

Producing energy is a big problem in Chronic Fatigue Syndrome (ME/CFS). In fact it’s probably the problem in ME/CFS, which is why the findings of mitochondrial dysfunction and lowered ATP production have made sense. When the metabolomics studies suggested that chronic fatigue syndrome (ME/CFS) was a hypometabolic state, the field seemed set: energy production (ATP) was low and the mitochondrial activity probably was too. Fatiguing disease and low ATP production: it seemed to make so much sense.
Then came the study with the eye-catching title: “Elevated Energy Production in Chronic Fatigue Syndrome Patients.” It suggested that far from being low, cellular energy production was actually abnormally high in ME/CFS patients. Even for a field that’s had more than its share of inconsistent findings, that was a real lu-lu.
The results, though, could not be ignored. They didn’t come from a small research group but from the Xinnan Wang Lab at Stanford. Last year the lab – which is devoted entirely to studying the mitochondria – made headlines with its potentially seminal finding in Parkinson’s disease. It uncovered a defect that prevented Parkinson’s patients from removing their mitochondria as their mitochondria start to wear out. That defect left those mitochondria pumping toxins into the brain. Because the defect was present in different types of Parkinson’s patients, it suggested that a “mitochondriopathy” might lie at the core of the disease.
Plus the patient samples in the Wang ME/CFS study came from some of our best ME/CFS experts. Plus it was funded by an ME/CFS group – the Chronic Fatigue Initiative – that hires only the best researchers. There was no looking past this result.
The Study
“Our results present an unorthodox view on CFS pathology: the fatigue is not caused by lack of ATP, and instead might be caused by a pathological process linked to non-mitochondrial ATP production such as glycolysis.” The authors
The study, which was funded by the Hutchins Family Foundation (Chronic Fatigue Initiative), used samples from 42 ME/CFS patients and healthy controls. (The ME/CFS samples came from Dr. Klimas, Dr. Peterson, Dr. Bateman, Dr. Levine and another doctor in Boston). It assessed mitochondrial architecture and energy production in immune cells in the blood called peripheral blood mononuclear cells (PBMCs).
Results
Even the researchers expressed surprise at the results. The ME/CFS patients’ cells were pumping out ATP at over double the rate of the healthy controls cells.
The Gist
- Immune cells from ME/CFS patients had double the rate of energy production of the cells from healthy controls.
- Because the study used immune cells outside of the serum, the factors in the blood that Ron Davis, Robert Naviaux and Fluge/Mella believe may be inhibiting energy production in ME/CFS patients cells did not apply.
- Even outside of the serum, however, the cells exhibited abnormal characteristics.
- Most of the increased energy production came from glycolysis – the same part of the energy production process fingered by several other studies. Increased rates of glycolysis could produce many of the symptoms found in ME/CFS.
- Increased levels of internal mitochondrial membranes called “cristae” suggested that the mitochondria also were under energy stress
- Immune cell activity could be triggering the increased glycolytic activity found outside the mitochondria, and pathogens or metabolic breakdowns could be triggering the increased need for energy production inside the mitochondria
Finally, the study found increased density of folds in the inner mitochondrial membranes called “cristae” in ME/CFS. Wang noted that the cristae are “exquisitely” regulated in reaction to the mitochondria’s need to produce ATP: the more folds present the greater the mitochondrial need to produce energy. That suggested that even though the glycolytic production of ATP was through the roof in the ME/CFS patients, their mitochondria were still under pressure to produce more ATP.
It was clearly a system under stress. I asked Dr. Wang how her lab got interested in chronic fatigue syndrome (ME/CFS).
I was introduced to this field by a project manager (Stella Lee) at the Chronic Fatigue Initiative. Because my lab’s expertise is to examine mitochondrial dynamics and function and there is an intriguing theory this debilitating disease is caused by malfunction of mitochondria, I decided to pursue this research by joining the collaborative effort by the Chronic Fatigue Initiative.
The high amount of non-mitochondrial ATP production (about doubled in the ME/CFS group) seemed to jive with past studies suggesting that something was going on with glycolysis. Glycolysis provides pyruvate for the mitochondria and produces some ATP. Most evidence to date suggests that glycolysis is the big problem in ME/CFS, not the mitochondria. Unfortunately, the glycolytic or anaerobic production of energy also produces toxic by-products that could conceivably be causing many symptoms in ME/CFS.

Testing the cells outside of the blood may explain why Wang’s study showed high rates of energy production
Still, the high energy production of the ME/CFS patients’ cells put the study finding at odds with a raft of other studies suggesting that ME/CFS is not a hypermetabolic disease – as this study appeared to suggest – but a hypometabolic disease.
Both Davis and Fluge/Mella have found evidence that something in ME/CFS patients’ blood is stopping their cells from producing normal amounts of energy. I asked Dr. Wang if the cells in her study were in the patients’ serum or separated from the serum. It turned out that they were indeed free from the serum.
