also had more bacterial diversity than the ME/CFS patients.
She also found that some patients have very high levels of lipopolysaccharides (LPS). Also known as endotoxins, LPS are large molecules that are found on the outside coats of gram negative bacteria that the bacteria release into the gut.
These toxins can send the immune system into a tizzy causing high amounts of inflammation. We don’t know how high the LPS levels were in these patients but high LPS levels cause a condition called endotoxemia which is a precusor to the sometimes deadly septic shock. Like all really good immune tweakers, LPS also appear to play a role in autoimmunity.
LPS are clearly bad actors and have gotten a lot of study. The 2011 Nobel Prize in Medicine was won by a man who demonstrated which parts of the immune system the LPS activated. A finding of high levels of LPS in a subset of ME/CFS patients would be significant.
Hanson’s work is not refined enough yet to determine which species might be disturbing the balance in ME/CFS patients guts, but she said she was “on the way to identifying some useful markers”. The Lipkin/Hornig group is also on its way to identifying some useful markers. It was encouraging to read that Hornig highlighted butyrate forming bacteria in her talk as well.
Hanson looked for gut products in the blood that which could indicate that damaged gut linings were allowing gut bacteria to leak into the blood and spark a strong inflammatory response. Using both blood analyses and bacterial composition Hansen was able to identify 83% of ME/CFS patients correctly.
Dr. Hanson is a careful researcher and she stated there’s no evidence yet that gut issues are causing ME/CFS; at this point she appears to be more comfortable saying they are more likely a consequence of ME/CFS than cause.
The gut is a complex place teeming with bacteria and it undoubtedly will take some time to get a handle on what is going on in there. The data thus far, however, suggests something is going on.
The Solve ME/CFS Initiative’s pilot gut/exercise study suggested that exercise was a) causing the gut flora to “bloom” and b) disturbing the gut lining enough to allow gut bacteria entry into the blood. Those bacteria that bloomed in ME/CFS patients (but not healthy controls) remained high for at least three days – long enough to contribute to the postexertional malaise so common in ME/CFS. As we just saw, Hanson has also found evidence of bacterial translocation in the blood.
These weren’t the first studies to find that. Back in 2008 Maes described a “novel pathway” of ME/CFS causation featuring weakened gut lining which allowed the escape of gut materials into the blood.
We’re not lacking for the possibility that pathogens may be effecting the gut either. One of the speakers at the IIME Conference focused on the effects viruses can have on the gut. The Whittemore Peterson Institute’s preliminary data suggests that endogenous retrovirus infected dendritic cells could be impacting the gut. Dr. Chia’s finding of enterovirus infections is well-known, if still, for some reason, not validated by an outside group, and Dr. Pridgen has found evidence of herpesvirus infection in the gut tissues of FM patients. Gut symptoms started Pridgen on his quest to develop antiviral protocols for FM.
Dr. Baraniuk
Baraniuk traced back the autonomic nervous system findings to the brainstem. It turns out that the nerves that power the motor and sensory systems of our body pass through the brainstem on the way to the lower body. The brainstem also regulates cardiac and respiratory functioning, the central nervous system and the sleep cycle.

Baraniuk appeared to reject the broader FM criteria created in 2010 when he argued that tender points are important signs in FM (the new definition rejects tender points) because they signal central hyperalgesia; i.e. central sensitization or a brain that produces pain.
Dr. Cambridge – B cell Biology and Rituximab treatment in
Patients with ME/CFS (Rituximab II)
Dr. Cambridge revealed that the situation with B-cells and Rituximab is more complicated than one might think. Most people probably know that Rituximab knocks out B-cells but that’s just the beginning of a fairly complicated story.
Rituximab whacks B-cells very quickly but in autoimmune diseases (as in chronic fatigue syndrome (ME/CFS)), the benefits don’t usually show up for months. That suggests the problem in these diseases is not the B-cells per se, but products the B-cells are producing, that linger in the blood for months after the B-cells are wiped out.
It’s when these products finally disappear and are not replenished (because the B-cells are gone), that patients feel better. When the B-cells come back and start producing those products again, they tend to get ill again.
Since people with high levels of autoantibodies tend to do better on Rituximab, that illness making product may very well be an autoantibody.
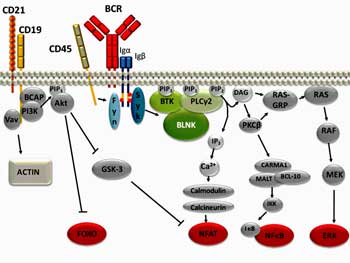
It’s clear as well, that since people on Rituximab generally relapse at some point after taking the drug, the drug doesn’t quite get at the core of the problem.
Thankfully, despite the fact that Rituximab was developed as a chemotherapy drug, side-effects are rare. Many people are worried that wiping many of the B-cells out will leave patients open to infection, but Dr. Cambridge reported that “the effects on protective immunity are mild and serious infections rare”.
Rituximab and ME/CFS
All that information came about from studying Rituximab’s effects on autoimmune disorders. ME/CFS is a bit different story. Dr. Cambridge stated that their journey with Rituximab and ME/CFS is just beginning, and it’s complicated by the fact that ME/CFS is such a heterogeneous disease. Put together a heterogeneous disease and the notoriously complex immune system and you have a knotty problem.
