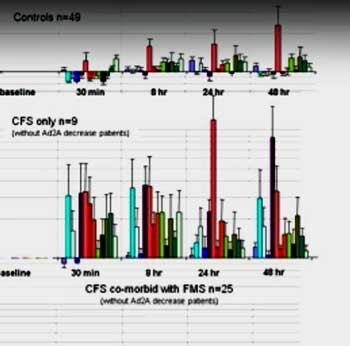
Dr. Light has said that he wants to do for fatigue what researchers have done for pain; that is uncover the molecular pathways that cause fatigue. Some time ago Light presented one of the most spectacular graphs of ME/CFS ever done. I still remember the gasp that filtered through the IACFS/ME conference when Dr. Light showed the slide below . It showed the levels of molecular receptors associated with fatigue and pain skyrocketing after exercise in white blood cells.
The healthy controls are on top and the ME/CFS patients are below. It was even worse than it seemed. In a 2011 Bateman Horne Center video, Dr. Light explained that the differences were so extreme that he had to transform the data into log scale format in order to fit the graph on the page. The expression of some genes was 10 times greater in the ME/CFS patients as in the healthy controls.
Even 48 hours later – still well within the post-exertional malaise (PEM) period for many with ME/CFS/FM – the gene expression was still incredibly high relative to the controls.
Light has found, by the way, that many people with ME/CFS (70%) fit the criteria for FM. When Light compared ME/CFS patients with FM vs ME/CFS without FM he found only a few differences. When he looked at FM patients without fatigue, though, they looked exactly like the healthy controls. That indicated that the Lights were really zeroing in on how fatigue is produced.
A key distinction between FM patients without fatigue and ME/CFS and FM patients with fatigue emerged when the Lights looked at the baseline findings. It turned out that ME/CFS patients looked like healthy controls at baseline but the “pure” FM patients without fatigue looked very different. The expression of three genes were dramatically elevated – so dramatically, in fact, that Light suggested that they might not be able to get any higher during exercise.
The fact that the rest of the genes were unaffected by exercise could explain why studies suggest that exercise seems to work so much better in FM than in ME/CFS. FM patients with ME/CFS characteristics do get knocked out by exercise, but the “pure” FM patients – which make up a considerable portion of the FM population – don’t.
Light did, however, find a startlingly different subgroup hidden within the ME/CFS population. After putting them on the bike this rather large group (40% of the study) looked exactly like the healthy controls with the exception of one gene called the alpha 2A receptor (AD2A).
This ubiquitous receptor causes the blood vessels to constrict in order to keep blood from pooling in our legs when we stand. The expression of that gene plummeted in the ME/CFS subgroup. That suggested that when those ME/CFS patients most needed their blood vessels to constrict in order to pound more blood into their muscles, the gene that did that pooped out. Not surprisingly, 70% of the people with this gene expression signature have orthostatic intolerance.
(Talk about from bench to bedside or vice versa. Dr. Light found this group when Dr. Bateman told him about a young man she could not figure out at all. Looking at the gene expression data, Light found zero increase in his ADR2A gene expression. Light then went back and specifically looked at this gene and found this major group characterized by orthostatic intolerance (OI). Unfortunately most OI drugs do not work on these patients; midodrine works for a short period but has side effects. What a nice example of a physician and researcher working together to break new ground.)
It’s important to note that the Lights were not looking at the cause of chronic fatigue syndrome (ME/CFS) – they were looking at the effects of the cause. Something, they believed – probably in the immune system – was causing the gene expression of their immune cells to go bonkers during exercise. It could be a virus, it could be an autoimmune process, it could be toxins, it could be any number of things.
The search for the cause came next….
Gene Variants (polymorphisms, mutations) ME/CFS/FM, Autoimmunity and Energy Production
In his talk at the Bateman Horne Center, Dr. Light somewhat apologetically referred to the incremental progress they were making but if this is what incremental progress looks like, I definitely want more of it.
First Dr. Light thanked an array of donors; Dr. Bateman without whom this work could not have been done, the Dept. of Anesthesiology at the University of Utah (good to see an ME/CFS/FM researcher getting departmental funding), plus The Solve CFS Initiative (SMCI) – a key funder early in the Light’s work – and the American Fibromyalgia Association, as well as patient donors.