The PBMCs we got were free of serum. So, we were studying the state of cells free of interruption from factors in the serum. It would be valuable if a direct comparison with and without serum could be made using the same cells.
I asked Dr. Wang if by-products of glycolytic production such as lactate could be causing issues in ME/CFS.
That’s indeed my speculation. There might be some intrinsic triggers in the patients, such as activation of immune cells, and/or elevation of cellular activities, that promote glycolysis – a similar situation when we exercise too vigorously and we feel sore and tired.
She suggested that a kind of runaway glycolytic process could be occurring in ME/CFS patients’ cells.
However, if we exercise too much and feel tired, we can stop the exercise, but the patients’ cells wouldn’t know how to turn off their activities which increases glycolysis and the by-products, and over time that may cause a problem.
When asked if the results in her study could shed light on the exercise problems in ME/CFS, she noted, again, that attempting to do that was like comparing apples and oranges. In the exercise studies the cells were in the blood; in her study they were not.
It is difficult to directly compare our results from cultured PBMCs with those from patients in vivo. The reason is that the cells we studied were dissociated from the patients and thus they didn’t have the same environment as those cells inside the body. To be precise, other cells, serum, and the environment surrounding PBMCs were not present anymore.
When asked why ATP production in the ME/CFS patients cells might be double that of the non-ME/CFS patients, Dr. Wang brought us back to a placde we always seem to end up at – the immune system.
It is possible that those immune cells (PBMCs) are activated, maybe due to a virus infection or other pathological conditions. It has been shown that when activated, immune cells shift from catabolism to anabolism which requires more energy, and become increasingly dependent on glycolysis for ATP production. This theory was raised by Dr. Maureen Hanson via personal communications. Ref: Fox, C. J., Hammerman, P. S. & Thompson, C. B. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol. 5, 844–852 (2005). (This study appears to show how T-cells, when activated by pathogens, increase their uptake of metabolic substrates.)
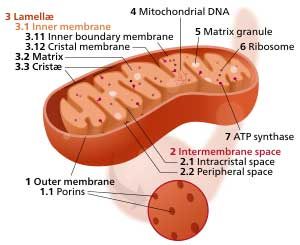
The condensed mitochondrial cristae in the ME/CFS patients suggested increased energy demands were present.
An abnormally high production of non-mitochondrial ATP was the main finding in the paper, but the mitochondria in the ME/CFS patients weren’t entirely normal either. Increased numbers of condensed mitochondria cristae (internal mitochondrial membranes) were also found in the ME/CFS group – a finding that was probably due to unusually high energy demands. I asked Dr. Wang to speculate on what might be causing such high energy demands in a very sedentary group of patients. She suggested that pathogens (immune activation) or metabolic problems could be present.
Condensation of mitochondrial cristae is usually caused by a higher demand of energy because increased cristae area can accommodate more oxidative phosphorylation reactions – the chemical reactions to produce ATP inside mitochondria. There might be a pathological condition the cells have to deal with, for example a virus infection, and thus the cells need more ATP. Condensed cristae could also be associated with a metabolic disease – for example these metabolomic papers suggest that the main energy source glucose is not being efficiently utilized, and the mitochondria may feel hungry and thus condense the cristae for compensation.
When I asked, though, which diseases from a mitochondrial standpoint ME/CFS is most similar to, Dr. Wang mentioned cancer. Since we know that the PBMCs Dr. Wang was studying do not come from cancer patients, it appears that we and the medical world are probably in for some surprises: something different is causing these cells to act the way they are.
Cancer patients have increased glycolysis rates and disruption of their mitochondrial metabolism – it is called Warburg theory. It believes that mitochondria do not function well to produce enough ATP in patients’ cells and as a compensation to meet the high ATP demand glycolysis is upregulated.
The Warburg effect – which can cause rates of glycolysis to shoot up 200x’s in some cancer cells (glycolytic rates Wang’s study were doubled) – may be caused in several ways. The mitochondria may be damaged by the cancer, or low oxygen environments may be promoting glycolysis, or cancer genes may be shutting down the mitochondria (to prevent them from helping the cell to commit suicide (apoptosis) or high rates of cell proliferation may cause it. Which, if any of these processes, may be occurring in ME/CFS is not clear.
When I asked if she planned to study this fascinating disease further. Dr. Wang expressed a strong desire to do so:
For one she noted that they could have done more mitochondrial tests if they’d had more cells. For another she’d love to do tests on muscle cells and on cells in and out of patients’ serum. Calling ME/CFS “absolutely a fascinating area” she said “I’m very interested to continue the research. My current plan is to find collaborators who work directly with patients.”
Conclusion
Despite its title, the Wang/ Chronic Fatigue Initiative mitochondrial study ended up mostly validating what we’ve learned recently about ME/CFS while adding a new slant on it.
It suggested that the glycolytic pathway may indeed be the key to the energy problems in ME/CFS.