Dr. Cambridge is approaching that problem, though, by learning as much as possible about what’s going on in the B-cells in people with ME/CFS. If I have this right, she believes that B-cells may be reprogrammed as they regenerate themselves to produce autoantibodies.
Thus far she’s confirmed that a protein called CD24 protein that has been implicated in immune and autoimmune diseases is being over-expressed on B-cells from ME/CFS patients. CD24 is expressed on many cells; as of 2010 the role it played in them was mostly unclear but it’s a subject of interest in diseases ranging from autoimmune disorders to cancer. Even back in 2010 it was clear that it played a role in T-cell stimulation.
Dr. Cambridge is tweaking B-cells from ME/CFS patients and healthy controls with different stimuli such as cytokines, antibodies and agonists/antagonists to see how they respond. Something, after all, has to trigger the B-cells in ME/CFS to a) slap that CD24 receptor on their surface and b) to get them to produce the autoantibodies she believes are responsible for ME/CFS.
If she finds a substance – a cytokine or some other factor – that gets the B-cells in some ME/CFS patients to go bonkers, she may get close to how the immune dysregulation begins. That would be exciting news indeed.
She’s also hoping to be able to identify who the drug is going to work in and who it won’t work in. If she can do that then expect a clinical trial that will surely work – and an FDA approved drug for a subset of people with ME/CFS.
Dr. Scheibenbogen -Autoantibodies to adrenergic and acetylcholine receptors in CFS/ME (Rituximab # II)
Dr. Cambridge believes autoantibodies are in play in ME/CFS and Dr. Scheibenbogen, a German researcher, and Drs. Fluge and Mella, are looking for them also. Scheibenbogen is particularly interested in autoantibodies that might be attacking receptors on neurotransmitters in ME/CFS patients.
Thus far they’ve found increased levels of antibodies against two types of receptors in a subset of patients. The receptors were
- the beta 2 adrenergic receptor – this receptor binds with epinephrine (adrenaline) and plays a key role in regulating the blood flows, muscle activity and breathing.
- muscarinic acetylcholine receptors – these receptors regulate the activity of acetylcholine, the neurotransmitter associated with the parasympathetic nervous system.
The two receptors that popped up in this study, just happen to regulate both branches of the autonomic nervous system and play a major role in blood flows. That, of course, fits ME/CFS in spades – a good sign.
The fact that the antibodies to these receptors dropped in ME/CFS patients who responded to Rituximab suggested that Rituximab was doing its good work by re-regulating the autonomic nervous system. The fact that several immune markers were also highlighted in these patients suggested that the autoantibodies were activating B and T cells.
Rather quickly, it appears we have the outlines of a multisystemic model that incorporates several key elements in ME/CFS. Some factor (pathogen) activates the B-cells which begin producing autoantibodies to receptors that govern blood flows, muscle contraction, etc. and sympathetic nervous system functioning. Rituximab knocks down the B-cells quickly but the autoantibodies hang around until they die off. When they do both the immune and autonomic nervous systems of at least some people with ME/CFS begin to return to normal and they feel much better.
It appears that about 30% of ME/CFS patients thus far have elevated antibodies to these B adrenergic or acetylcholine receptors. This isn’t the first time one of these autoantibodies have been found in ME/CFS, by the way, a Japanese study found elevated muscarine receptor autoantibodies in about 50% of patients.
Dr. Scheibenbogen proposed that the kind of ME/CFS you have may depend on which autoantibodies are present. According to the tweets, she suggested treatment programs consisting of Rituximab, high dose IgG and M3 acetylcholine stimulation might be helpful.
The stars seem to be aligning a bit for Rituximab. As researchers have expanded their understanding of what the drug is doing in ME/CFS they produced a model that seems to fit ME/CFS very well.
I don’t think anyone thinks Rituximab will help everybody with ME/CFS but if it works in a substantial percentage – and if researchers can pinpoint who those people are – Rituximab will be way ahead of the game.

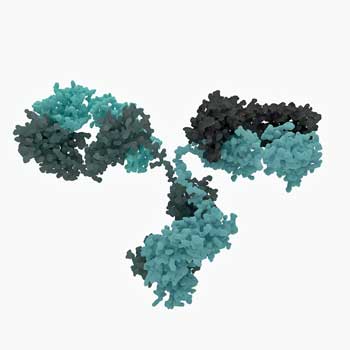




“Thankfully, despite the fact that Rituximab was developed as a chemotherapy drug, side-effects are rare.”
What patient population are side effects rare in? Non-ME/CFS or ME/CFS? I’m betting side effects are much more common in ME/CFS, especially hypersensitive patients.
Is it possible to get tested for some of the relevant autoantibodies or are these tests not widely available outside of research labs?
I think that was in autoimmune diseases in general although I haven’t heard of significant side effects in ME/CFS yet (???).
I don’t know about those autoantibodies but I wouldn’t be surprised if this is research lab stuff. Some may know better than me though.
Whitney Dafoe’s current situation is a result of Rituximab if I’m not mistaken?