Several recent studies suggesting an autoimmune basis to at least a subset of ME/CFS patients had caught their eye. Intriguingly, both Fluge and Mella and a German group lead by Loebel found evidence of autoantibodies to some of the same adrenergic receptors that had popped up in the Lights’ studies. Furthermore, declines in the antibody levels Loebel found in patients doing well on Rituximab treatment suggested that those autoantibodies – and Light’s dysregulated adrenergic receptor – could be key in chronic fatigues syndrome (ME/CFS).
Evidence emerging that postural orthostatic intolerance syndrome (POTS)- or at least a significant subset of it – had an autoimmune component further piqued their interest.
Wondering whether an autoimmune process was whacking patients as they exercise, the Lights teamed up with an Oklahoma researcher to explore that question. A small pilot study with 18 ME/CFS patients with either POTS or another form of orthostatic intolerance ensued. Almost all (14) also met the criteria for FM. They looked for evidence that the immune system was attacking those receptors that had been so remarkably elevated during exercise.
The results were remarkable: autoantibodies to at least one of the receptors (beta adrenergic / muscarinic) were found in almost all (15/18) of the ME/CFS/FM patients.
This suggested that autoantibodies were attacking the receptors involved in increasing blood flow and other aspects of exercise. Immediately they moved on and asked why these autoantibodies were found.
Back to the genes, they went to determine if gene polymorphisms – slight shifts in the makeup of the genes that produced these receptors – could help explain why the autoantibodies were present. Using a technique that allowed them to measure both the mutations present at birth and those acquired afterwards, they looked at the mitochondrial DNA of those white blood cells.
Why mitochondrial DNA? Because somatic mutations – mutations gained later in life – in the mitochondrial DNA of immune cells could lower the energy levels of in those cells. Lower energy levels in white blood cells could translate into a reduced ability to filter out autoimmune antibodies; i.e. immune cells with low energy could be producing more autoantibodies than immune cells with normal energy levels.
It turns out that mitochondrial DNA is more susceptible to mutation than normal cellular DNA because of the environment tit exists in. As cells produce energy they also spew out free radicals which can damage DNA. Throw in the increased levels of free radicals typically seen in ME/CFS and you might have a recipe for increased mitochondrial DNA mutations produced after birth.
We haven’t seen much evidence of mutations in the mitochondrial DNA yet, but the Light group found at least one mitochondrial mutation not seen in healthy controls in everyone but one of the 40 ME/CFS patients. Plus more than 70% of them had a variant likely to have a high or moderate impact on the production of a key mitochondrial protein; i.e. most had a gene variant likely to cause a serious problem in energy production. The fact that most of the ME/CFS patients had multiple mutations further suggested the mutations were having a real impact.
Significantly, all of the variants were acquired; i.e. none was present at birth – as the result of something in the environment the ME/CFS patients had bumped into. They were scattered throughout the complexes that make up the mitochondrial energy pathway.
About 80% of the ME/CFS patients also had mutations in autoimmune genes (beta adrenergic, acetylcholine and PKA genes), but different genes or gene combinations were found in each patient. All the patients with mitochondrial gene mutations or polymorphisms also had autoimmune gene mutations.
This set up an intriguing scenario; ME/CFS/FM is not caused by a specific gene mutation but by any of a number of broad mutations in mitochondrial genes and in genes associated with the ability to respond to things like exercise (such a blood flow).
It takes the low energy state conferred by the problems with the mitochondrial genes plus a tendency for autoimmune issues to create chronic fatigue syndrome (ME/CFS).
That was exciting, but then Light went further. What he asked, could be causing all these polymorphisms or mutations to crop up? The big clue was that the ME/CFS patients did not appear to be born with these mutations – at some later point they showed up. It appeared that something they had bumped into somewhere had tweaked those genes.