Wang wasn’t able to compare cells in and outside of serum, but her findings of high ATP production in cells outside of the serum suggested two things at least to me: that Ron Davis and Fluge/Mella are correct in their hypothesis that something in the serum is knocking these cells for a loop and that the serum may or may not be the entire answer (???)
Even when analyzed outside the serum, the cells, after all, were abnormal; they produced too much ATP (!), their glycolytic pathways were going bananas and their mitochondria were struggling to produce more energy. The big surprise may be the greatly increased rate of glycolysis found in the cells outside of the serum.
So why are the cells still so abnormal when taken out of the serum? Did something in the serum damage them beforehand or are they infected with a pathogen or do they have a metabolic defect? (Or am I just misinterpreting this?).
I asked Dr. Wang about this. She replied that she was not an expert in immunology but suggested the following could be taking place
I’m not an expert in immunity so I’ll have to speculate. The immune cells undergo significant structural and signaling changes after activation – such as receptor binding to a ligand on the plasma membrane, intracellular transduction of signals, and eventually the signals end up in nucleus to change gene transcriptions. I don’t know how long these changes last once the cells are out of the body, but maybe the cells still keep some of the internal changes in our culture so we are able to detect it. It’s also entirely possible that these phenomena are responses of the cells after removing inhibitory factors in the serum – to prove this theory, a direct comparison with and without serum is needed.
Whatever medium they’re put in, something appears to be inherently off with the production of energy in ME/CFS patients’ cells. Either they’re producing too much energy when left outside of the serum or they’re producing too little when left inside of it.
Another serum issue has cropped up in ME/CFS. When taken out of the serum NK cells appear to function normally. That again suggests that something in the serum is harming them. Unfortunately mitochondrial tests have not been done in those cells.
More study is clearly needed. Wang would like to carry out further mitochondrial analyses – we’re not done with the mitochondria – and a lot more is coming down the pike from other researchers. Three Solve ME/CFS Initiative (SMCI) funded researchers are focusing on a mitochondrial/immune connection.
- Isabel Barao will assess the energy index of NK cells in one study (“The Bioenergetic Health Index of NK Cells as a Diagnostic Tool for Chronic Fatigue Syndrome“) using a new test called the “Bioenergetic Health Index.
- Another SMCI study “Metabolic Analysis of B-Cell Maturation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome” will assess whether mitochondrial problems are affecting the B-cell problems that Rituximab may be fixing in ME/CFS.
- A third study “HHV-6 Mediated Mitochondrial Modulation and Its Association to ME/CFS” will determine if HHV-6 infection is affecting mitochondrial energy production in ME/CFS.
Plus the Simmaron Foundation is going to give us our first look at the metabolomics of ME/CFS patients’ spinal fluid, Naviaux’s follow up to his striking ME/CFS metabolomics study should be done this year (and published next), and of course, there’s Ron Davis, Fluge/Mella, Maureen Hanson, and the Australians. It’s going to be interesting…
Next up on the energy production front – the recent Armstrong paper suggesting that gut problems may be contributing to the low energy state in ME/CFS.








This is great stuff, Cort! Thank you for the clear synopsis.
Wondering about the correlation between the subset of us who have had, or will have, cancer and ME/CFS…. and how this relates to Thomas Seyfried’s “Cancer as a Metabolic Disease.”
“When I asked, though, which diseases from a mitochondrial standpoint ME/CFS is most similar to, Dr. Wang mentioned cancer. Since we know that the PBMCs Dr. Wang was studying do not come from cancer patients, it appears that we and the medical world are probably in for some surprises: something different is causing these cells to act the way they are.
Cancer patients have increased glycolysis rates and disruption of their mitochondrial metabolism – it is called Warburg theory. It believes that mitochondria do not function well to produce enough ATP in patients’ cells and as a compensation to meet the high ATP demand glycolysis is up-regulated”
I think the only cancer connection is the understanding that has evolved from it about how to get cells to act this way. Proliferation is one way – it seems to be a key for cancer cells. Low oxygen environments are another and the blog mentions still others. One of these or possibly another appears to be operating in ME/CFS. I think cancer is kind of fig leaf; it doesn’t really apply.
First both diseases are driven by a virus. Here is my understanding of cancer: Cancer is a viral disease. Virus destroys mainly end cells (matured cells in Q compartment), to which the organism responds with chronic inflammation and
increased proliferation to restore the organ and clean the debris. In this chronic inflammation tumor may emerge. When stem cells are hit, the entire Tissue Proliferation Unit disappears, to which the organism responds with dysplasia, carcinoma in situ, carcinoma, etc… According to the hypothesis of professor Zajicek MD, tumor protects against cachexia (muscle loss, wasting manifested as fatigue and weight loss). Every cure, which silences the disease driver, namely virus, cures cancer. Muscle loss is seen in all cancer types. Cancer is one disease. Example: Fever is a defensive mechanism. Plus two degrees protects while plus five kills. As long as tumor does not block any vital function or causes pain, it should be left untreated. Patient should keep her tumor as much as possible. Some people find alternative treatments, like
Ann Vigmor diet, very helpful. With a treatment strategy of tumor dormancy and with host resistance to virus , only minimal doses of medication may be required. Tumor dormancy is a state:when tumor shrinks or stop growing. In any case patient feels healthy.
