

“The pathological nature of the fatigue experienced by ME/CFS sufferers is its inexplicable persistence, severity and its inability to be sufficiently relieved by rest.” Armstrong et. al.
The Naviaux metabolomics study with its findings suggesting that a hypometabolic state is present in chronic fatigue syndrome was thrilling, but it wasn’t the first or even nearly the first ME/CFS metabolomics study. The Aussies (McGregor, Gooley, Butt, and more recently Armstrong) have been plugging away at metabolism and metabolic work for years, and their 2015 study – ignored by most – was as exciting as the Naviaux paper. Ron Davis glommed onto it early and praised it. Looked at in light of Bob Naviaux’s work, the paper, with its similar core findings and somewhat different interpretations, is exciting indeed.
Christopher Armstrong, the lead author, is an example of the kind of young researcher this field needs so much. An Australian researcher working with Neil McGregor – a metabolomics pioneer in the ME/CFS field – Armstrong represents hope for the future.
Armstrong was not at IACFS/ME conference but Dr. McGregor was. McGregor’s mostly been working in the shadows for years but he’s out of the shadows now and appears to be in considerable demand. He was not being totally ignored before; a year or so ago the Australian group’s work caught the eye of an ME/CFS researcher – Dr. Fluge – who doesn’t miss much. Dr. Fluge’s subsequent metabolomics work was one of the highlights of the IACFS/ME Conference.
When I asked Armstrong how he got involved in chronic fatigue syndrome (ME/CFS), he noted that Dr. McGregor and Dr. Henry Butt have studied ME/CFS since the early 1990s. After Butt and Gooley published a paper on gut microbes in ME/CFS. Armstrong’s Ph.D. study examined D-lactate in blood, fecal and urine samples. He then got funded for the larger study that was published in 2015.
The Metabolic Profiling Paper
- Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients, Christopher W. Armstrong, Neil R. McGregor, Donald P. Lewis, Henry L. Butt, Paul R. Gooley. Metabolomics
DOI 10.1007/s11306-015-0816-5 - Metabolism in chronic fatigue syndrome. Armstrong CW, McGregor NR, Butt HL, Gooley PR. Adv Clin Chem. 2014;66:121-72. Review.
- Solve ME/CFS Initiative with Chris Armstrong
The idea that energy production is impaired in chronic fatigue syndrome is inherently appealing. As Armstrong and McGregor noted in the 2015 paper, both fatigue and post-exertional malaise are often the result of impaired energy metabolism.
Tossing the discussion of what initiated this illness aside, they suggested a focus on the “maintaining factors” of the illness and suggested that problems with oxidative stress could be whacking the mitochondria.
Virtually every study that has looked for oxidative stress in chronic fatigue syndrome (ME/CFS) has found it. Perhaps the most exciting oxidative stress studies in ME/CFS have been done by Dikomo Shungu whose brain imaging studies have found increased lactate levels and decreased antioxidant levels. Shungu believes oxidative stress could be causing the cognitive problems in ME/CFS.
Two Metabolomics Studies – Two Approaches
Armstrong has access to both of the machines used in metabolomics; nuclear magnetic resonance (NMR) and mass spectrometry (MS). While the Aussies have been using nuclear magnetic resonance to study the metabolome, Naviaux has been using mass spectroscopy. I asked Armstrong what the difference was between the two.
Armstrong suggested that both should be used to study metabolites. NMR is much more reliable but MS is more sensitive (can pick up more metabolites). Because mass spectrometers pick up more metabolites you can do statistical studies on them such as multivariate analyses to ferret out subsets.
With NMR’s you can analyze the same samples on different machines and get the same result. You can’t do that with MS. In fact, MS’s produce variable enough results that you can’t even measure samples on different days with the MS and get the same results; it’s best to do them all in one shot.
The reliability issues are overcome by using large sample sizes, by analyzing the same sample repeatedly and by using quality controls. Armstrong suggested using both machines in the same study but acknowledged that it was expensive. He noted that running his NMR samples on a mass spectrometer resulted in generally the same results.
Instead of the mass spectrometry used in the Naviaux metabolomics study, the Australian group used a process called nuclear magnetic resonance (NMR). They did an untargeted search; i.e. they looked at all the major biochemical pathways in the body in an attempt to find metabolites they could use as diagnostic biomarkers.
Metabolites are very small molecules left over when larger molecules or compounds are broken down. Our genes produce proteins which do the work of the cell. In the process of doing that, they interact with and get broken down into metabolites.
Unexpected metabolites that show up, or metabolites that show up in higher or lower concentrations than expected, indicate that some sort of alteration in metabolism has occurred. It’s these breakdowns or signs of disease or disturbance that the metabolomics studies are looking for.
Metabolomics studies are tricky, though; your metabolites can alter in response to the slightest changes in the body. Because they’re so sensitive Armstrong believes metabolomics studies could be an ideal way to delineate the subsets found in ME/CFS. On the other hand, that sensitivity comes at a cost; metabolomics is best assessed using longitudinal or a series studies that can sample the ME/CFS population again and again – arriving at a set of core metabolites.
The group’s 2014 review paper found that ME/CFS metabolism studies found evidence of decreases in mitochondrial output, decreased amino acid production, increased levels of oxidative stress and problems with nitrogen.
The 2015 study examined the metabolites in both the blood and the urine. Increases in the concentration of a metabolite in the blood suggest that problems with a biological process is present.
Urine, on the other hand, is the body’s way of excreting waste and/or toxic products. Urinalysis is important in metabolomics because when faced with unhealthy concentrations of metabolites, our bodies will attempt to eliminate them through our urine.
If a metabolite is too low, then another metabolite may be dumped in order to maintain balance. If a metabolite is too high, then it may be eliminated through the urine.
Unusual levels of metabolites in the blood generally reflect a process that is happening at the moment the blood is drawn. Unusual metabolites in the urine, on the other hand, generally reflect a chronic or ongoing process of metabolite disruption which has become significant enough for metabolite dumping to begin.
Blood and urine metabolite levels are generally interconnected. Metabolic problems that are found in the blood but not in the urine could either reflect a temporary metabolic fluctuation or problems with excreting the metabolites via the urine.
The study included 34 females with ME/CFS who met the Canadian Consensus Criteria and 25 female healthy controls.
Study Results
Blood
Six blood metabolites were significantly altered in the ME/CFS group. Glucose levels were increased whereas acetate, glutamate, hypoxanthine, lactate, and phenylalanine were decreased.
A PCA analysis found a clear separation between the two groups using relative abundance data.
Another analysis indicated, interestingly enough, two metabolites whose abundance was not significantly altered, formate and acetate, played a significant role in separating the ME/CFS patients from the healthy controls.
Urine
Significantly decreased concentrations of five metabolites (acetate; alanine; formate; pyruvate; and serine) were found in ME/CFS. Comparing the abundance of metabolites relative to each other they found eight altered metabolites; the five decreased metabolites found above plus decreased valine and increased allantoin and creatinine.
A PCA analysis of the relative abundance data for urine demonstrated a moderate separation; i.e. the ME/CFS and healthy controls were moderately different.
Emphasis on Anaerobic Energy Production
At this point, an unusual problem that had surfaced earlier in the study came to the fore. Creatinine levels are often used as a kind of set point to standardize the concentrations of other urinary metabolites, but when the group used creatinine in this fashion all the metabolites registered as decreased. Considering that a questionable result the researchers normalized each sample to the total metabolite concentration.
The Gist
- Amino acid concentrations suggest that the clean, powerful aerobic energy producing system is being inhibited, while the dirtier and less effective anaerobic energy production system is being used more in ME/CFS
- The anaerobic energy pathway – glycolysis – which provides pyruvate for the mitochondria and small amounts of ATP is operating strangely
- Instead of glucose, amino acids such as glutamate are being used to provide fuel for this pathway.