Hi Troy,
That’s not my sense. I think its possible that Whitney tried it (I think I might have heard that ?) but he’s tried many things and nothing has halted the decline. I don’t think anything outside of the fact that he has a very severe case of ME/CFS is responsible for where he is at.
Great article Cort. We have started checking the PANDAS autoantibody panel (Cunningham panel) and are finding a good correlation with symptoms (the test is expensive, however). Been hesitant to do Rituxamib because we have found almost all CFS/ME patients have abnormally low CD19/CD20 cells, so not sure if want to lower them even more?? This is great research. Been doing LDN, IVIG and peptide immune-modulators with some success. Looking into doing plasmaphoresis.
Thanks
Thanks Dr. Holtorf. I didn’t know about that panel. For those who are interested here’s some information about it
These tests measure circulating levels of autoantibodies directed against specific neuronal antigens, including: Dopamine D1 receptor (DRD1), Dopamine D2L receptor (DRD2L), Lysoganglioside GM1, and Tubulin.
The 5th test, CaM Kinase II (CaMKII, Calcium-dependent Calmodulin Protein Kinase II) activation, produces a laboratory value (expressed as a numeric score) that reflects the percent above or below baseline CaMKII activity in a human neuronal cell line. CaMKII is a key enzyme that is involved in the upregulation of many neurotransmitters such as dopamine. CaMKII is also understood to increase the “plasticity” or sensitivity and responsiveness of neurologic receptors to neurotransmitters.
Our goal, at this time, is to assist the physician by determining if there are elevated anti-neuronal antibodies and neuronal cell activating antibodies circulating in the patient’s blood…
http://www.moleculeralabs.com/cunningham-panel-pandas-pans-testing/
Thanks for retrieving this much of the jist of the conference, Cort.
One little question I have is about Dr. Baraniak’s current view on FM criteria. I couldn’t understand your sentence. Did you mean he still does believe in the earlier definition requiring the tender points or he now likes the new criteria which requires pain, but not that specific form of it?
It was my sense going off the tweets that he felt the older criteria was better. That was an interpretation and its possible something got lost in the translation.
The Muscarinic and Acetylcholine receptor autoantibody testing from what I understand is not available unless you are in a clinical trial. Is this correct?
I know the standard Acetycholine rescepotor autoantibody test is available for Masthenia Gravis. But these ones talked about are different.
If it these tests are available does anyone know where?
http://forums.phoenixrising.me/index.php?threads/celltrend-test-for-me-cfs-prof-dr-carmen-scheibenbogen-university-hospital-charite-berlin-etc.41232/
About a year into my illness with ME/CFS (4 years ago) a doctor ran a Acetylcholine receptor antibody test on me to rule out Myasthania Gravis. The test came back positive. The doctor was perplexed as outwardly I didn’t exhibit any of the physical characteristics of Myasthenia Gravis. He ran the test again to be sure a mistake hadn’t been made. Again it was positive. I was then sent to Myasthenia Gravis specialists who eventually concluded I didn’t have it. In total 3 Acetylcholine receptor antibody tests were done and all came back positive. It’s interesting to now read of these findings by Dr. Scheibenbogen. Does anyone know if Rituximab is available in the UK?
Great work Cort, thanks for making this information available and somewhat understandable. You do a great job and make research exciting! ?
Thank you for another great job of reporting Cort!
Thanks Pat for your support!
I administered Rituxan for years (in my former life as an oncology nurse) and found side effects to be very rare and easy to treat. Usually Benadryl and Tylenol did the trick. It’s nothing like chemotherapy. I wouldn’t hesitate to try it myself.
That’s very good to know Aggie. When I hear chemotherapy I think disaster! So thanks for passing that on.
Aggie- What tests are needed to be completed to be eligable for the Rituxan? Are the tests available in the US? Or in the SE area. Thank you and also thank you Cort. Great Reporting!!!
Thanks Carole.
I want to thank you for posting all the articles and information. You offer the one thing that is difficult to hold on to….hope. Being 62 and having ME/CFS for 14 years lately I am prone to groan, “I’ll never be well”. Probably many others do the same. I have such severe neurological symptoms and I often wonder how in the world the damage could be reduced, yet alone be eliminated. It seems a long, long road for researcers to figure out what causes this, yet alone find a ‘cure’. But, then I’ll read one of your articles and find that renewed HOPE, that perhaps there will be a way forward and hopefully in our lifetime.
Thanks Stephanie. As someone who’s had this for over thirty years I can well understand how easy it is to see a future that looks like the past has. Hopefully it will not – and these and other findings will end up in a way forward in our lifetimes. Here’s to that!
Hi Stephanie and Cort, I too am a veteran of some 31 years and just when I think i’ll never be normal again (now 64 years old) a glimmer of hope appears. Quite honestly I would settle for a fraction of my former health. Thank you for keeping us going.
Thanks for your efforts and for the important Information, hoping that the researches related to the CFS/ME will get more attention and Funds to find a cure for this mysterious disease
Cort, I’m curious how the elevated antibodies to the beta 2 adrenergic receptor and/or muscarinic acetylcholine receptors affects production of epinephrine and acetylcholine respectively. Downregulated? Upregulated? Both?