Learner, the answer to your question has been researched and documented in this expert’s book, “Cancer related fatigue handbook” more than 10 years ago. I always take “new” research with a grain of salt from chronic fatigue researchers. Even Dr. Davis is folllowing the paths others have gone before albeit with slightly new twists.
Cancer related fatigue can show up before the actual cancer cells appear, and when it does it’s called paraneoplastic syndrome. Read page 117 second last and last paragraph and page 118. I can’t cut and paste as it’s part of Google books. They do refer to chronic fatigue as well, and even at that time felt that chronic fatigue could be autoimmune. https://books.google.ca/books?id=dI9loJJutC0C&pg=PA118&lpg=PA118&dq=cancer+autoimmune+fatigue&source=bl&ots=B-deLFUCSJ&sig=QSspJm-uN5Qt6NAriCLLag7XRNE&hl=en&sa=X&ved=0ahUKEwjl3q2FrbDTAhVM8IMKHXqnCKoQ6AEIXjAJ#v=onepage&q=cancer%20autoimmune%20fatigue&f=false
Mayo Clinic description of Paraneoplastic syndromes of the nervous system: http://www.mayoclinic.org/diseases-conditions/paraneoplastic-syndromes/home/ovc-20315084
that is interesting because i have cfs and had breast cancer 2 years ago. i asked the doctor if it would make me tired and she said usually only if it has spread. i was in severe fatigue before i was diagnosed with cancer, it was just off the charts for me.
paraneoplastic syndrome is defined as damage to remote tissue or cells from a malignant tumor or its metathesis. From my experiment its mainly the ability of the patient to control his muscles. Please see Cancer is a Triad [Tumor, Para-neoplasia, Cachexia], A New Cancer Hypothesis by professor Gershom Zajicek M.D
Early on Dr. Komaroff published a paper on metabolic pathways, simple compared to this. However, he said if scientists could find out why patients were alcohol intolerant, (ethanol metabolism), that might be a key finding.
When I read the summary of this complicated finding, I was reminded that glucose metabolism is complicated. I went to this :https://en.wikipedia.org/wiki/Glycolysis which is even more complicated. Up regulation makes sense to me. I imagine in the recesses of my brain I have reasons for this from past, extensive reading about glucose and my own onset of Type 1 Autoimmune diabetes in 1982. That onset was AFTER I became ill in 1980 (enterovirus). Note that the wiki reference places ethanol metabolism in this cascade of chemical reactions.
Interesting! Who knows how this disease will turn out!
Cancer patients seem to benefit from anaerobic exercise and aerobic ones makes their muscle worse. According to professor Zajicek cancer is a triad: one local manifestation tumor and two systemic ones. One of them being sarcopenia, which is the cause of viral infection, which destroy stem cells (a puncher, so to speak, in an organ, cause the tissue to degenerate and is aggravated in the aerobic pathway).
Hey Cort,
I took your very informative article and ran with it (possibly off a cliff, but we will see).
http://forums.phoenixrising.me/index.php?entries/my-current-understanding-of-me-part-4-lactic-acid.2186/
I would love it if you could ask Dr. Wang or Prof. Hanson about any of the possibilities (summed up at the end of the blog), although I know you have excellent questions of your own to ask them when you interview them.
Anyway, thanks for the very informative articles.
Does that mean Ron Davis hypotheses is not entirely accurate? Cort it feels like we are moving forward and then going backwards again. For those of us who are severely affected, are we getting anywhere nearer treatment being found? Its such a long road….
I think the Stanford work largely indirectly validated Ron’s hypothesis but I logically, if the cells are operating abnormally outside of the serum that would suggest that something is going on with the cells themselves as well (?) Perhaps it’s possible that something in the serum damaged the cells or caused them to damage themselves leaving them in this altered state when they were tested outside of the serum. Dr. Wang suggested this might be what’s happening.
Science is full of surprises and I expect that we will have many more before we are done.
I think the most important part of Ron’s, Naviaux’s Fluge and Mella’s findings are that something in the serum is affecting the energy state of these cells and this study findings indirectly supports that.
The changes in the cells could be an adaptive response to increased energy needs. Thus developing structural changes that remain after removed from serum. That makes sense to me. So I would not think of those cells as faulty, but as potentiated.
If the body is asking for more energy it makes sense that all possible means of energy enhancement are turned on.
The question is why. I recently found through a hair kit analysis that I have hugher levels of the Toxic metal Palladium .
If you search the web ion how it affects the body, it is classic CFS symptoms including metabolic, Mitochondrial, inflammation, abnormalities.