- Increased usage of glutamate, however, could be contributing to reduced levels of the main antioxidant in the body (glutathione)
- A similar pattern seen in sepsis and starvation suggests ME/CFS may, in some ways, be similar to those diseases/conditions
- If ME/CFS patient’s cells exist in a chronic state of mild starvation, a specialized feeding program may be needed to help them recover
- Both the Naviaux and this paper agree that a hypometabolic state characterized by amino acid depletion is present in ME/CFS
- Several further Australian studies are underway including a longitudinal study examining treatment options
Confirmation of that hypothesis came when creatinine was negatively correlated with glycolytic processes in the healthy controls. This suggested that as the aerobic energy production pooped out, creatinine levels were increased in order to rush phosphates to the muscles to produce more ATP.
Impaired Glycolysis
These correlations, paired with a decrease of amino acid concentrations, implicate an increasing utilization of amino acids as a source of energy production through the citric acid cycle, largely via glutamate. Armstrong et. al.
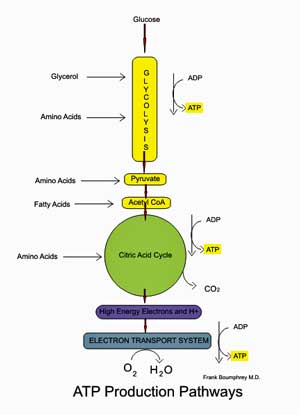
The aerobic energy producing pathway; note that glycolysis provides some ATP but that its main function in aerobic energy production is to provide pyruvate for the mitochondria.
Glycolysis is the metabolic pathway that converts glucose into pyruvate while producing small amounts of ATP. In glycolysis no oxygen has been used and only small amounts of ATP are produced.
Pyruvate then enters the mitochondria and gets oxidized in the aerobic energy production pathways to produce high amounts of ATP.
Glycolysis, then, is a part of both the anaerobic and aerobic energy production systems. When not enough oxygen is present for the aerobic energy production pathway to function properly – such as during intense exercise – glycolysis becomes a particularly important source of energy. Not much energy is produced, though, and the by-products of anaerobic energy production such as lactate are toxic.
Note then that anaerobic energy production or glycolysis is always occurring at least to some degree, and a healthy body is well adapted to take care of the small amounts of toxic byproducts produced by it during normal functioning. A decline in the aerobic energy production process appears to have left people with ME/CFS more dependent than usual on glycolysis to produce energy.
Glycolysis appears to have problems in ME/CFS as well, though. Glucose is the preferred substrate for glycolysis. The high blood glucose levels in the ME/CFS patients combined with the decreased concentrations of the metabolic endpoints of glycolysis (alanine, pyruvate), plus reduced levels of acetate, suggested instead that glycolysis had taken a hit in ME/CFS.
Reduced blood lactate levels could reflect decreased glycolysis as well. All in all, the ME/CFS patients appeared to be having trouble converting carbohydrates (glucose) into energy in the glycolytic pathway.
If I have this very complex subject right this suggests both energy producing pathways – the aerobic and anaerobic – are not functioning well in chronic fatigue syndrome. The fact that glucose – the best fuel for glycolysis – is not being used in will impact aerobic energy production but it’s not clear to my befuddled brain if that is the main issue or if other problems with aerobic energy production exist.
The Glucose / Amino Acid Switch
What then were the people with ME/CFS using to produce the substrate – pyruvate – used by the aerobic energy production process? The low levels of amino acids found suggested they were breaking down non-essential amino acids (alanine, glutamate and proline) to produce it, instead of carbohydrates.
(Armstrong noted that the high glucose levels could also be the result of increased gluconeogenesis. Gluconeogenesis maintains glucose levels by breaking down non-carbohydrate substrates such as amino acids and lipids.; Note that increased gluconeogenesis would also result in increased glucose levels and reduced amino acid levels).
“…suggests in ME/CFS a depletion of nonessential amino acids in the blood is being used to fuel the citric acid cycle and produce ATP in the absence of sufficient glucose usage via glycolysis.” Armstrong et. al.
The amino acid depletions suggested that levels of an enzyme called aspartate transaminase (AST) used to break down amino acids should be dramatically increased. They weren’t able to test that hypothesis themselves but a recent Stanford/Columbia study finding threefold increases in AST levels suggested that the Aussie team’s findings and hypothesis regarding amino acid depletion were correct. The study also found increases in other enzymes used to support amino acid use in the TCA cycle. Enzymes associated with producing aerobic energy in the electron transport chain were reduced.
Glutamate
The differences between blood and urine glutamate levels between the two groups may tell the tale. Glutamate levels were higher in the blood of the healthy controls and lower in their urine. (This presumably indicated glutamate levels were “in bounds” in the blood of the healthy controls; there was no need to dump glutamate into the urine). Glutamate levels, on the other hand, were essentially the same in both the blood and the urine in ME/CFS patients.
The authors believe this may be because glutamate in the blood of ME/CFS patients is being transformed into glutamine and then into amino acids for use as an energy substrate. (When amino acids are used in this way glutamate is usually the amino acid that is used up.) With the glutamate in the blood being used up as an energy substrate, little glutamate is left to be dumped into the urine; ergo, glutamate is low in both the blood and urine of ME/CFS patients.

Instead of glucose, glutamate, an amino acid, was being broken down to provide energy. (Glutamate enzyme)
The negative correlation of blood glutamate levels and urine creatinine levels also suggested that glutamate being used as a substrate for ATP production in ME/CFS.
This suggested that ME/CFS patients were using glutamate instead of glucose as an energy resource.
Glutamate, interestingly, given Shungu’s findings of low glutathione levels in the brain, plays an important role in glutathione synthesis. If glutamate is being used as an energy source it may not be available for the synthesis of glutathione – the body’s chief antioxidant.
Creatinine
As markers of anaerobic metabolism (formate, glycine, hypoxanthine, and lactate) increased in the ME/CFS patients, the levels of another marker of anaerobic metabolic activity, creatinine, decreased in the urine. The urine decreases suggested that creatinine – which is used to increase anaerobic energy production during intense exercise – was being used up quickly in the blood. That creatinine depletion suggested, as have the other findings, that ME/CFS patients’ bodies were in the kind of energy deficit healthy people only reach when they exert themselves very vigorously.
These findings, of course, fits very well with the exercise studies showing that aerobic energy production has gotten hit hard leaving a significant number of patients relying on the dirtier, less efficient and far less powerful process of anaerobic energy production to produce their energy. At the IACFS/ME Christopher Snell said the metabolomics findings correlated perfectly with their exercise results.
Starving for Energy?
Why is this happening? Armstrong suggested a couple of reasons. He noted that many of the metabolomic anomalies he found in ME/CFS are also found in sepsis and starvation. All show reductions in amino acids and lipids and increased levels of glucose. In both diseases proteins and lipids are used to produce maintain low energy levels while glucose is used for other matters – such as immune cell proliferation in sepsis.
Armstrong speculated that an infection or autoimmune process may have triggered a sepsis-like condition which then lead to a state of chronic starvation. During sepsis immune cells rely entirely upon glycolysis to proliferate wildly. They are so energy hungry during this process that they can deplete the system of essential cofactors perhaps leading to a state of chronic cellular starvation.
In starvation amino acids and fats are preferentially used to feed the TCA or Krebs cycle instead of glucose. Likewise, in anorexia the mitochondria switch to amino acids and lipids to fuel ATP production. The Aussie team believes the inability to use glucose properly may be contributing to a kind of low-level chronic starvation of mitochondria.
This state of low-level starvation is not particularly easy to escape. When the body begins starve it robs the tissues of many of the cofactors (vitamins/minerals) needed to utilize foods. If those cofactors aren’t provided along with the food a problem called refeeding syndrome can result. That’s an intriguing issue given the problems some severely ill patients have with gaining weight.
Armstrong speculated that the treatment protocols similar to those used to safely bring people out of starvation might be able to help in ME/CFS. Those protocols involve providing nutrients in specific stages based on their metabolic state. Bob Naviaux has also endorsed a stepwise approach to solving ME/CFS patients’ metabolomics issues.