The Gist
- in 2011 the Light’s found that the gene expression of receptors on white blood cells which responded to muscle metabolites skyrocketed during and after exercise
- Their findings suggested a large and unusual immune response occurred during and after exercise in ME/CFS/FM
- One subset of ME/CFS/FM patients with orthostatic intolerance had a very different response
- Six years later in a small study the Light’s found evidence of acquired mitochondrial mutations that could be impacting the energy levels of these immune cells
- They also found widespread evidence of mutations in some genes associated with autoimmunity
- They suggest that the low energy state in the immune cells of ME/CFS/FM patients results in the increased production autoantibodies
- They believe these autoantibodies may be targeting processes vital to producing energy and the ability to exercise
- A large NIH grant will allow them to greatly expand their study
Light suggested that a process called molecular mimicry may be occurring. It turns out that the beta adrenergic receptors affected in ME/CFS are similar in structure to proteins found in streptococcus bacteria. Being exposed to streptococcus bacteria could, therefore, have produced an immune response that mistakenly targeted the beta adrenergic receptors instead of streptococcus bacteria. Being exposed to other pathogens could result in the immune system attacking other aspects of the “fatigue response”.
This process may have been given a boost by the low energy state ME/CFS patients’ immune cells may exist in. If their immune cells are not filtering out autoantibodies they could be at increased risk of autoimmune disorders.
An intriguing scenario seems be emerging:
- Exposure to a pathogen results in genetic polymorphisms or mutations that whack the mitochondria in their immune cells.
- Those low energy states that ensue inhibit the immune cells from filtering out autoantibodies.
- After exposure to a pathogen those autoantibodies attack the beta adrenergic and other receptors involved in blood flow and other processes. The result is extreme fatigue associated with low levels of exercise.
Thankfully the NIH – in this case NINDS – agrees this is exciting stuff. In 2017 Kathleen Light scored a nice $1,000,000 ROI grant to greatly expand (n=300!) their search for mutations in the mitochondria of immune cells in people with pure FM, ME/CFS and ME/CFS/FM as well as (thankfully) other diseases (migraine, depression).
The most difficult part of any study is not the technical stuff – it’s finding participants. This is a big study and the Lights are looking for patients (particularly the more severely ill) and healthy controls.
Calling “the boss” – his wife (smart man!) – up to the mic to tell the audience when the study was going to happen, Dr. Kathleen Light said the study would begin this month. They’re looking for everybody and would love to get severely ill patients. As far as studies go, it’s an easy gig – one blood draw and an online questionnaire. It involves no exercise. If you can participate please bring along your significant other to serve as a healthy control.
Dr. Light said information on how to participate in the study would be posted on the Bateman-Horne Center website.
If you can convince your doctor to get the blood draw, you don’t have to be in Salt Lake City to be in the study. Otherwise, the study is only taking place in Salt Lake City, Utah. Regarding outside participants Dr. Kathleen Light stated:
Thank you so much for helping us get the word out to potential participants about our new study. Because our sample processing is not the usual type performed by most blood drawing labs, we can only test patients from elsewhere if they come here to Salt Lake City (as some have done), or who can find a researcher or lab near them who is willing to practice and then follow our protocol, which we can provide to these other professionals. It also involves shipping them tubes with the buffer solution we use, and they must flash freeze their samples and return the samples to us frozen on dry ice. Because this is pretty complex, we are hoping to get the majority of our 150 patients from Utah and close surrounding areas.
Aside from the blood draw, the only other part of participating is completing questionnaires for any patient who already has a doctor’s confirmed diagnosis of ME/CFS or fibromyalgia, and that is done online at home. We are also testing parents and siblings of these patients to determine whether gene variants that we identify are shared in other first degree relatives. If patients and other family members wish to come together, we can do their blood draws during the same visit.
If you have further questions please contact Dr. Alan Light at Alan.Light@hsc.utah.edu. (Because of problems with customs and having to keep the sample frozen getting samples from outside the U.S. is not feasible. )
The Future
They’re also applying for a new NIH grant to look for more autoantibodies….ME/CFS researchers take note! The Lights have been good at getting NIH grants and the CFS SEP grant review panel is now manned by an ME/CFS expert. Getting a grant is still a very competitive process but the door, so to speak, is more open than in the past.
The Lights and Dr. Akiko at the University of Utah will also soon begin a propanolol/midodrine trial for ME/CFS/FM patients with autoimmune induced receptor problems. Dr. Akiko has apparently been using this drug combo to good effect in her fibromyalgia patients.
The Light’s aren’t the only ones on the hunt for energy problems in the immune cells in ME/CFS. It’s fascinating to see the work in this area evolve. As we’ve seen after the Lights found that receptors on the white blood cells of ME/CFS patients were responding in a bizarre way to exercise, they incorporated results from mitochondrial and immune studies and hit paydirt. Seizing upon recent similar findings, the Solve ME/CFS Initiative (SMCI) is funding several studies aimed at examining energy production in immune cells.