This is amazing news, thank You to every doctor and scientifics for putting so much esffort to solve and help this much afflicted SUFFERING community thats has a Life WORTH than HIV,AIDS… But my theory after carefully reviewing and Researching solo much is that a RETROVIRUS IT HAS TO BE BEHIND THIS HUGE BODY IMMUNE CHANGES…my Wife and i have it and We are 2 different genetic individuals , i have see changes of immune funtions in us thru out this Last 5-6 years till now We started to have All this symptoms and tested + for EBV..this and soo Many things including DRAMÁTICLY increase in cases over this Last few years specially in US.. Here the clinics are full of people with cases every Day .the seg prevalence is when people are MORE SEXUALY active with some excepto a With All do respect no offense to anyone this has to be a retrovirus thats spreading in us SAME way that other retroviruses does , SEXUALY, BLOOD,,,,it could be small porcentage of people that were born with defficient immune system and developed the illness but also could of been blood transfused or direct line.. There is several similaties with Htlv-1 TSP HAM HTLV-1 could be in a person for years before develope to TSP ,, most of people never do, the underlaying cause of All this immune desregulations could be in that family or MLV or lukemia..THIS IS NOT NORMAL.
There is soooo much darkness in this horrible devastating suffering illness from all governments institutions cdc, nih,fda.. It’s More than clear that this cronic illness that it’s affecting afflicting millions of Americans is do to an underlaying cause, a virus, a retrovirus something that Me/ Cfs suffers get at some point in their life that’s starts debilitating their immune system alone the years to a point when they less expected and unaware of what they have inside of them , they aquire a new infection like EBV or CMV or a flue and this viruses have a huge impact and do a lot of damage do to the poor defense of the immune system, there is tons of studies that proof that, in most cases are the trigger to turn progress on me/ Cfs,,
almost thesame way happens in people with immune deficiency like HIV, HTLV-1 and other viruses, it may be thru a different mechanism but the bottom line is thesame, htlv-1 is a retrovirus from the lukemia family that affect humans, it has a lot of similarity to me/Cfs just with out the profuse fatigue, but the prevalence it is more among woman 80% more than man, both have severe neurological and immune desregulation,overactive immune system, also in htlv-1 doctors find higher indices of cancer cells as they do on me/Cfs patients,, both are debilitating,Htlv-1 is a virus from the lukemia family and could be on a person for years -3-5 years in cases 10 years with out progressing to htlv/1 tsp/ ham..or lukemia cancer,, that are really the cronic part of the illness,, all this indications and many more make me believe that a retrovirus or a virus from the lukemia family could be behind this devastating illness.
the most surprising part what amaze me the most is that years ago many scientifics found evidence of a lukemia family virus call XMRV,, in many patients with ME/CFS,, no one , but many from different places, there was even a test for XMRV and many patients tested positive but it was taking out of the labs by the guvernametal institutions and FDA and they said the test were contaminated and that’s why the results were positive,BUT THEY NEVER DID SOMETHING TO KEEP LOOKING OR DIGGING IN WHAT WAS CAUSING IT, THEY DIDNT GET THE BEST SCIENTIFCS TO LOOK FOR THE CORRECT RETROVIRUS THATS POSSIBLY ALMOST 99% SURE IS IN THE LUKEMIA FAMILY( or) MLV.even till this Day phycitians doctors still talk about the conspiracy theory behind this actions),,but what’s more amazing is that they never brough back a test that will meet All the requirements and standars with FDA regulations and With Out contamination,,becouse they said XMRV was a mice ( mouse) virus and do not infect humans) why they dont have that test aveilable in the labs????
If there is tests for other animal inffections that affect humans humans , there is tests for many other viruses regardless if they are the cause or not of an illnes,, But more surpricing is that this test was takikg Out of the lab in 2012 they dismissed that this virus could inffect humans but years later the blood Banks come up with aa a molecula to detect and destroy MLV ( XMRV) VIRUSES IN BLOOD BANKS,,LATER 2015 WAS INVENTED A FILTER FOR MLV VIRUSES????COMUN SENSE IF BLOOD BANKS USE FILTER FOR MLV VIRUSES FOR LUKEMIA VIRUSES MEANS THEY BELIEVE THE POSSIBILITY THAT MLV AND LUKEMIA VIRUSES CAN BE TRANSMISIBLE IN BLOOD AND IF THET COULD BE TRANSMISIBLE IN BLOOD MOST LIKELY COULD BE SEXUALLY TRANSMISIBLE,
GO TO FACE BOOK AND SERACH FOR WALTER IRVINE, YOU CAN SEE THE VIDEO THAT HIS WIFE RECORDED BEFORE SHE COMITTED SUICIDE, ITS ALSO IN YOUTUBE,,SHE CLEARLY SAD HER HUSBAND GOT IT FROM HER 8 YEARS AFTER MARRIED.. THIS IS A TRASMISIBLE DISEASE AND SHE ALSO SAID THE GOVERMENT CLEARLY KNOWS THAT AND HAS IGNORED THAT FACT FOR 30 YEARS, (( YES NOW THEY ARE PREVENTING BLOOD BANKS TO TRANSMIT THE DISEASE THRU BLOOD BANKS LIKE THEY DO WITH HIV, HTLV-1 NOW!!!BUT WHAT ABOUT THE MILIONS OUT THERE WITH IT ALREADY TRANSMITTING IT SEXUALY OR VERTICALY OR DIRECT LINE TO THEIR CHILDRENS AT BORN)!!! WHAT WOULD OF BEEN OF THE WORLD IF HIV-AIDS WOULD OF BEEN OUT OF CONTROL AND PREVENTION)))
XMRV is From the MLV family.. Why people WITH ME/ CFS can not dónate blood if there is no viruses on them?? A doctor in molécula Dr mikovits found Out this virus i most of her CFS patients in an esffort to understanding the cause of CFS,, she found out that blood banks could of been contaminated and millions of americans could be harboring this virus , that at some Point in their Life will become cronic ill, like it happen Many years ago WITH HIV( AIDS) .. And this findings turned her Life into aches, she was arrested ,
You can find All details by Google( dr mikovits CFS , I have done alot of Research and reding , From doctos, scientifics the more that i Research and read something doesnt make sense , becouse in most of publications the position of the govermental institutions like CDC and Many others it have been to dismissed this illness , to downplay this illnes and the possible involment of a virus perphas XMRV or a virus From the MLV , lukemia family..