Naviaux has reported that he hopes to start small clinical trials in the upcoming year and the Australian group is doing likewise. They’re providing ME/CFS patients with combinations of metabolites, vitamins, and minerals and then monitoring their metabolomics over time to see the effect they’ve had.
Broadly Similar Findings Thus Far
Armstrong stated that thus far broadly similar findings pervade the ME/CFS metabolomics studies. They suggest that:
- Increased oxidative stress is present;
- Lipids (fats) are being used to produce ATP;
- Issues of purine metabolism and with folate cycle and methionine are present;
- Reduced glycolysis/ increased glucose and increased use of amino acids for ATP production are present.
Treatment
Armstrong said it was too early to recommend specific treatments based on metabolomics results. The longitudinal studies underway, do however, include treatments the researchers believe may help to improve ME/CFS patients’ metabolic profiles.
Armstrong suggested that Rituximab could be helping, at least in part, by reducing the energy drain caused by expansion of B-cells.
Armstrong didn’t mention paleo or ketogenic-type diets. Do the problems with glucose metabolism suggest that high fat/ moderate protein and low carbohydrate diets are indicated? I don’t know but I’ve been very happy with my results from a paleo-type diet over the last couple of months.
- For more on ketogenic type diets check out our Diet Resource section
Next Up
Next up for the Aussie group include studies on
- Longitudinal metabolomics and genomics while providing interventions;
- Metabolomics of immune cells;
- Producing a large-scale symptom database for patient stratification;
- Large-scale genetic markers and metabolite population studies.







Thanks, Cort, for this excellent write-up. I heard Christopher Armstrong’s webinar, and I thought it was such an exciting development. I have had many of these abnormal test results for years, and no one knew why (I did about every test imaginable). I did not know how to explain the webinar to my doctor, so I will send him your write-up. He’ll be fascinated. Thanks!
I hope I got it right; I must say that this field is very complex and very new to me…Hopefully its mostly correct.
Cort this is a great article. I am wondering as I have been on a Gluten free diet for 3 years now-but really did not see any improvement until I increased my Probiotics . I started doing VSL#3 when I had an IBS flare and then kept with Klaire Pro 5 Probiotics by 3 AM and 3 PM. No fried food at all, many salads,
Some chicken and some fish. Many vegetables. Also a Gluten free protein drink with Avocado 1 whole and fresh Blueberries, Coconut water and high PH water. I have been able to go off of my Immune globulin. My IBS issues have gone. I do still take 3 cc B-12 injection weekly. Before I was on 3 a week. Powder I use in drink is called Love & Peas (gluten free) Vanilla flav.made by Natures Sunshine Products . This is a high Pea Protein powder. I also am using Brown Rice and Beans alot on and with my salads. Only water or Herbal teas. I do have coffee in the am.Not saying I am over everything, but I am functioning better and can think more clearly. I am also on Vit D, Adrenal lig supplement. No sugar!!!! No fried foods at all. I have no physician to advise me. I have an Environmental Dr who is more than please with my progress. Would like some feedback from other readers. Thanks for all you do Cort.
Cort- I forgot to say- Sorry-That I am also on a Lactose free diet.
Article is great.
Thanks.
I look forward to learning eventually whether anything similar is going on in Fibromyalgia. My hunch thus far is that the problems in FM are far more to do with actual physical malfunction in “stuck” muscle layers. The fact that ketogenic diets help in FM, may be because the by-products of burning sugar for energy, are part of the cause of the stickiness in myofascia ground substance.
It would be interesting if ketogenic diets also help CFS but in a completely different way! Or perhaps they are more similar than I am thinking.
Wondering if eating chlorella and spirullina will help. These algaes are used successfully to treat starving animals in my field of wildlife rehab.
Also wondering how prior anorexia may influence occurrence of CFS. Do you know if anorexia correlates with CFS rates?
Fascinating article.
Cort, I agree with your experience. I started The Candida Diet by Dr. Crook in the 80’s, consuming animal protein, non-starchy vegetables and nuts and drinking only pure water. If I eat carbs, I get tired and gain excess weight. In recent years, I’ve learned about methylation problems that I have and have been careful to avoid grains, especially those that are enriched with folic acid. I’ve also gone to a functional medicine doctor who gives me regular detox IVs containing glutathione. They’ve made a huge difference in my energy levels. Thanks and keep up the good work.
I wonder how important detoxification is; I think it could be critical. I also lost some weight on the new diet – another benefit.
Very interesting. I have always found also that a diet high in carbs drastically reduces my energy levels. When I am very low I crave red meat, although I have to be careful as I have recently been diagnosed with the milder form of haemachromatosis. Lots of butter, coconut oil and olive oil are definitely beneficial.
CORT, This new research has answered many of my questions regarding my diagnosis of only FM when I have all the symptoms of ME/CFS as well, except for the fact that I do have some energy reserves, but I do have to be careful not to overdo. I am on AMINO ACID REPLACEMENT and it has been the most beneficial treatment I’ve received in the last 21 years! Is this not the answer for all of us, until they can figure out how to get the body to produce energy again using glucose? For further information on my treatment find my 2 articles 17 YEARS UNDER THE CARE OF A NATUROPATH DR. on HEALTHRISING, click HOME and scroll down to SUPPLEMENTS. I was tested for my AMINO ACID profile through DOCTOR’S DATA, a lab in the USA. and when I received the results, the prescription I needed was included. A compounding pharmacy then prepared all the AMINO ACIDS I needed in a granular form and all I take is 1 rounded teaspoon 2x a day. It gave me back 80% of my function back, including brain!
Thank you so much for your relentless efforts and perseverance! Because my me/cfs is so bad at present, I’m having great difficulty in deciphering long and complex reports. I wonder if, for those of us who are struggling with brain fog, you’d be kind enough to include a simple précis of your reports. I just find it too hard to wade through the terminology and, to be honest, long words at this time. (I used to be an RN with a post grad in A&E, and more recently double qualifications in CPE, theology & Christian Ministry. So this cognitive impairment is purely since contracting this chronic disease.) Please would you be kind enough to add a simple dot-point summary in simple layman’s terms for those of us too ill to cope with long reports. Thanking you in anticipation.
Actually I just put a column called The Gist which provides a bullet point summation. I’ll be doing that for all the blogs from now on. It’s on the right hand side.
Thank you, Cort, for the Gist. Much appreciated. Interestingly my illness began with sepsis from a dog bite. Interestingly, Sophia, I’ve put myself on a strong probiotic, “Multiflora”, which after being on it for a couple of months now, seems to have some slight benefit.
Cort, are you able to recommend any dietary adjustments to assist this problem with amino acids? Kind regards Mandy
I have no idea on amino acids. Naviaux said that the issue is more complex than just adding the amino acids one is low in and from what I gathered from Chris’s talk he believes the same. Fortunately both groups are or will be trying interventions to see if they will help. I think we just have to wait.
thanks Cort, I couldnt get through this article and found The Gist column useful. I really needed that.
Mandy – I am probably replyng to the wrong question, but I could not find a place in your other comment. I tripled my Probiotics 2 times and still stay on that. That has helped me more than anything at the moment. May change but my IBS issues were terrible a year ago and I happen to read that another patient cleared hers with just increasing all her Probiotics. Mine started getting better with everything after a few weeks I also have GERD and that too improved . I was listed a PreDiabetic. That has resolved according to new blood tests-Also arterial placque has improved. They even redid an Ultrasound of my neck arteries to recheck them. They said it looked like it was Dissolving???? It has to be the Diet and the Probiotics-my answer!! I am seeing the same Dr for my testing. I have had CFIDS/MS for 28 years.
I do use EXtra Virgin Olive OIl.
As an Aussie with ME/CFS, I’m really proud of our researchers :-)) and tried watching Armstrong’s seminar, but found it very technical and difficult to follow.
Thank you so much for this plain-English summary, Cort.
This is very interesting to me as my cfs developed after a bowel parasite that caused my body to be in starvation. The parasite has been treated but I have struggled to recover my functioning. L glutamine is something I have found helpful in my recovery process. L lysine another amino acid is a recent addition to my protocol and I have had a noticeable energy increase. I also crave butter since gettin cfs and I always hated it in the past.