Apparently because of an immune activation to an element that just circulates and is not removed.
Had CFS 24 years and just found out this.
I wonder how many of us have the same issue. Palladium was not tested in my blood toxic metal test 17 years ago. But my body is excreting it theough my hair.
This reminds me of other CFS patients who reported full recovery after removing a toxic metal.
When cells produce abnormal amounts of energy via compensatory pathways, it is quite possible that is causing Adenosine Monophosphate to be released from the cells into serum. That may be how the cell is causing effects in serum. There is some chicken and egg involved here.
Incidentally when MyHill et all checked energy producton they found the two different compensatory pathways were deployed to differing degrees in two subclasses of pateints.
The first subclass utilised a pathway generating more Lactic Acid. the second group utilised a pathway that converted two molelcules of ADP to produce one molecule of ATP and one of AMP that was susbequently released into serum.
This finding is very much in line with the high volume of food profoundly ill bedridden patients eat. The hypo-metabolic theory bothered me because it didn’t explain why bed-ridden patients can be eating huge volumes of food, far far more than their active, working carers of similar body size.
This is a what if……comment – my doc (Stephen Fry) found Protomyzoa Rehumatica (protozoa similar to malaria) and is now finding glomus fungus/mold in his patients (this can cause vascular tumors. Article in peer review on this now.) These are in the blood and the mold/fungus can be in organs (found in my thyroid biopsy and has been found in prostate biopsy of others). Since these can be external/foreign invaders our body creates an autoimmune response and there will be compensations for it.
I know with my POTS, I feel, a lot of the compensations -are considered by most as symptoms. My feelings are different – it’s the body’s way of trying to balance things out. May not be the best thing to try to supress what may be keeping us alive. Fine line between what is acceptable and what we can actually tolerate.
Interesting info. One of my wrong DX before my accurate POTS DX was Parkinsons. Treatments didn’t work and made me way worse. But one of the treatments was massive CQ10. It didn’t help. Things continued to progress in a negative way. I occasionally take CQ10 – but don’t really find it that beneficial. May in fact be too stimulating. This would explain why!!!
Issie
I think we have 2 ME subgroups: 1. with an underactive ANS, ‘ATP’, slow moving, sleep all day, many infections,dauer state etc., 2. with an overactive ANS, ‘ATP’, hyper state, can’t sleep but are very exhausted, POTS, etc..
I think we are switching back and forth but no body is seeing it because they are not monitoring anyone while exposing them to triggers vs. avoidance of triggers
for example: after I became ill I was not sleeping , after I realized I needed to avoid things like chemicals,voc’s,mold,ect. and made sure my bedroom (basicly my whole house) was free of triggers I started sleeping
Yes, I agree.
Issie
I was agreeing with GiGi – I think we have different subsets for sure in not only CFS – but definitely in POTS. What works for me, may be totally wrong for someone else. Until we get the subsets figured out – we can’t all be treated the same. And then when we get in a subset there will be variations as we are all unique and individual.
Issie
Do you know the name of the Boston doc who contributed samples?
I don’t…Maybe Komaroff or Felsenstein? The name might be on the CFI’s website.
Is it not the other way around? Cells produce normal levels of energy, but something in the serum neutralises/eats it, so the cells ramp it up with cristae, but that still isn’t working so the glycolysis ramps up but causes more problems than it solves…
Great analysis, thank you – not sure how much of it I took in but hey.
Yes it could be that the glycolytic issues remain, at least for a time, after the cell is removed from the serum.
wouldn’t a blood transfusion, replacing the serum with healthy serum, be a possible, tho temporary, solution?
I’m not an expert but it makes sense to me that that would…It might be necessary, though, to provide specific factors that are missing. Chris Armstrong is trying, right now, I think a staggered treatment plan to bring the metabolomics back into balance.
The National Centre for Neuroimmune and Emerging Diseases NCNED, who have found genetic problems on the TRMP (?) SNP – also glycolytic issues are a problem. The way to reduce glycolytic issues is rest.
@cort johnson: I’ve actually had a blood transfusion during the time I’ve been sick and I must admit I didn’t really notice any difference in my fatigue. But, maybe it is something that you would need to happen more than once?
Even though I am just a ‘study of one’ I figured I’d put in my two cents since I just happened to have the odd luck that I have had a blood transfusion. 😉
Thanks for doing that!
I am fascinated with the research now being done. Don’t think we’ll ever go back to the dark ages again . Cort, you do such a crazy good job of culling this all down, making it interesting to read and mostly understandable! Thank you .
As always Jeanie – thanks for your support 🙂
In Australia we are still in the dark ages Prof. Andrew Lloyd is currently disseminating “educative material” to our medical professionals via e-leaning. He got a $60,000 grant from the Mason Foundation who fund CFS to see if its a good way for medical professionals to learn. He published saying in BMJ saying that he would release the learning material to interested parties BUT won’t unless you are clinically working in the field. The learning material is GET and CBT and his very own graded cognative activity therapy. The underlying basis is the cochrane review…..a la Peter White,Trudi Chaldler, Michael Sharpe, Simon Wessley and the insurance industry….