Dr Nathan in charge of the new 2016 NIH initiative clinical trial with 40 patients with me/Cfs said he bilieve the action is in the Immune system, but that was said 20-30 years ago!! A lot of doctors scientifics suspect the problem was in the immune system and posible that a virus most likely a RETROVIRUS.. Could be the cause of the illness as we see it on HIV, AIDS, HTLV-1…
Why now after so many years the realize that, that would of been easy to realized when You see a family husband Wife that are to different genetic with this illness and All the symptoms perhalf even their children as a result of ditect line transmition as its been documented, im not a scientific, but base on the facts im a strong believer that the real cause of all this cronic illness its a virus most likely a RETROVIRUS that Many milions of americans are unaware they have and most Likely is Transmited by blood or sexual Transmited like most of this.
Viruses are, HIV,, HTLV-1..HTLV-2..ALL OF THEM ME/CFS,, FIBROMILAGIA… THIS IS THE TIME WERE WE ALL HEALTHY, OR SICK SHOULD RASE OUR VOICES AND HANDS TO ASK THE GOVERMET TO TAKE REAL ACTION BEFORE THIS BECOME EVEN MORE EPIDEMIC LIKE ONE DAY AIDS WAS,,RIGHT NOW ITS ALREADY EPIDEMIC NUMBERS RISE ITS BY MILLIONS WITH ME-CFS .. MORE MILLIONS WITH CFS LIKE SYPTOMS,,WHAT IS NEED IT FOR THIS TO HAPPEN????30-40 MILLIONS OF AMERICANS LEYING SUFFERING IN BED???? OR THE INMEDIATE FAMILY MEMBER OF A PRESIDENT OR A SENATOR TO BECOME CRONIC ILL WITH THIS DEBASTARING ILLNES?? NO THE TIME TO DO IT IS NOW, IT DOESNT MATTER WHAT WAS DONE WRONG IN THE PAST !!!
WHAT MATTERS IS TO FIND THE CAUSE AND TREATMENT FOR THE MANY MILLIONS SUFFERING , BECOUSE THIS NUMBERS ARE ON THE RISE, EVERY DAY PASS THERE IS MORE PEOPLE WITH THIS ILLNESS,,THERE IS ALREADY TO MANY MILLIONS SICK,WE NEED PREVENT THE OTHER MANY MILIONS OF US CITIZENS AND PEOPLE AROUND THE WORLD TO HAVE SAME FAITH!!!!!!NO MORE MISLEADING INFORMATION NO MORE MISLEADING CLINICAL TRIALS AND OBCURE WAY OF TREATING THIS,, IT HAS BEEN ALREADY TO MANY YEARS OF THAT TO MANY PEOPLE SUFFERING DEEPLY FOR THIS TO CONTINÚE HAPPENING,!!!
BESIDE DR NATHAN IN THIS 2016 CLINICAL TRIAL IT SEEMS ONE MORE TIME SOMETHING ODD, SOMETHING VERY CONTROLED,, SOMETHING NOT CLEAR NONE SENSE, HOW THEY ARE GOING TO PUT IN CHARGE AS PART OF THE TEAN RECRUITING 40 PATIENTS FOR THE TRIAL, A PERSON THAT ALL THIS MANY YEARS A GO HAS DINIED ME/CFS AS A REAL CRONIC ILLNESS CAUSE IT BULY DISFUNTION OF THE IMMUNE SYSTEM, HE HAS BEEN ONE OF THE BIGGEST PROMOTERS THAT CFS IT WAS A PHYSICOLOGICAL DESORDER, BRIAN WALITT !!