Great article Cort. I was not even aware of this Australian research. Very interesting. I am trying at the moment to get more insight into the energy metabolism dysfunctions in ME/CFS, and this really helps.
I have been thinking recently that if we better understood how our bodies are trying to adapt and compensate for these dysfunctions, by using alternative energy production pathways, we perhaps might be able to figure out how to support these alternative pathways, even if we cannot for the moment fix the primary energy metabolism dysfunction(s).
I like the suggestion that ME/CFS patients are using glutamate as an alternative fuel supply. I wonder if this means that glutamine supplementation will help supply energy, as glutamine and glutamate easily interconvert.
What I wonder is whether this glucose problem is “the” problem regarding energy production or if something else is going on in another part of that very complex aerobic energy production process (?)….
Glutathione IVs have helped me immensely. So has NT factor and LDN. I wonder if this sepsis like state might be the leaking of our gut bacteria into our blood stream. A few studies this year have indicated this.
Sophia how much glutathione do you take; also which NT factor and what dose of LDN?
My troubles started with appendicitis. With the onset of fatigue I found carbs left me feeling empty. Eggs and whey protein (immunocal platinum is best) were a real boost along with lots of vegetables.
Thank you Chris
Immunocal helps me tremendously. But it cam also incrrase inflamation. SomI have to find the sweet spot which varies with many things like the seasons.
Am I unusual in feeling better when I have carbohydrates like oats? Even high Gi sugary things give me a boost. I’d feel really unwell on a low carb diet. Healthy diet with a high good carb intake works best for me, if depending on how it makes me feel. That doesn’t mean that it’s best in managing the disease though. I know I’m not the only one who doesn’t get on with the Paleo type diets. There was a lot of starvation back in the gatherer-hunter (the correct way round) days. Discovering how to do arable farming wasn’t so bad.
I also feel better on a moderate to high carbohydrate diet (although not oats or gluten grains.) Whenever I’ve tried a Paleo type diet I have trouble sleeping, feel miserable, and actually gain weight! My sister also feels bad and gains weight on a Paleo type diet. We both have tried it at least 3 different times and for varying lengths. It’s a strange reaction I know. People are so different in what helps and what harms. Genes probably play some role in this.
🙂 We are a variable group. I did have trouble sleeping at first on the paleo diet and at times felt really spacey and awful. That passed after about 10 days but I think I still wake up more in the middle of the night.
just an FYI you might look at your blood type and diet. type A doesnt do well on Paleo. hard to digst and weight gain. Gluten and GMO grains are good exclusions.you can look up blood type diet and body ecology diet as well to help with CFS. Body ecology diet sent me into a tail spin with my health but im an O type so peleo is my go to.
Very interesting Renee. My sister and I both have A blood type.
I have also a good experiences with low carbs diet. I was on ketogenic diet for half year and i got 20 % more energy almost immediately what had a great impact on my life. But after 6 months I started to feel suddenly hypoglycemic and I had to add more carbs but i still eat much less carbs than before the diet. What is possitive that even after adding carbs the energy level seems to stay the same. Also i had always problems with high triglycerides. With this diet they are perfect – does this indicate that we have problems to processing carbs? Does someone have similar experiences?
My triglycerides dropped sharp after a (quite moderate) low(er) carb diet. Cholesterol dropped a little even when consuming twice as much fat as before.
However, I consumed way to high a % of carbs after years of reducing fat (and therefore also less proteines in a regular diet) because of constant “too much cholesterol” messages from my doctor. After consuming near no cholesterol, I learned that for some people you produce your own cholesterol if you don’t get it with your food. As I seemed to only produce bad LDL I thought to give it a try to add more fat rich of HDL to my diet. It kinda worked.
Concerning the triglycerides: if a person with ME would have lower needed caloric intake than a healthy person (few motion and brain activity possible) and a high % of carbs (of which most convert to glucose), then the entire energy need of the body or more could be met by glucose alone. High glucose levels in the body are dangerous. Maybe it is because of that that people with too high carb intake have been seen with very high triglyceride levels: there is a direct conversion mechanism from carbs too triglycerides.
Once again, great article. It jogged my memory about some tests I had done 9 years ago. I wish I could repeat them through a Homeopath, but I don’t have the energy to see a doctor again right now. But, the tests were done through Designs for Health and I see on their website they still offer them through a doctor. They were Blood Spot Fatty Acid Profile, showing Omega 3&6 level breakdown, Trans Fatty Acids and Ratios), Organix Profile showing 32 different ‘-ates’ from Pyruvate to Malate to Adipate, ect. which identify B-Vit Insufficiency, Cellular Energy, Neural Function and Detoxification and a Lipid Peroxide-Urine which shows how your body might be failing to control the rate of formation of free radicals. They included the summary of abnormal results, which were easy to read and a supplement recommendation summary. For me, it recommended more B-Vit specifics, Magnesium Malate, CoQ10, Arginine, Carnitine, Omegas and Alpha Lipoic Acid (ALA). I wasn’t able to tolerate the ALA until just recently when I discovered the Timed Release formula.
It would be great to have a list of all the metabolic tests that can be done – I had no idea there were so many….That said as I remember Naviaux thinks it will probably take other than the usual supplement recommendations to move many of us out of that low energy state.
I initially thought my son had a Mito issue and trialled on Mito cocktail plus a stack of other things along the way.
Think my son has mild mast cell activation as cause of CFS? Maybe. But the supps that help are starting to make sense.
What works for us through years of trial and error, activated b group vitamin, Creon digestive enzymes, Natural Sport Nitric Oxide (has one of the amino acids listed above as being used to compensate), and a few other things like Ubiquinol.
It doesn’t fix the issue but it improves function from bed bound 95% of the time to around 60%. Most importantly, reduced gut pain, mind numbing fatigue and fogginess, joint and body pain reduced too.
So, it’s something.
Thanks. Yes, that is something. The alternative is much worse and lets hope he will improve much more.
These types of studies are the ones I’ve waited for forever. For so long doctors would run the usual panel of bloodwork and most things came back normal, therefore you’d get that look from the doctor that said “there is nothing wrong with you”.
I would think “If only you knew more”. Finally they know more.
Fluge said at the conference “just give us a year and we will know so much more..” 🙂
could viruses be taking that energy themsélves, thus starving us??
Armstrong said that immune activation in reaction to pathogens could be producing a sepsis-like state which evolves in a state of chronic cellular starvation.
I like the sepsis idea. We have the same symptoms. But there is more.
Wow Cort, I commend you for this in-depth report. I certainly makes a lot of sense to me.
It will be good to know what causes this hypometabolic state, time will tell and hopefully not too much time.
Since I started reading your blog there have been many advances in research, not enough for sure but at least through your reports we can see that some head-way is happening.
One of these days we will celebrate when we hear from one of these research studies “I’ve got the answer”
Maybe sooner than later if Fluge is right and we get more money. Who knows?
Thank you very much Cort this writeup is excellent. I have to study this more to understand it completely, but I feel in my gut that this is right on for me. I “hit the bonk” years ago and haven’t been able to find my way out.
Thanks once again, Cort.
This may be part of the explanation why my Total Protein and Globulin levels are so low in spite of all the protein I eat. And my Homocysteine level so very high.
I love the glimmer of Understanding!
Diane
Hi Diane This is exactly what happened to me. Our AMINO ACIDS are low because we cannot synthesise Protein. Then, if we are using our amino acids to produce anaerobic energy, no wonder they are low! My ND. had me do a 24 hour urine collection and it was sent to a lab in the USA called Drs Data. With the report they had included my prescription stating exactly the amino acids that were low and the amounts my body needed. My ND then sent the prescription to a compounding pharmacy to be filled. I take this granular preparation as follows: I rounded tsp. 2x a day. This gave me 80% of my life back! Why should we still be in bed suffering, when this miracle is available to us now and it’s natural to our bodies. It’s so important to be tested as using the wrong amino acids and amounts can cause more problems.