We now have Ron Davis at Stanford, Dr. Naviaux in San Diego, Armstrong and McGregor in Australia, Fluge and Mella in Norway, Maureen Hanson at Cornell and the Simmaron Research Foundation in Nevada looking at energy production via metabolomics and other means.
If the medical gods look kindly on this work it’s possible that future work could establish that a low energy state – perhaps as the result of exposure to a pathogen – exists in the immune cells of ME/CFS patients, which has triggered autoimmune response which then whacks glycolysis, the mitochondria, the blood vessels and other aspects of energy production causing severe fatigue, PEM, etc.
The Lights recent work, of course, has relied on small sample sets so it has to be regarded as preliminary but with the funds to test their findings out we should get a definitive answer at some point.








Is there a link or contact email to become a research volunteer for the Lights?
The Bateman Horne Center works really closely with the Lights on their studies. I would think they would be the one to contact. Their web address is here – https://batemanhornecenter.org/ and their contact information 1-801-359-7400 | support@batemanhornecenter.org. Their research portal is here – https://batemanhornecenter.org/patient-centered-research/
I think we really lucked out having a doctor of Dr. Bateman’s caliber who is very interested in research being located right next to Dr. Light at the Univ. of Utah. 🙂
Check out the research studies the Bateman-Horne Center is currently engaged in!
https://batemanhornecenter.org/how-to-participate/
Here is a better link. Jennifer is the one who coordinates with those to be in research at the Bateman Center. I’ve got a message in to her to get more info on this Light study and who to contact.
Issie
Thanks for the information Cort!
Hey Cort
Have you seen this article yet?.
http://www.meassociation.org.uk/2017/02/the-science-behind-queensland
Thanks Steve. I was not caught up on the Aussies work. I knew something big had happened but not what.
🙂
You’re welcome mate. It’s great news.
This Aussie research sounds like a real potential breakthrough. Article on it please Cort. You are a champion
For sure. The studies are a bit daunting but they are VERY high on this work.
It makes so much sense. They have identified how trauma – infections, trauma, high stress- can trigger it. Speaking personally, my cfs was triggered by a combination of a period of high stress and an infection I contracted in Malaysia
I know with me, GastroCrom – is not only a mast cell stabilizer – but a mild calcium channel blocker. It has been one of my better meds. I have thought there was an issue with calcium channels. And also issues in glutamate/GABA channels/conversion. Hoping my hypothesis checks out. Glad people are researching it that can hopefully make a difference.
I’m soon on my way to Salt Lake. I’ll let you know if I learn more.
Issie
Thanks, Cort, for such an interesting article. The Lights’ work is amazing. I still remember my jaw dropping over that graph!
As you say, recruiting participants is often the real problem for this kind of study and I think we should be doing everything we can, as a community, to speed this up.
I’ve reposted the paras where you talk about recruitment onto the Phoenix Rising forum (with a link-back here, of course) and I hope other forums will also repost it. I hope you might consider approaching #MEAction or other platforms and get them to also promote this study to PWME.
All it takes is getting a blood draw and shipping it and filling in a questionnaire – lots of us can do that, even many of the severely affected who are so often left out of research.
Let’s speed this thing up!
Dr. Light said that was possible; only one patient had done that so far but that it was possible. How great it would be to fill up this really very exiting study fast. Thanks for posting 🙂
Dr. Light quickly replied
“We have been successful in at least two cases were we have trained people at other locations to do the blood separation and then freeze and send the white cells on to us. The procedure for the separation used to be the standard way to harvest white cells, but this is now all done with differential centrifugation, so few know how to do it any more.
If the patient can talk their physician into it, we have a video that explains how to do the separation and centrifugation, freezing and storing of the blood. We will work with them to make sure the assay will work.”
I added the final word from the Lights into the body of the blog.
Maureen Hanson? You have Kathleen.
I must say that I’m just terrible on names – fixed – thanks
Hi Cort,
I am a severelly ill cfs/me patient. 98% of the time completely bedridden, homebound . I may be happy if I can eat and wash myself a little bit on a chear. could I also be in this trial because I live in Belgium. Who do I have to contact? thanks!
Sorry to hear that you’re in such bad shape. I wonder about being able to ship the sample quickly enough to get it to SLC in good shape. I really have no idea.