Ouch!
I have heard that Dr. Lloyd is doing neuroinflammation a neuroinflammation study but his strong focus on CBT/GET is disturbing….
In a Norwegian ME Facebook group, someone wanted this blog translated into Norwegian.
Is that alright for you, Cort?
Anyhow thank you for your wonderful blog.
Eirik Randsborg
Vice chairman
Norwegian ME Association
So much of this research is profoundly confusing for us lay persons, but I so appreciate the information in an understandable format. All the theories about ATP and it’s production/non-production are equally confusing. But, perhaps this explains why I haven’t had any noticeable difference in my severe ME/CFS symptoms, including the debilitating fatigue, by experimenting with ATP in capsule form, which I buy online from Swanson. I’ve tried from 200-400 mg. at different times of the day for at least a year, hoping I’d see some kind of extra energy. Not so much……
Thank you again for presenting this research. I really appreciate that I can go to your site to fine write ups on all the major research being done on CFS.
Well done, sir!
Thanks 🙂 Hopefully it’s mostly right. I would say always take these blogs with a grain of salt. My understanding is wide but shallow!
Thanks Cort, your blogs are a great help.
Thanks Georgina 🙂
Maybe cleaning of the blood like plasmapherisis. Is also being used in autoimmune conditions.
But less invasive would be full body cleanses and detox. Get immune system to recognize what it should and leave alone other things it shouldn’t attack. Working on known methylation issues and supporting the body… will be a big help. Heavy metal detox, parasite cleanses, digestive system support, microbiome support, hormone balance, balanced nutrients and assimilation, self check of perception – etc. These things we can do for ourselves…..takes some digging to figure out how and then willpower and persistence to keep the course.
Issie
I looked into plasmapheresis a while back. Both studies and anecdotal accounts seemed to say that Plasmapheresis didn’t really help (with CFS)
Great summary Cort.
As you already focused in on, the key to Dr Wang et al’s finding of higher ATP levels in the cell of ME/CFS patients may revolve around the fact that these cells were taken out the serum, and the serum may contain the factor that inhibits and blocks mitochondrial ATP production.
So when these cells are suddenly released of this blocking factor by being taken out of the serum, they may rebound to creating higher than normal ATP levels.
Dr John McLaren-Howard’s explanation of Dr Wang’s paradoxical results and of this serum blocking factor is to be found in this post:
http://forums.phoenixrising.me/index.php?threads/47443/page-6#post-801982
Thanks Hip – I didn’t know that McClaren had responded to the study. In the article he says
“It demonstrates the ability of the ‘new’ cells to produce increased levels of ATP.”. Honestly, I’m a little leery of the “rebound effect” but what the heck do I know?
I think the big question for me is why glycolysis was so upregulated in the ME/CFS patients cells outside the serum? That’s one of the issues that we think is dogging cells in the serum. If the increased energy came from the mitochondria – that would be different – but the increased energy is still coming from the dirty, energy-producing pathway. That suggests that the cells are still whacked even outside the serum. Maybe, as Dr. Wang suggested, something in the serum may disturbed them beforehand….
Disentangling this will make for some interesting experiments.
Thank you!
1) We shouldn’t just assume that the underlying cause of the disease is hypometabolism. There isn’t really any good evidence to support this yet and it leads to simplistic assumptions that will likely turn out to be wrong.
2) We shouldn’t use white blood cells for doing this kind of research, assuming they are a good surrogate for muscle, organ, or brain cells. The metabolism of immune cells is complex and decoupled from the rest of the body, controlled by cytokines, binding of antigen receptors, and activation of other immune signal receptors. PBMCs (as were used in this study), such as T cells, when activated engage in warburg metabolism, aka aerobic glycolysis. It has been shown repeatedly that ME patients are in a state of immune activation. It’s likely that these cells are behaving exactly as should be expected, and we shouldn’t assume that what they are or aren’t doing metabolically has anything to do with what’s going on in the rest of the body of an ME patient. My guess is that the further we dig into the metabolomics of this disease, the more things are going to end up pointing back at the immune system.
More complex info to sort through:
“Intranasal VIP safely restores volume to multiple grey matter nuclei in patients with CIRS”
https://www.survivingmold.com/Publications/VIP_AN_GALLEY_PROOF_3_20_2017.PDF
The research is fascinating and mentions “nuclear encoded mitochondrial gene activation” … “The most prominent changes in gene regulation were found in ribosomal and mitochondrial activity” … “In addition, the authors posited that since the mitochondria have their own ribosomes, mitoribosomes, also containing the evolutionarily conserved sarcin-ricin loop, these mitoribosomes may also be vulnerable to microbial toxins.”
I don’t know if this applies but some people are using intranasal insulin to good effect as well.
Very interesting! Just read about it. Thanks for mentioning it.