Edie,
Thanks for your reply, your info is very interesting. I’m going to look into it. It just makes good common sense. No guessing!
Edie how long did it take on the aminos before you felt a difference?
had you ever tried taking whey protein powders or similar before?
I was 80% better neurologically within 1 week! It, however, did not change my pain level. There are many other things that can help with pain. You should get tested before using Amino Acid therapy. Google Doctor’s Data for arrangements. I have tried various protein powders that were lactose free, but I did not notice any changes in my health. If you can’t synthesise protein, that probably applies to protein powders as well, but I’m not an expert. I only know for sure what helped me. Hope this helps Diane.
Cort, is it possible that our disease is due to an uncontrolling problem in the body to control pH levels in our entire system? Maybe this could lead to a new theory.
I wouldn’t be surprised if pH is involved.
The prospect of studies being able to elucidate nutritional therapies (both diet and supplements) that might help us is exciting. I don’t think the evidence we have to date gives any good, solid information that can guide diet or supplements, but am delighted to hear that the Australian researchers and Naviaux’s team are looking at exactly this, and they must at least have some theories on what’s worth trying experimentally. In Ros Vallings’ summary of Maureen Hanson’s IACFSME presentation, Vallings says “Amino acids, fat metabolism and energy and sugar metabolism are all affected.” This, and the fact that Naviaux et al did not refer to possible benefit of manipulating carb/fat/protein proportions, leads me to think we really don’t know yet whether there’s even a theoretical rationale for doing so.
Even with a theoretical rationale, actual intervention studies on people with ME/CFS will be key. After all, mild-moderate exercise is theoretically beneficial for the immune system, but that doesn’t work out so well for the immune systems of people with ME/CFS, to put it mildly. For me, paleo/stone age diet makes me feel much, much worse, but given Naviaux et al’s findings of high variability in individual metabolic dysfunction, it makes sense that different diets might well work for different people with ME/CFS, or perhaps for different people at different times. I’ve landed on a super-nutritious diet that works for me but hasn’t changed my ME/CFS – I like to think that it is setting the scene for other treatments to be extra-effective in the future, though! This idea of a very targeted, personalised stepwise nutritional treatment plan based on metabolomics (rather than just blood tests or mitochondrial testing) is very appealing. Bring it on!
Great point… We really need interventional studies – I agree.
It seems that the leaky gut fits in here. My immune system reacting to all the substances leaking into the blood stream and the idea of sepsis seems to fit together. In spite of my limited knowledge, I’ve long had a hunch that it’s related to too much lactate and a problem with mitochondria
After reading this article, I went in search and found this dated today, and although it’s starts off in 2005, the article ends with two drug trials that are going on right now!
Cort, you rock!
http://www.medscape.com/viewarticle/871787
Good for Miriam Tucker and on Medscape as well! Thanks for sharing that 🙂
Everyone should read this article – it’s very hopeful.
From Mella
Fluge and Mella have done so much for this disease…
Fascinating! Yet another report that is tying everything together for me personally.
I have had my own metabolic testing done showing abnormalities so am very eager to see how my results compare to these studies as well as to be able to share them with medical doctors who up until this point have not thought much of them.
In 2011 I had a metabolomic testing panel done in Ottawa by a company called Nutrichem. They are based in Ottawa and headed by a brilliant pharmacist Kent Macleod who is appearing to be well ahead of the game. He does a body wellness test of blood and urine in which 40 or so metabolites are measured that indicate how systems such as energy production and gut health are faring.
Part of the panel of testing is of organic acids excreted in urine that show how the Citric Acid Cycle ( Kreb’s Cycle) is working. Mine was completely abnormal and showed abnormal spilling of intermediates indicating severe mitochondrial deficiencies. For instance my FUMARATE was +138% of normal.
I also had extremely low metabolites that indicated blockages in the cycle. CITRATE was as negative 12% of normal and ISOCITRATE was at negative 8%. Also low were PYRUVATE, CIS-ACONITATE, 2-KETOGLUTARATE, MALATE and OXALOACETATE). LACTATE was on this panel and mine was super high at 120%.
Also in this analysis is a panel entitled “Organic Acids-Gastrointestinal Health” in which 6 organic acid metabolites are measured in the urine. I had an increase of 4-HYDROXYBENZOATE which indicated overgrowth of gut bacteria, possible Celiac Disease. This led to me being tested for Celiac, testing negative but having positive antibodies to anti-gliadin antibodies so being prescribed an anti-gluten diet by a gastroenterologist. Now a faulty gut microbiome is being found in ME/CFS.
Another panel in the testing looks at organic acids that indicate problems with general metabolism. Interestingly I scored off the charts with a high 2-HYDROXYBUTRYATE at +140%. This is formed when glucose can not be used for energy. According to the blurb provided with the report a value over 40% is problematic!
When I researched this metabolite is says that it is formed in excess during oxidative stress, lactic acidosis among other things.
HIPPURATE, OROTATE, ADIPATE, SUBERATE, 3-hydroxy-3-METHYL-3-METHYLGLUTARATE are also on this panel– I tested very low for all of these. Not sure what that means.
BENZOATE is next on this panel, a marker for bacterial overgrowth in gut. Again I tested high.
PYROGLUTAMATE was last on this page and indicates depletion of glutathione. Mine was high indicating depleted low glutathione. Again fitting with the research.
COENZYME Q10 and VIT D also on the panel and I also tested very low.
The great thing about having this test done was that I was then prescribed a regime of supplements to support my specific issues. NAC to replenish glutathione ( Dr Shengu just did research to show that NAC does pass the blood brain barrier and does increase brain glutathione-Yay!), acetyl-L-carnitine, magnesium, coenzyme Q10 ( all for mito’s), methyl folate and methyl cobalamine among other things.
I can’t wait until ALL patients with MECFS, in fact all people in general,can have this type of testing as part of a routine physical. And for medical doctors to respect this type of testing instead of scoffing at it and acting like it is some sort of voodoo.
Wow…
Anyone kniw who does this near Stuart, Florida or just Florida?
hi Claire did this lead to any useful treatment?
Im on a very low carb diet with high fats as this is what works best for me but I’d put that down to me having hyperinsulinemia to which this diet is good for.
Dr Henry Butt has done a lot for the ME/CFS community. He’s also was part of a group who worked out and put my states(Sth Australia) MCS Public hospital policy in place and then later on was responsible for getting a safe place built for MCS patients into one of our main hospitals (I guess this was done, last I heard it was in process of being built). I really hope he’ll be around for a while yet, he’s quite aged now. (Unfortunately most of the ME/CFS drs in my state now are very aged or have died and arent being replaced by other drs).
Interesting read. My son appears to have some issues in these areas but darned if I can get help. He can produce energy – albeit really slowly. He doesn’t appear to replace ATP at the rate he uses it.
We tried a LCHF diet a few years ago and it made us all so sick – my son (10 years old now) naturally avoids a lot of proteins and seems to do better on a high carb diet. I find this all so confusing. He was definitely starving a few years ago and had to have a nasal gastric tube inserted. It also made him sick. We found that adding glucose to his diet helped fix low blood sugar? Recently, he’s improved slightly with low histamine diet, anti-histamines and DAO but not to the point where I would say we had found what was wrong…
Aaargh.
Hi Justine; I’ve had ME my whole life, just started with gastric symptoms 2 years ago. Wanted to see if I could offer anything helpful for your son; has he had a kidney function test? Has he ever had anxiety/lightheadedness during bowel movements? Carbs&low histamine diet – anything processed, or as whole foods as possible?
Hi Laura,
We have done failsafe diet (low chemical), LCHF, high carb, whole food, and easily digested food (so processed).
He naturally avoids proteins – specifically animal protien unless it’s a bit of mince in a mountain of rice.
We have just gone low histamine, made a bit of difference – removed some body pain and headaches, added the anti-histamine – took away severe gastric pain and rest of join issues, added DAO – made no difference really. But then started on quercetin last week – and hola! He’s out of bed. He’s playing and laughing and singing.