There is also the issue of cost of shipping. I suppose the patient would have to bear the cost of the blood draw and shipping…I guess (??)
Cort, someone mentioned that when they had their blood drawn as part of the Lights’ (earlier?) study, it had to be done at their office. Is it worth double-checking that they’re willing to take on people who can’t travel to SLC?
At the end of the video Dr. Light said that although only one person – from Georgia – had done it – it was possible. I think we need to find out more how it can be done…I will try and find out.
Thanks, Cort – if PWME can help speed this up, we should be all over it!
Hi Cort,
Fascinating research going on all over the place!
Any specific instructions for participation in this study as you say they need participants? I’m not in SLC but have a doctor and a hospital lab where I could get blood drawn if there’s a way to get it to them / make it work and I can post on my FB page etc…
hi Cort,
thank you for youre answer. But where and what is SLC? was not yet capable to see the video, only could read the text you wrote. Is no problem if I would have to pay for it and was once told could ship it with fedexx or something like that. they could draw blood now tuesday and then ship it but can not remember when it had to be there. Please can you help me? Also not shure if from belgium is ok for them?
thank you!
Konijn, SLC is Salt Lake City, in the USA. I believe Cort is checking to see if it’s possible to take samples from abroad – the time they take to reach that part of the USA could render them useless.
thank you taokat for the information!
Dr. Light replied:
We have been successful in at least two cases were we have trained people at other locations to do the blood separation and then freeze and send the white cells on to us. The procedure for the separation used to be the standard way to harvest white cells, but this is now all done with differential centrifugation, so few know how to do it any more.
If the patient can talk their physician into it, we have a video that explains how to do the separation and centrifugation, freezing and storing of the blood. We will work with them to make sure the assay will work.
thank you very much Cort for the work you did for information with Docter Light. My docter who comes at home does not take any blood. He finds it a waist of time, even does not believe in cfs/ME.If it is necessary it is a nurse from the hospital who comes at my home. And they are, sadly enough for me, allways in a hurry to go to the next patient etc so they would never take time to watch the video from docter Light. Freezing it in and shipping it would be no problem. It is sad that I am again not in a studie and am to ill to go somewhere else if Dr Light mentioned 2 places in Belgium. I was last night even to ill to go dowstairs to eat although I felt hungry. That got me verry scared.But thanks again for youre work!
Thank you as usual Cort for a brilliant write up of this very exciting research. Although a non scientist it makes complete sense to me and it completely relates my condition. It’s just so frustrating that we have to wait for even more years for his findings to be proved.
I am being selfish because I am 69 next year and have been ill for over half of my life. At the moment I am in a complete crash having had several things happen which has lead to me getting at least 5 different viruses this year, all in my throat and the ME symptoms have come back with a vengeance even though I had done so well last year. I know I have an autoimmune illness (Hashimotos) and I also have POTS plus I have been exposed to many viruses over the years as I was a teacher in a local school.
Unfortunately I cannot help out with this study because I am in the UK but I hope that they soon find all the participants they need and thanks again for your wonderful work, I really appreciate it. Knowledge is king although I know that isn’t too well accepted in today’s world!
I know…It takes so long! Hopefully though with these findings from different fields hopefully merging together we’ll build up some momentum finally – and things will move more quickly. I hope you recover from this difficult past year and can get back to feeling better…
Pam, you are totally not selfish! never think off yourselve that way!!! If a healthy person had what we have for only one month, they would cry all day and we have to live with it. To ill to cry or just no more tears over… I hope for you, for all of us that they find soon an or some answer(s) and help. Please keep strong untill then!
Pam I am 67 and have been suffering as you have for yrs now, and this past yr and especially the last month has been horrible. It is a struggle to do some of the smallest things, so I can relate. My question to you is do you have RH negative blood? I have a theory about it being a contributor to auto immune conditions either by the fact that you are absent of the D antigen or the mix of a positive D from a parent or family member. If I had a medical degree I think I would have already figured it out.
“Being exposed to streptococcus bacteria could, therefore, have produced an immune response that mistakenly targeted the beta adrenergic receptors instead of streptococcus bacteria. Being exposed to other pathogens could result in the immune system attacking other aspects of the “fatigue response”.