WHY THEY DONT MAKE UNREFUSABLE OFFERS TO THE BEST WELL KNOW DOCTORS AND SCIENTIFICS ON CFS???LIKE DR MONTOYA, DR KLIMAS, DR NANCY REY, AND MANY OTHERS THAT KNOW BETTER THAN ANYONE THE BIOMARKERS, THE SYMPTOMS WERE TO GO WERE TO CONENTRATE THE SEARCH, WHY IF THEY KNOW MOST OF THE IMMUNE DESORDERS ARE CAUSED BY RETROVIRUS WHY THEY DONT PUT ASIDE WHAT HAPPEN IN THE PAST AND BRING IN ONE OF THE BEST RETROVIRUS HUNTERS IN THE WORLD FRAK RUSCETTI ( HE FOUND HTLV-1 RETROVIRUS, WHY THEY DONT BRING ON BOARD DR MITKOVITS ?? ONCE AGAIN THIS CLINICAL TRIAL IT SEEMS TO BE VERY CONTROLED BY INDIDE PEOPLE OF THE NIH AND PEOPLE THAT HAVE DISSMISED THIS ILLNESS FOR MANY YEARS FOR ALL WE KNOW THE PATIENTS HAVE BEEN CAREFULLY SELECTED AND CONFIRMED THAT NO RETROVIRUS HAVE BEEN DETECTED IN THEM TO KEEP THE OBSCURE AND UNCLEAR EVENTS THAT HAPPEN IN 2011-2012 WHEN THE XMRV WAS DISSMISED BY THE GOVERMENTAL INSTITUTIONS…
WHEN WHAT THEY WERE SOPUSE TO DO WAS TO PULL ALL THEIR RESOURSES AND ESFFORTS INTO FINDING WHAT WAS THE RETROVIRUS THAT WAS THE CAUSE OF THIS TERRIBLE DESEASE… THEY SHOULD OF COME OUT WITH A NONE COTAMINATED TEST AVEILABLE FOR EVERY CITIZEN..,WHY WASNT DONE LIKE THAT?? IM A TRULY BELIEVER THAT SOONER OR LATER ALL THE THRUTH WILL COME OUT SOME HOW,, BUT LIKE I SAID BEFORE NOW ITS THE TIME TO CHANGE THIS FOREVER AND NOT LET MILLIONS OF AMERICAN , PREGNAT WOMANS, NEW BORNS,,INTIRE FAMILYS, CHILDRENS IN THIS SELDOM DEATH THATS THIS CRONIC ILLNESS OF ME/CFS,, THATS PROGGRESIVE STATEGES IS COMPARE TO LATE STAGES OF AIDS!!!!!!
THATS HOW BAD ITS WHEN DOCTORS WITH A DOUBT SAY THEY MUCH REATHER HAVE HIV AND NOT ME/CFS… LETS UNITED OUR HANDS OUR VOICES TO FOREVER CHANGE THIS AND ASK NIH, ASK OUR PRESIDENT TO CHANGE THIS FOREVER AND THINK IN THE MILIONS OF US CITIZENS BEFORE THINKING IN WHAT THE CONSEQUENCES COULD BE OF THE WORLD KNOWING THAT THEY COULD OF STOP THIS MANY YEARS AGO AND THEY DIDNT!!!!!!!!! (( AND YES ARE SEEN DOCTORS FOR ME/CFS AROUND THE WORLD LOOKING FOR ANSWERS , WE HAVE ALL SYMPTOMS WE TEST NEGATIVE FOR HTLV-1, WE SHOW NO DAMAGE ON BRAIN MRI (( WE ARE THE EXAMPLE I WAS SAYING BEFORE OF TWO DIFFERENT INDIVIDUAL WITH DIFFERENT GENETICS ,SO NO WE DIDNT BORN WITH THIS DISFUNTION, COMUN SENSE, LOGIC AND ALL THAT I MENTIONED BEFORE INDICATE THAT ONE OF US MAY OF HAVE THIS ( UNKNOW NOT DISCOVERED YET( OR MAYBE DISCOVERED BUT CONTROLED AT HIGH LEVELS FROM GOVERMENTAL INSTITUTIONS)) VIRUS O RETROVIRUS SUSPECTED FOR MANY YEARS IN THE CAUSE OF THIS DESORDERFOR AND PASSED TO EACH OTHER ,, UNAWARE OF IT , THE WHEN WE BOTH GOT EBV NO SURE EXACTLY WHEN WAS BUT WE TEST POSSITIVE FOR HAD IT AT SOME POINT THE ILLNESS WAS TRIGGER TO PROGRESS TO CFS!!!!
OUR 5 YEARS OLD IS SHOWING SYMTOMS TO??I WILL GO TO THE END OF THE WORLD TO FIND THE ANSWERE OF THIS FOR HIM!!!!!!MY QUESTION IS HOW WE ALL COME TOGETHER TO ONCE FOR ALL MAKE JUSTICE FOR ALL THE CHILDRENS AND ADULTS THAT HAVE DIE , EITHER FROM COMPLATIONS OR HAVE COMMITTED SUICIDE FROM SO MUCH SUFFERING AND THE MANY OTHER MILLIONS DEEPLY SUFFERING!!! LETS ALL COME TOGETHER!!!!!!
Hi William,
I think one reason that the government is not interested in XMRV anymore is that they only found it in the blood that had been stored at the WPI. When they looked at blood from ME/CFS patients from other facilities it wasn’t there. That suggested that while XMRV is real that it got into the samples at the WPI – not into the patients themselves.