If folks want to learn more about Dr Shoemaker’s CIRS (chronic inflammatory response syndrome) work, we put together a “101” guide for patients. http://tinyurl.com/moldcirs101
Great write up Cort!
A major piece missing here is that Ron Davian said the ME/CFS cells become normal (as measured by electrical impedance) when placed in normal serum and normal cells become abnormal when placed in ME/CFS serum. Even if the ME/CFS cells are abnormal outside of serum, it seems there is something in normal serum that resets the abnrkmal cells (???). This would allude to the poster’s point above for blood transfusions. Indicidentally there’s a startup in Monterrey, California selling “young blood” transfusions from teenagers to those with difficult illnesses
Anway the work of these two researchers need not contradict
Thanks Jesse…Nice point! We have three different scenarios now – cells in ME/CFS serum; cells in healthy people’s serum and cells outside of any serum….Three different environments – perhaps its no surprise that they are reacting different in each. Good point!
Blood serum or plasma is actually connective tissue. White blood cells (mast cells are a type of white blood cell) sending out cytokines are not just for immune purposes but they also repair connective tissue. White blood cells are shape shifters and smaller than red blood cells and they travel (unlike red blood cells) to the lymph system to dispose of toxins from the blood.
How many of us have been diagnosed or suspected to have Ehlers Danlos Syndrome which is not just about collagen but about connective tissue, and also under researched re POTS, MCAS, etc? What if what’s going on is at least two parts of connective tissue disorder – leaky blood and lymph vessels allowing viruses, bacteria and blood antibodies and antigens travel to where they shouldn’t be within a serum that’s degrading and not doing its job and our white blood cells are trying to fix everything, connective tissue plus clean up and send to the lymph system the garbage (bacteria, toxins, fungus, virus, etc)?
Another thing – ATP cells are stored in adipose (certain type of fat) tissue. Adipose tissue is also connective tissue. ATP cells have no cell membranes. What if they’re being stored and immediately depleted because of degraded adipose tissue? Then when they’re being called upon during normal anaerobic metabolism, they’re not there. Newer research suggests that we call upon anaerobic energy at the same time as aerobic energy, not that we use one or the other exclusively. (Please read from 7.2 Phase 2 Fat Mobilization and Use, starting with, Fat from the adipose is used to make ATP in most tissues when glucose in the blood is low.” https://www.med.upenn.edu/biocbiop/faculty/vanderkooi/chap7-9.pdf )
So I think we’re all a little bit right – but I think blood transfusion is too risky – there are hundreds if not thousands of blood differences between each individual accumulated over our lifetimes. Better but also a temporary measure would be blood filtering outside of the body – maybe someone else knows about that and can add that if they’ve had it or heard about it. More permanent and the flavour of the month in therapies for genetic disease is stem cell transplant. Blood cells and serum are made in our bone marrow. Replacing the mesencymal stem cells which make adipose tissue, etc. may be our answer as currently understood. https://en.wikipedia.org/wiki/Mesenchyme
Here’s a story of a boy withe “multi system issues” which a gene was finally found for, but really it didn’t matter as the therapy is the same for all gene multi system issues, stem cell transplant: https://www.thestar.com/news/gta/2017/04/03/toronto-doctors-discovery-of-new-disease-comes-as-real-relief-to-boy-10.html
LY I think your comment about the serum or plasma being connective tissue is intriguing.I did not know this. One day when I have energy I would love to do a write up on my own path. What I can share as succinctly as possible is that I was diagnosed with CFS in 2008 and from that time until 2013 my main complaints were energy issues (including PEM) and neurological pain.
From 2013 onward I began to have muscle and then skin / tendon involvement. And in 2014 a type of interstitial lung disease (Non-specific interstitial pneumonia) was added.
Even as late as Nov. of 2016 I tested negative for everything including plain old ANA and the ro52 antibody. In March more tests were run. I now test POSITIVE for the ro52 antibody and Jo-1 antibody. BOTH CONNECTIVE TISSUE DISEASES.
The question for me is did I have a connective tissue disease all along? Just one that they could not identify? Or did I have whatever ME/CFS is for so long that my body developed the identifiable connective tissue diseases?
I highly suspect that my doctors will continue to ignore the ME/CFS as they have all along and move to treat the now known connective tissue / lung disease issues.
Really interesting Tina…With the Ehlers Danlos connection to ME/CFS I wonder if this is part of your ME/CFS path? I think there are going to be lots of pathways.
Cort, they have not even told me what autoimmune disease they will categorize me with yet. I think I will have a more concrete diagnosis in a couple of weeks with a recommended first line of treatment, which will probably be steroids. I will update when I have that information. The words I heard kicked around were dermatomyositis, polymyositis and antisynthetase syndrome. What came to my mind was your article about the “atypical subset.”
Two years ago when I started having the muscle and skin issues I brought up Rituximab. They looked at me like I had three heads, because ya know I only had cfs. I was told to join a support group and exercise. I don’t think they are thinking it is so crazy now. Although they will have to go through steroids before going on to immunosuppressive drugs.