So, it appears he has a mast cell issue?
He was chronically constipated for a few years, then almost diarrhea (sorry) recently – think mast cell again.
Heaps of anxiety – linked to being sick. Especially around food and feeling sore.
All tests are normal. Has been through them all for the past 7 years. But got an appt next week in Melbourne with Dr Lewis at CFS Discovery for him? That’s going to be a very expensive week but I am hoping he can shed more light?
It looks like he cannot create ATP properly – it’s so slow to replace what he has used.
And he craves carbs, like crazy craving. He has to have them. I try to balance it but he eats so little that at times all he can stomach when he’s sore is rice and a bit of butter.
I have no idea what I am doing now – I am just getting through. Thanks Laura!
Justine,
I take an ATP Energy, a supplement made by Progressive Laboratories, since I have CFS/ME and methylation difficulties. I purchase it online in the US. It has helped me. See if it is available to you. Best wishes.
Hi Justine,
Poor guy! Glad your latest intervention got him out of bed and singing and dancing. So, I have no medical training, I’m just brainstorming with you as another mom and having experience with CFS&chemical sensitivities, which may or may not be like your son’s. I’m going to assume you’re taking any info I share with you to a qualified doctor.
A clinician at the IACFSME conference in Ft. Lauderdale in Oct said that it isn’t one or two things that help CFS,it’s a number of things that will eventually help a person’s orbit revolve around health rather than sickness. Another metaphor of riding a bicycle relates to our state of homeostasis. Our metabolism is always in motion and needs to be balanced. We take in oxygen and nutrition to create energy, and we need to detoxify the wastes from that process as well as any poisons entering our bodies from various means (crap food, virus, bacteria, pesticides, etc.). When something goes wrong in this process we fall our our bicycles and it can take awhile to heal and get back on again. It’s really important to talk with your son and bring him on board so he’s making his own food decisions. He’ll choose healthier foods when he takes ownership of his own health.
So, you wondered, could your son have a rare mast cell disorder? Maybe, but I think a doctor will first turn around his diet to see if the mast cells stop putting out alarm bells (histamines, etc.) My naturopath says “let food be your medicine or medicine will be your food.”
Your son may need to see a gastroenterologist to rule out IBS? If your son is constipated a gastro doc may recommend Laxaday at 3X the regular dose once a day until bowel movements become like pudding. Which could take a couple of weeks.
I can’t eat almost any processed food anymore. White rice and bread will make me produce histamines and I’ll be bloated. However, it is sometimes hard for me to digest other food.
I start my day with a little fresh squeezed lemon juice in room temperature water to detoxify and set my stomach to take in food. I make smoothies with coconut milk, tumeric and avocado and a little local wild honey. I’ll also eat a little minced meat or slow cooked pulled pork with yams or sweet potato (not constipating like rice and it will provide needed fibre). Dried prunes for iron and fibre. I take magnesium glycinate by Douglas Laboratories from 1-5 a day for relaxing muscles and digesting. Glycinate is the most bioavailable magnesium. A person with IBS may only be able to tolerate 1 or 2 a day.
Whenever I’m not feeling well enough to lie in bed I’ll dampen a cloth with apple cider vinager and water and put it on my head. That somehow helps lower the histamines in my foggy brain and helps add alkaline to the body when there’s too much acid.
Water is very important for moving the digestive system and detoxifying. But if he can’t stomach 1 1/2 litres a day, try making an natural electrolyte replacing drink(s) for him. Some recipes here; http://dailyburn.com/life/recipes/homemade-sports-drink-recipes/ Sometimes I crave salt. So I’ll put Himilayan salt in room temp water to my taste and drink. Gives some energy.
Other than food – if there’s pain in the body, (upper back and neck?) osteopaths can help with massage to flush out toxins from the body. Some osteopaths have their own techniques, but I’m not sure if it’s all the same. There’s the Perrin Technique” (he presented at the conference in Florida a few weeks ago) and the Bowen Technique. There’s also youtube videos giving do it yourself instructions.
Hope some of this might help! Good luck next week with Dr. Lewis. As much as possible, have Dr. Lewis talk to your son rather than while you just listen on and ask questions you have later. You’ll be surprised how carefully your son will process the information and then bring it up as you’re planning meals together. Kids love to be treated like adults when it comes to talking to adults outside their family.
Interesting Laura. My son has gotten progressively worse over the years (because we haven’t know what the hell this is and the doctors weren’t very ‘helpful’). I reckon he probably started off with leaky mast cells or he reacts to things he shouldn’t (but what? is the next mystery as nothing comes up with allergy testing). And with every passing ‘crash’ he gets sicker and sicker. I’m not sure if it’s a methylation issue but certain supplements help (nitric oxide, vit b) and others don’t. It certainly looks to me (also unqualified) to be an ATP issue. And so many things could be causing this – I just don’t have the skill yet to understand this (going back to uni next year). Fingers crossed Dr Lewis will be able to help here.
The difficulty has been identifying what he can and cannot eat. I think overreacting mast cells cause all the gut pain because it dissipates with anti-histamines or on quercetin. He has avoided anything hard to digest because his tummy simply couldn’t cope.
A few years ago, he was diagnosed with eosinophilic oesphatigitis – which is an allergy based disease. All the protocols made him so sick, he had to be hospitalised. He has been diagnosed with IBS but only because they don’t know why his system is constantly inflamed. Constipation has given way to really loose stools these days (everything changes with his symptoms).
We have done smoothies, juices – the works. I add amino acids to them to improve his protein intake. Raw foods, of course, are hard to digest. Steaming affects their texture which he hates. So now he has to come up with solutions so he gets more nutrients. If I do it, he turns his nose up and this way, he has ownership and eats more.
I used to dehydrate and process vegetables to make them easier to digest and retain nutrients but it’s a big, time-consuming chore.
We are eating low histamine at present – and it’s really helping. Like you said, several small things. Maybe the CFS diagnosis is wrong? I just don’t know.
I find the information I get online has been more helpful than the medical folks where I live (it’s a long story but because they couldn’t find anything, the suspicion for my son’s issues falls on me – despite no evidence of events like poisoning etc). Hence us having to fly interstate to see a doctor familiar with symptoms and issues like my sons – they aren’t unique, just unusual.
I really appreciate your information and your explanation of the bicycle – it looks like small breakthroughs are making a little difference in him every day but, like anyone, I’d love to know what is happening so I can actually help him.
Kathleen, so you supplement with ATP? I’ll have to look up and see how that works. Thanks for the product tip – we have our new doctor appt in a week so we have to be supplement free for that and then I have to wait and see what he finds. But I’ll do some research and keep it in mind – thanks very much.
You mentioned that your son had food allergy tests. Has he had the IgG Standard Food SENSITIVITY Assay? Perhaps you already know about this but for those that don’t it’s a blood test. Mine, which I had done through Immuno Laboratories in Florida in 2008 was immensely helpful. Previously, I had tested negative on every standard ‘food allergy’ test. Immuno Labs tested nearly 100 different foods in this one blood draw. It shows which foods you are not sensitive to and then those you are, rating them on a scale from 1-4 on how your blood serum reacts to the food antigens. Once identified, you first eliminate all the foods you’re sensitive to. Then the next step is rotating all the foods you AREN’T sensitive to, so that you don’t develop any sensitivities to those. It was a 4 day cycle. I know some doctors recommend an elimination diet, but I would have NEVER figured it out on my own. This takes all the guess work out of it. So, for example if you can have carrots, you only have them once every 4 days. Later on I did an additional test called Extend Food IgE which tested even MORE foods, herbs, nuts and so on. That one was done by Allos Reference Laboratory in Mountain View, CA.
Just posted a reply to you and another commentor, but forgot to add this:
CFS is pretty much a body’s inability to deal with stresses which the psychiatric industry has twisted to mean psychological, and governments have withheld funding so that biological researchers around the world are often reinventing the wheel as far as research is concerned, and CFS/ME sufferers worldwide are not even aware of what we have. I’ve just become aware of researchers, clinicians and support groups only about a year or so ago. I thought I just had weird health problems and low energy from time to time. People like Cort Johnson, David Tuller, Miriam Tucker, the IACFSME are doing a great job connecting the CFS/ME world.