Then they found that XMRV came from the substance used to culture the blood – that cleared the mystery of where it came from up. That’s my understanding anyway.
Hi cort Thanks for replaying, in regards to what You just said, You probably know more than me, but they were others scientifics around the world that also proclamed findings XMRV in me/ CFS patients, statements that were later retracted when Our goverment finally with small CONTROLED by them studies said the contamination theory, but beside mitkovits also a famous retrovirus hunter Frank ruscetti the Guy that Many years ago found HTLV-1,, but beyond that there is a theory of conspirancy with all this XMRV ,, and the dismissed and downplaying behavior of the goverment is not understandable.. There is been suspected for Many years a retrovirus or similar as and underlaying cause that turns our bodys immune system that way.. Thats more than CLEAR why they havent Research and do as they did with HIV / AIDS Many years ago?? This is EPIDEMIC 17-20 millón people SICK around the world. If it was not something transmisible there is no reazon why my Wife and i will have it.. Do You agree with All this????
I certainly agree fully William that this disease deserves tons more research and if and when it comes who knows what they will find.
As to transmissibility – I don’t think the studies bear out much transmissibility although perhaps some forms are; it could be that a really bad bug that spreads kicks off some ME/CFS. In that scenario it could be transmissible.
There’s certainly evidence for a genetic predisposition – which could, unfortunately, explain your daughters symptoms if she should end up having this.
There’s a lot we don’t know….who knows what researchers will ultimately find?
All do respect dont get me wrong i do not mean to offend any body , but its not my DAUGTHER its my Wife and my Wife and i dont Share any genetic, and We have no reazon to have thesame illness of this kind if it wasnt SEXUALY transmisible.. Many other theories believer that its transmisible Google the vídeo of char Urvine before she comit suicide she clearly say that this was a trasmisible disease her Husband got it From her 8 years after married.. How would you explain that?? In miami there is no such bug … How come my Wife and i got it after EBV that trigger it.. But any body gets EBV and nothing happens unless immune is compromise before this my wife and i were very healthy individuals… Its been documented Husband and Wife and son that happen direct line From mother as it happens with htlv-1 ..
Hi cort,
Hope You are not upset at me, im just just new with the illness and the more i read and analize it the less i understand how for Many years this cronic illness is been kept in shodow and dismissed by NIH, CDC and Many other goverment institutions and treated as phsycological illness when its more that that its caused by viruses and infections. In desperation to find a Doctor with Good knowledge than can help us out we just travel From miami to new York to see a doctor that has been a Researcher and CFS doctor for Many years, he has helped thouthands of patients with Me/CFS .. He has work earlier in his career in NIH.. He was amazing with us in every way ill rate this Doctor a 5 Out 5,he lisent take his time explain and has a very Good knowledge about the illness, one of the things he Share with us is that CDC, NIH.. They long know that this is cronic illnes that its not phsycological or anything similar that since the very first days there is strong evidence of viral and microbioal factors involved, evidence of a very disfuntional IMMUNE SYSTEM..he told us the suspicions of a retrovirus involved as the unknow underlaying cause its been proposed to CDS, NIH .. Many times and they have dismissed and continiusly turned down. In other words he said they dont Care to find it, actually THEY DONT WANT TO FIND IT… Thats why they have dismissed discredit this illness.
He even believe as i do and many of the Me Avócate GROUPS that this sooo call NIH iniciative súper comperhensive with only 40 patients its no more than a polítical show to say they are doing something about, he Share same opinión that if they really wanted to crack this up and finally find the cause, the real MECHANISMS of this illness they would of bring on Board to that study the very Best virologist in the world to work on it , they also would of bring the doctors and Researchers with lots of experience on ME/CFS… THIS SO SAD WITH SO MANY YEARS OF SO MUCH SUFFERING OF KIDS, ADULTS, OLD PEOPLE, WOMAN, MAN, ON AN ILLNESS THAT AT SOME POINT IS COMPARED TO LATE STAGES IF AIDS AND THEY STILL NOT PUTTING ALL FIRE POWER TO SOLVE IT. .. SOOO SAD
William: Who is this dr in NY and did he help you. If so,
what did he do/prescribe that helped you?
Honestly no reazon what so ever of why my Wife and i will become ill around SAME time( ME/ CFS ) itself may not be transmisible but the cause that makes immune system deteriorate after years im 300% sure its transmisible.. The rise in cases in UNITED STATES and in the world EXPLAIN it.. My case Husband and Wife to have it around SAME time makes it clear, We have test negative to HIV, HTLV1-2.. Lyme everything,, We both have 95-99% of symptoms and im assuming We are just ok the onset, miofacial pain, pain spreading All over body , tons of food allergies if We eat bad later and next Day no energy , Brian fog, pain awfull All over, always tired, sleep desorders, irritable bowel, dizzenes and náusea standing up,, tingling twiching.. Profuse sleepiness memory lost!!! Why?? Two different genetic will have MECFS??? And yes my son only 5 its showing symptoms wich subgest what ever either of us got From some one else Many years ago We passed into each other and Our son at birth probably got ir direct line breast feeding.. As its documented in HTLV-1,, There is Many more familys like that !! can You explain me Logic for this?????