”
I wonder if this can explain some patient’s temporary improvement on antibiotics. Can lowering the levels of even “healthy” streptococcus (or other implicated bacteria) also lower this immune attack?
Visiting my brother in SLC next month! Putting the Lights on my itinerary now. Glad to finally be able to do my part!
Good for you and good luck! If you can get your brother in there that would be great. It can be hardest to get healthy controls. Have a good trip 🙂
Dr. Fellsenstein at Mass General with Harvard has been compiling a bio bank if that helps
http://www.massgeneral.org/doctors/doctor.aspx?id=16629
The graph and video (or YouTube link) are missing from the pdf
Yes, in response to requests we turned off having images show up in the PDF.
Kerogenic diet can help to address mitochondrial mutations. see this research http://www.ncbi.nlm.nih.gov/m/pubmed/15389892/
and the end of this presentation by Nobel prize winner Douglas Wallace
http://high-fat-nutrition.blogspot.com.au/2014/04/dr-doug-wallace-on-mitochonria.html?m=1
Wonderful to read of all the research being undertaken. If the problems we have with the ATP, ADP, AMP cycle could be solved I’m sure we would all be a lot better off.
The proposed trial with Metoprolol & Midodrine sounds challenging. I was given Metoprolol for high B/P, it caused my BP to go even higher as the concentration dropped off after 7-8 hours, also severe shaking internal & external, couldn’t eat or write. BP much better since it was stopped but the withdrawal period was hell. I have read other ME/CFS people can’t tolerate Metoprolol either. After having ME for 35 years I have experienced bad reactions to many drugs.
Propranolol is the one drug that enables me to part-function. Without it I’m a shaking mess and cannot stay upright, even sitting.
I have POTS along with the ME/CFS, and the auto-immune beta-adrenergic cohort sounds exactly like me. Even on the Propranalol, my HR can hit 140 just standing making a simple meal, or loading the washing machine. I wish I was able to take part in this study, and I hope they find the patients able to help soon. There is hope at last with this great work, the Norwegians, Australians and Ron Davis’ metabalomics work. Amazing what some funding can do!
Hello ,,,I’m with you ,,,I think I’ve had it all my life ,bad for the last 30 ,ruined my dreams ,I inherited it from my mother ,which you do get your mitochondria from your mother ,it destroyed her life too ,I finally was diagnosed w hashimotos after thirty years but the old geezer Dr has no clue ,put me on t4 which didn’t do jack ,,,,because the problem is so much bigger than a pill ,,he has no idea so I stopped wasting my time ,,,but I follow Dr Izabella wentz protocol and Dr Terry Wahls protocol ,u should check her story on utube ,she had severe ms ,crippled and is now running marathons ,she now treats her patients w thenWahls protocol,great book ,and she said it matters less what the name of the disease is than the treatment ,,,FOOD ,,,,Wahls paleo ,all autoimmune whether it’s lupus ,ms ,hashimotos,Krones,diabetes,etc ,etc are mitochondrial problems
A HUGE thank you for this wonderful summary of this research. I had ‘one of those days’ again today with my doctor who still chooses to be completely ignorant about this illness and I have no one else to go to. I have learned to ride that wave after more than 40 years with this illness, but news like what you wrote about makes the ride so much easier. Forget treatment and cure (for the moment); I just look forward to having SOMEBODY in my life (outside a support group) understand and acknowledge that my illness is very real and very serious. Forty years is a long time. Maybe that will happen.
I so agree Lizzie, I first got sick in 1979 but I didn’t get severe energy issues until 1996 when I think the Hashimotos kicked in but I wasn’t treated until 2002.
The Light’s explanation seems to make more sense to me than just concentrating on Rutuximab.
I got almost well last year and was managing daily steps of 8500 for over a year and I did this by taking many of the herbs in the Cowdon Protocol for over 18 months plus my usual thyroid/adrenals meds and rounds of Rimfaxamin for my gut issues.
However since January its the viruses I have picked up that have floored me so a very weak immune system and probably a predisposition to Strep throats are the reason I am sick with ME again. It is such a frustrating illness and can feel like a life sentence at times.