A word on psychology: I firmly believe that the anxiety that arises within CFS/ME or any patients who haven’t been diagnosed is a direct result of not having access to accurate biological information so we can manage our health and rehabilitate our bodies to the extent possible. You used an accurate word – poisoning. Even CFS/ME doctors don’t seem to understand how medications can send our problems from bad to worse. We end up having to jerry rig our energy inputs and triage outputs like NASA engineers figuring out how to get a space shuttle in trouble back home. I find taking on the attitude of a Navy Seal to make decisions under tough circumstances and regulating our breathing when we’re in physically trying circumstances in order to not lose it and make decisions helpful. Mark Divine has some great youtube videos on this: https://www.youtube.com/watch?v=osWQuPtvRlQ and here he talks about box breathing and meditation, which I think is great for boys who don’t see yoga as manly: https://www.youtube.com/watch?v=GZzhk9jEkkI
So Laura, you’re basically saying that psychological stressors have NOTHING to do with illness? That’s just — laughable.
There’s indeed a huge connection between long-term chronic stressors, including childhood and adult trauma — and chronic illness, including ME/CFS. Instead of automatically assuming this means ‘blaming the patient’, it DOESN’T.
It means here’s a tool you can use to stop the ‘fight or flight’ cycle, and switch it to rest and digest. Both of which we with ME/CFS don’t do well at all.
The metabolomics that Naviaux discovered are not like dauer; they resemble the geriatric state.
Dauer is a highly controlled, efficient process. The digestive system is sealed off at both ends. There’s more energy for detoxification of the organism. The homeostasis of this organism is not thrown off. The nematode worm larvae does not try to make energy and fail which shortens its mortality; it systematically turns down its metabolism which increases its longevity. http://eprints.maynoothuniversity.ie/515/1/Burnell_etal_ExpGerontol2005.pdf
ME patient metabolism is like a factory filled with old machinery which sometimes works sometimes doesn’t or never works, according to the individual. Homeostasis, including redox homeostasis, is thrown off so much more easily. This is not the body preserving itself. This is the body out of control because it can’t produce enough energy to perform necessary functions. This is just like the aged who continually have complex health problems because their bodies don’t have enough energy to function while responding to its environment. Who else wears compression stockings for POTS? Is sensitive to medications? Needs to take naps during the day? Experience tachycardia during bowel movements? Who else has elevated IL-6 plasma levels secondary to infection, trauma or stress? Abnormal red blood cells? Etc. etc. The elderly.
I know the researchers didn’t mean any harm with their dauer metaphor; it’s simply academics talking. Unfortunately they squandered a great opportunity to humanize our condition in the media and compare it to a mammalian condition which the average person can relate to if not empathize with if they’re elderly.
http://bioecologie.over-blog.com/article-28161677.html
https://books.google.ca/books?id=zLm7sO1sZ6sC&pg=PA292&lpg=PA292&dq=metabolism+geriatric+phospholipid&source=bl&ots=lj-l_htyXh&sig=g1-WSLCYxfCxRffj2H_rgXyy98c&hl=en&sa=X&ved=0ahUKEwiG94StoM7QAhUo5oMKHba2DLgQ6AEIPTAG#v=onepage&q=metabolism%20geriatric%20phospholipid&f=false
Thanks for your interesting perspective Laura. I might mention that several studies have suggested ME/CFS resemble aging including a CDC one (still unpublished I believe) which found telomere shortening.
Thanks, I’ll look that up!
I think Dr. Naviaux would be surprised to learn how people are interpreting his study just from articles, not reading the study. Speculating about body “hibernating” on treadmills; hypometabolism is only ever a “lifesaving” body mechanism; deer go into dauer after running from a forest fire…sigh. A goldmine of a study which now promotes misunderstanding of ME in public and patient community due to this dauer (spurious?) correlation.
Have you looked at the possibility of a primary mitochondrial disease, I.e. genetic?
Thanks for the great reporting of this essential article Cort! Great job.
I tried to connect the dots trough these blood and urine chemicals in another way. It is a VERY long stretch (sorry for the length, could not tell shorter):
* For easy of reasoning I start with following preconditions:
– Massive oxidative stress is generated by vast amounts of mitochondria going in Cell Defense Reaction producing loads of hydrogen peroxide, an essential building block for all Reactive Oxygen Species (Naviaux).
– B-cells, T-cells and Natural Killer cells are all severely exhausted (Mella, Fluge and others), therefore I believe white blood cells in the blood are too weak in order to deal even with weak opportunistic pathogens. Long term survival chances are nill without a powerful backup mechanism. This resembles the long term variant of sepsis.
I assume oxidative stress levels are that high so that near all anti-oxidants going from the food trough the gut in the blood stream are rapidly consumed so that they do not reach the cells that have an oxidative stress burst in sufficient quantities. I believe the same holds for recycled glutathione coming from the liver in the blood stream.
Therefore I do believe that the body is producing as much glutathione from cysteine, glutamic acid and glycine as it can. I believe this process is likely limited by enzymes rather than available proteins. “Calcitriol (1,25-dihydroxyvitamin D3), the active metabolite of vitamin D3, after being synthesized from calcifediol in the kidney, increases glutathione levels in the brain and appears to be a catalyst for glutathione production.” As many ME patients have low to very low levels of vitamin D3, it seems that we may produce and consume loads of glutathione rather than few. Mine was very low while I was still better and took no supplements: “A level lower than 10 ng/mL (25 nmol/L) is associated with the most severe deficiency diseases”. Mine was 5.5 nmol/L).
Yet, there seems to be much remaining oxidative stress and it is likely much worse at the source. Outlasting the danger as Dauer does is hard if peroxide damages the cells at the source. While a mytochondria in CDR does not seem to need antioxidant, adjacent working mitochondria are very vulnerable. A stash of private anti-oxidant would be welcome. Such source may come in the form of uric acid: a “waste” product of deconstructing proteins to for example glucose. Uric acid and urate oxidase are anti-oxidants. Prioritizing proteins as a source for glucose thus provides a source of anti-oxidants at the very location of exertion at the very time of exertion. Using it anaerobically increases the production of uric acid and hence anti-oxidant per amount of produced ATP. Dumping ATP out of cells, as Naviaux observed as something extremely rare, increases this rate further. This may be related to the creatine marker.
Glutamine is also one of the few amino acids that can directly cross the blood–brain barrier. So it can be prioritized as brain food making anti-oxidant as a waste product. Also glutamate “is used by every major excitatory information-transmitting pathway in the vertebrate brain, accounting in total for well over 90% of the synaptic connections in the human brain.” So all things glutamate and derivate are in very high demand.
One problem must be overcome first: glucose and carbs store only in low quantities in the body. Excesses get the blood sugar levels to diabetic levels, a dangerous condition reducing life expectation that must be avoided. Burning “original” glucose fast in an anaerobic way gets faster trough this overstock. Lactate can be converted to pyruvate, pyruvate to acetyl-Coa and acetyl-Coa to triglicerides so this process burns trough excess glucose and increases ability to convert proteins to anti-oxidant.
The brain has a problem however: they work aerobically and hence can produce uric acid only slower. However, pyruvate has been proven to strongly protect neurons from damage by peroxide http://www.jneurosci.org/content/jneuro/17/23/9060.full.pdf. Pyruvate can be made locally in the brain from consuming lactate. Maybe it is therefore that lactate is considered by some scientist prime brain food. It is also prime food for the equally aerobic hart. Pyruvate is anti-inflammatory and anti-oxidant https://www.ncbi.nlm.nih.gov/pubmed/16641887. This again favors anaerobic consumption of glucose.