Thank you Cort for all your hard work! If it weren’t for your
research, review, extensive knowledge & reporting, I could not keep up with or make sense of all the important results of the studies being done re CFS/ME.
My question regards Dr. Hanson’s research and finding re high levels of lipopolysaccharides. I had been planning on starting a specific probiotic saccharomyces, boulardi and mannanoligosaccharides. Do you know if these are lipopolysaccharides? Also if part of the equation of CFS/ME is gut leak, do you know if the leak can be stopped & how?
Hi lynne,
This Doctor was really helpfull, he is a doctor with Many years of experience have treated thouthands of patients with Me/ CFS and Many other diseases.. He is very knowledgeble, he will check individually in every symptoms you have and beside the facts of been a result of the ME he will explain you why and what it could be and order test for it, since Our immune system is do Impared beside the viruses EBV, HHV6, CMV etc.Also little micro organisms that dont do any harm on healthy people in us they do, All im saying is based only in the 1 consultation we had with him. Im going to wait a little longer till my results come back treatment etc to recomend it to you and to anyone that he can help, i know thats one of the worth Parts of this cronic disease that very few doctos have real knowledge about the illness, i dont want to rush recomending him them later end up like Many others.. One thing for sure , as i mentioned before is that our goverment , NIH , CDC , they dont have any interest in helping us, thats why ME/ CFS is one of the most cronic suffering illness of this era and there is so small amount of phycitians doctors to treat it, We Owner that to them( THEY ARE HIDDING SOMETHING THAT HAVE KEEP US IN THE DARK THE SILENCE ALL THIS YEARS, WE NEED TO SHARE RISE THIS EVERY WERE WE GO EVERY PERSON WE SEE AND TALK TO!!!!!THE RESEARCH THEY ARE DOING NOW WITH 40 PATIENTS ITS IS JUST POLITIC TO SHOW THE AVÓCATE GROUPS AND THE WORLD THEY ARE DOING SOMETHING AND THEY CARE.. WE NEED TO AVÓCATE FOR A RESEARCH WITH REAL WORLD CLASS VIROLOGIST AND DOCTORS AND RESEARCHERS WITH REAL EXPERIENCE TO FIND THE TRUE CAUSE OF THIS IMMUNE DETERIORATION AND DISREGULATION IN US!!!WE NEED TO STRONGLY AVÓCATE FOR THAT SO WE CAN SEE A POSITIVE CHANGE AND QUICK..THIS IS TO COMPLEX OF A DISEASE WITH TO MANY DUSFUNTIONS FUXING ONE WONT SOLVE OR HELP MUCH, FINDING THE CAUSE THE ROOT LIKE THEY DID WITH HIV MANY YEARS AGO ITS WHATS REALLY GONA HELP US!!!
Hi cort,
Hope You are not upset at me, im just just new with the illness and the more i read and analize it the less i understand how for Many years this cronic illness is been kept in shodow and dismissed by NIH, CDC and Many other goverment institutions and treated as phsycological illness when its more that that its caused by viruses and infections. In desperation to find a Doctor with Good knowledge than can help us out we just travel From miami to new York to see a doctor that has been a Researcher and CFS doctor for Many years, he has helped thouthands of patients with Me/CFS .. He has work earlier in his career in NIH.. He was amazing with us in every way ill rate this Doctor a 5 Out 5,he lisent take his time explain and has a very Good knowledge about the illness, one of the things he Share with us is that CDC, NIH.. They long know that this is cronic illnes that its not phsycological or anything similar that since the very first days there is strong evidence of viral and microbioal factors involved, evidence of a very disfuntional IMMUNE SYSTEM..he told us the suspicions of a retrovirus involved as the unknow underlaying cause its been proposed to CDS, NIH .. Many times and they have dismissed and continiusly turned down. In other words he said they dont Care to find it, actually THEY DONT WANT TO FIND IT… Thats why they have dismissed discredit this illness.
He even believe as i do and many of the Me Avócate GROUPS that this sooo call NIH iniciative súper comperhensive with only 40 patients its no more than a polítical show to say they are doing something about, he Share same opinión that if they really wanted to crack this up and finally find the cause, the real MECHANISMS of this illness they would of bring on Board to that study the very Best virologist in the world to work on it , they also would of bring the doctors and Researchers with lots of experience on ME/CFS… THIS SO SAD WITH SO MANY YEARS OF SO MUCH SUFFERING OF KIDS, ADULTS, OLD PEOPLE, WOMAN, MAN, ON AN ILLNESS THAT AT SOME POINT IS COMPARED TO LATE STAGES IF AIDS AND THEY STILL NOT PUTTING ALL FIRE POWER TO SOLVE IT. .. SOOO SAD
I have had CFS/ME for 32 years and tried almost everything for my very severe case of CFS. The paleo diet & probiotics have really helped my pain, stomach problems, etc. probably because of the inflammation & gut imbalance. It took over 6 months or so for it to start making a difference and gradually it’s gotten better and better over the past 4 years. (The first 6 months were very difficult) I’m wondering if large doses of antibiotics and several different herpes viruses played a big role in my illness.
Good to hear Nancy…Has the diet impacted your energy?