Hello ,,Lizzie ,I’m with you ,,,I think I’ve had it all my life ,bad for the last 30 ,ruined my dreams ,I inherited it from my mother ,which you do get your mitochondria from your mother ,it destroyed her life too ,I finally was diagnosed w hashimotos after thirty years but the old geezer Dr has no clue ,put me on t4 which didn’t do jack ,,,,because the problem is so much bigger than a pill ,,he has no idea so I stopped wasting my time ,,,but I follow Dr Izabella wentz protocol and Dr Terry Wahls protocol ,u should check her story on utube ,she had severe ms ,crippled and is now running marathons ,she now treats her patients w thenWahls protocol,great book ,and she said it matters less what the name of the disease is than the treatment ,,,FOOD ,,,,Wahls paleo ,all autoimmune whether it’s lupus ,ms ,hashimotos,Krones,diabetes,etc ,etc are mitochondrial problems ,,,and like I saw mentioned on here about ketosis ,Terry Wahls uses ketosis for her diet ,in her third tier Wahls paleo plus ,,,,
Hi Cort..Thanks again for a great summary…How do these findings sit with Ron Davis’s belief that “the mitochondria work just fine but aren’t getting what they need and/or are getting a bad signal.” Aren’t Dr Light’s findings indicating that there are genetic issues with the mitochondria?
This is a difference. Both Ron Davis and Naviaux have said that their metabolomics findings suggest the mitochondria are fine. Plus Davis finds ME/CFS patients cells work fine in healthy serum.
From what I can tell the Lights are finding genetic issues with the mitochondria that others have not. I suppose, though, it could be that both are right; the main issue is that enough pyruvate is not available from glycolysis and the mitochondria also have some inhibitions as well which make the pyruvate issue worse.
We are seeing some differences in the studies but the low energy aspect seems the same. Time will tell.
Are the mitochondrial antibodies the Lights found available to test for by major labs in the USA such as Quest or Labcorp?
I don’t know but I would be shocked if they were…
Gulf War Syndrome. Anthrax vaccination was given with a whooping cough pertussis (untested – although once tested on rats around the same time 1/3 died or got very ill – as did the veterans USA and UK 1990 – 1991 Gulf War plus 2003 2nd Gulf War). Pyridigistimine Bromide tablets bwere ordered to be taken every 7 hours also affecting the already damaged central nervous system
25 years later many are seriously ill with illness such as in this document.
Hypopituitarism, found often in tested ill veterans, is a major factor as it affects the major hormone production, immune system and overall health.
We are not being tested enough and research is being blocked because of the political uproar that may ensue….
I attended the hale fatigue clinic 18 months ago for my cfs.
They did some tests and their conclusion was immune system was insulted by a variant of streptococcus.
Strep Bovis was the major strain that showed up.
Another thing that just occurred to me after watching the video – did I hear somewhere that me/cfs patients have a high frequency of sinus infections? I know I do. I’ve read that the most common infectious organism in sinusitis is strep bacteria. Maybe there is a more direct connection than just generally suppressed immune function.
Ha! That’s interesting. I’ve had a mild sinus infection for years. For those who don’t know strep bacteria causes a variety of respiratory infections….The Bateman-Horne Center has a video on sinusitis that HR will be covering
Ironically, I have been in bed sick this week with Strep.. so sick…ugh
Don’t know if my Dr. can do the blood draw, or if I will be well enough to participate.
Colloidal silver sprayed in nose. Mixed with xylitol if tolerated. There is a compounded nose spray that has these ingredients and an antibiotic included in it. Also used in those with Mold exposure. (Look into MARCONS.)
My family is in the Lights study. Will do blood draws when I get there, and there are 4 questionares. From what I understand, they do need you to go to SLC for blood draw. (They are working on having other labs to be able to do draws.)The study will be 3 years in length. May be awhile before data is completed. We have also been asked to be in Dr Batemans study. My nephews are under age, so waiting on a few more months. They are needing families with seeming genetic involvement. Must have confirmed DX of CFS and/or FMS.
Issie
Its lyme disease and mycoplasma!
Hi Cort, any follow up on this? It’s been a while, I found this one of the most interesting blogs you have done. Was it Dr Kem at Oklahoma they were collaborating with in these studies? https://www.healthrising.org/blog/2017/02/23/genes-mitochondria-autoimmunity-chronic-fatigue-syndrome-alan-light-talk/
Haven’t heard a thing…I imagine that the work is going forward though. I don’t know if Dr. Kem is involved or not but he’s doing great work! Not often one person can turn a whole field around. Hopefully he will.