Formic acid, derived from formate, combines rapidly with peroxide to form performic acid. It is an unstable chemical, but it may last long enough to scavenge peroxide and remove it out of the working mitochondria and cell. Peroxide is relatively stable as well and hence most of it can be removed timely from the cell, but only perioxide can form far more reactive ROS. Buffering it in the cell inhibits hence formation of far more radical species near delicate areas. It also allows to stabilize peroxide long enough to be able to move it to the blood stream and/or less affected areas where uric acid or other anti-oxidants can neutralize it or worst case can take the hit to spread damage to less affected organs that may be easier to repair.
Note: “A major use of formic acid is as an antibacterial agent in livestock feed.”
SuperOxide Dismutase can buffer and transport ROS in comparable ways. When SOD is depleted, red blood cells can take over as less powerful ROS buffers. This may explain why ME patients often have low oxygen saturation of red blood cells: cells buffering SOD can’t carry O2. This may also explain beneficial effects reported of several breathing techniques. The fact that optimal 02 supply reduces peroxide generation could help too: too less 02 increases peroxide and anaerobic metabolism. Too much increases peroxide sharply. It may also link too high-fat diets. Glucose consumption generates 1 molecule of CO2 per delivered molecule of O2. No vacancies are created. Fat consumption generates about 20 to 40% less CO2 for the same O2 delivery leaving vacancies in red blood cells that arise exactly at time and place of exertion. They can be immediately filled with peroxide to be buffered and carried away.
The high fat diet may as well benefit from higher protein levels and no need to remove glucose first. Another plausible path is the reaction of ketones with hydrogen peroxide to ketone peroxides. Information indicates that by combining those two chemicals it can be formed slowly. But even in an oxidative stress storm, the number of ketone bodies vastly outnumber the peroxide molecules so that increases speed of peroxide scavenging quite a lot. Potential catalysts can add to that. While those are nasty chemicals to have in the brain, so are free radicals.
It may also solve the odd observation that the two most helpful diets are either paleo (high meat) or the nearly opposed vegan. But vegan diets are often moderate calories reducing need to get rid of glucose. It also uses lots and lots of fibers reducing glucose peaking. It is among the best anti-oxidant diets. It also is often high in proteins (nuts, seeds, tofu, seitan…) and vegetarian oil (nuts, seeds…). And last but not least, quite a number of plants have decant anti-bacteria, anti-viral and anti-parasite properties.
Hypoxanthine is a necessary additive in certain cell, bacteria, and parasite cultures as a substrate and nitrogen. It is very good that it is removed when under stress of pathogens. Some xanthines such as caffeine are anti-oxidants are broncholidators and astaxanthine (not synthesisable by humans) is one off the most powerful anti-oxidants there is. So it may be better to make those and deprive pathogens from a key resource for growth.
Phenylanaline is a protein that can be burned for uric acid production, but it can be better used as precursor to dopamine, norepinephrine and epinephrine. ME patients make large amounts of the latter two as part of the fight-or-flight response. While epinephrine has very strong disadvantages, it is the prime anti-inflammatory medicine, is a broncholidator and can according to some studies boost white blood cell activity so that is no small feat.
Alanine is used in glycogenisys so it is needed reforming proteins to glucose and not to be wasted.
“Serine is important in metabolism in that it participates in the biosynthesis of purines; It is the precursor to several amino acids including glycine and cysteine, and tryptophan in bacteria.”
Removing it out of the body may be beneficial to hamper pathogens, but “Serine; precursor for D-serine, which is a neurotransmitter” so it may be a trade-off to keep it.
Valine is an essential amino acid used in the biosynthesis of proteins. Removing it out of the body may be beneficial to hamper pathogen but as it is essential is may be a trade-off.
Allantoin: “In bacteria, purines and their derivatives (such as allantoin) are used as secondary sources of nitrogen under nutrient-limiting conditions. Their degradation yields ammonia, which can then be utilized. So removing it may be good to hamper pathogens, but it may be more useful to convert it to anti-oxidant rather than discarding it.
SUMMARY:
* As white blood cells are impaired, massive oxidative stress and CDR may need to take partially over functionality of the impaired white blood cells.
* This creates heavy load of ROS and other toxic stuff in the bloodstream, further exhausting the white blood cells needing to maintain this Mexican standoff.
* Going anaerobic and using the odd protein to glucose pathway so much likely produces key anti-oxidants at the correct place at the correct time. This likely helps protect the own cells from oxidative damage whilst still damaging the pathogens. This further adds to Dauer.
* Several byproducts of this process are anti-pathogen. This further adds to Dauer.
* Reducing some key proteins that pathogens need further adds to Dauer. As the own cells do only need repair and maintenance over growth they are at an advantage.
=> Maybe the energy production pathways are not broken but are reused to maximize Dauer efficiency? It may indeed be the starvation disease: starve the pathogens.
Can you edit for the typo, my brain does not seem to be able to read between the lines any more? Fourth line down. Thanks matey.
If I have this very complex subject right this suggests both energy producing pathways – the aerobic and anaerobic – are not functioning well in chronic fatigue syndrome. The fact that glucose – the best fuel for glycolysis – is not being used in will impact aerobic energy production but it’s not clear to my befuddled brain if that is the main issue or if other problems with aerobic energy production exist.
A very interesting and important area of research which is yielding real results. I can’t help but think, however, and I’m no expert, that since encephalitis causes fatigue, that the main source of the trouble is in the CNS. I understand that measuring metabolites in the blood is far easier than sampling nervous tissue.
My own symptoms run in a predictable cycle: crushing fatigue one day, followed by painful headache and pressure the next, then I feel somewhat better the third day and the cycle gradually begins again, on about a weekly interval. It feels like my immune system keeps fighting something, but never wins the war. I was told years ago that I had a virus of the CNS and that it would last a long time and not to waste my time and money bugging doctors. Good advice.
Hi I have suspected a viral infection of the vagus nerve for the last three years and last week I had an appointment with a new natural therapist who specialises in diagnosis based on genome analysis. She diagnosed me with a viral infection of the vagus nerve based on my symptoms. She has a treatment protocol which I will start after some other treatment she has commenced first, based on my genetic profile. She has treated other people successfully for this. Feeling hopeful.
what is the treatment prescribed? please keep us informed.
I’m wondering if Jarrow Beta Glucans might help your situation. I had tried a company that contained only 100mgs in their preparation and did not find any benefit. Then I started on Jarrow’s 250mgs Beta Glucans and my 21 year sore throat(and lump in throat) disappeared. The swollen lymph nodes in my neck also shrank. I haven’t tried 500mgs yet as I forgot to bring it up with my Naturopath Doctor. I’m hoping that the one I’m on now will stop any viruses this coming Winter.
Thanks. I’ll look into it.
Hi Dave. I forgot to tell you, that if you start on 250mgs of Yarrow Formulations Beta Glucans, you can expect to have a couple days of flu like symptoms as this product goes full force against the viruses that are activated in our bodies. To maybe avoid this reaction, you may want to start taking a 100mg dose for a couple of months. I had run out of the 100mg Beta Gucans for 2 months, so that’s probably the reason I got the 2 day flu symptoms. All in all, it was well worth it! Would really like to know if this helps you. Good luck.
Thanks Cort! Great write up as usual!
How does this line up with the work that Dr Myhill in the UK (http://www.drmyhill.co.uk/) has been doing? She has been chasing mitochondrial dysfunction ever since Dr Cheney suggested it about ten or so years ago.
Hi Vic,
I imagine it certainly fits broadly; I have no idea about specifics – i.e. if they believe the problem in energy dysfunction is in the same part of the energy cycle or not. The core finding, though, seems to be the same.
I get a bit confused about the creatinine..first it says that creatinine is increased in the urine…and later on that it’s decreased, also in the urine…what is correct..? Great and interesting information!
“Chronic Fatigue Syndrome” should be renamed “Mitochondria in Chaos Disease”. OK,it takes a good sense of humor to live with this medical condition which has been ongoing for me in the last 29 years. I with internist would get up-to-date with this research so they can get off their the merry go round “it’s all in your head”.
Well said!
Did these studies lead to any treatment solutions or trials? Thanks.