When I find interesting articles, like this on,I like to pin it on my ME board on Pinterest. When I go to the doctor it is easy to find. I let the dr. read it as I am at a loss to explain. But I missed the Pin option on this article. Just wanted to let you know I love that option.
Thanks for letting me know. Stavya just got it up there- I’m surprised we didn’t have it before (lol). Thanks for improving Health Rising!
Hi Cort, thanks for the great writeup once more!
Rather than a paradox, it might be an answer to an old paradox: “How can so many ME patients live for decades with such low blood volumes? Some claim reductions up to 60 or 70% of normal values and many claim that survival rates of otherwise healthy people who lose that much blood due to an accident are low.”
This paradox appears to be so daunting that most doctors refuse to see the low blood volumes of ME patients as proof that there is something physical wrong with us, it just couldn’t be true.
The answer may be: we adapted in such way that, if toxic byproducts could be kept under control/removed, our tissue could produce 100% of our energy needs in an anaerobic way. That is similar to what is happening: in a test environment where waste is potentially diluted we produce 100% of our energy needs aerobically and another 100% anaerobically.
This may add to my idea posted somewhere else on this forum that our blood volumes are so low, and that variation downwards upon these already low volumes are really large after exertion, that we should have prolonged episodes of *very* poor blood flow in part of our body and mainly in the tiniest hair vessels. Add to it that the large amounts of ROS that Dauer generates kills NO and hence contracts blood vessels. From a hydraulics points of view it is almost impossible not to have such periodic catastrophic events.
Recently I learned it should be even worse: viscosity of blood increases as its flow speed decreases so the liquid is worse than water. There is an interesting link here: http://ndnr.com/cardiopulmonary-medicine/blood-viscosity/
* blood viscosity goes up with inflammation
* physical and mental exertion and stress cause inflammation
* blood viscosity goes up with infection (cytokines make it clot more)
* the three above could point to common “entry points” into ME
* blood viscosity goes up with low temperature, something a large subgroup of us has
* high red blood cell count increases viscosity
* according to the link, men are far more vulnerable to high blood viscosity then woman; however women are far more vulnerable to ME and fibro
* however, according to my theory high viscosity only comes into play when blood volumes are far to low
* according too: reference.medscape.com/calculator/estimated-blood-volume woman have on average 20% lower blood volume then men for the same body weight; this could help explain why women are much more vulnerable to ME and fibro; their often lower body temperature does not help either
* according to the blood-viscosity link the viscosity of blood drops during pregnancy; this could potentially be linked to the many observations of ME symptom withdrawal during pregnancy (but complications with pregnancy increase viscosity)
* this hair vessel problem resembles arteriosclerosis a lot, but it is theorized to happen in the hair vessels as opposed to the bigger blood vessels
* it should be a highly inflammatory disease, but could be just as undetectable as the highly inflammatory arteriosclerosis due to its similarities.
One problem remains: during prolonged very poor blood flow tissue damage should become undeniable due to the toxic waste produced during long periods of combined lack of sufficient oxygen and anaerobe metabolism. For example lactic acid should go up very high. Near the hair vessels with poor blood flow, lactic acid removal should be too low and buildup should surpass dangerous levels.
Here an unlikely ally could help: massive oxidative stress. According to https://www.researchgate.net/publication/38050622_THE_OXIDATION_OF_SODIUM_LACTATE_BY_HYDROGEN_PEROXIDE sodium lactate and hydrogen peroxide react to form acetaldehyde. It is an autocatalysing reaction meaning that high amounts of both lactic acid and peroxide increase the conversion rate. It’s hard to guess if the conditions in the body would allow for sufficient conversion rate without extra information. My guess is it should work with lactate and peroxide as well in the blood stream as it is likewise and sodium lactate may be available to some extent in the bloodstream too. With sufficient oxygen it further breaks down. Lacking sufficient oxygen elevated amounts of somewhat toxic acetaldehyde remain both local and throughout the whole body. According too https://en.wikipedia.org/wiki/Acetaldehyde acetaldehyde is also a byproduct of alcohol breakdown and may cause hangovers. That may contribute to feeling sick, desiring to vomit and feeling disorientated. It may also help explain why many of us don’t tolerated any longer alcohol.
So how could peroxide (H2O2) be formed if there is insufficient oxygen? The answer may be: peroxide is a signaling molecule starting nearby cells and cells nearby those to go into CDR too and produce peroxide as well. Those cells could be located toward the thicker ends of hair vessels having sufficient blood flow and hence access to oxygen.
So how could peroxide reach the cells who suffer from very poor blood flow? Where red blood cells are “huge” and hampered a lot in their movement under these conditions, peroxide is a tiny molecule that diffuses well even with no movement in the liquid at all. It’s like dropping one drop of paint in a glass of water: it is a physical process that goes fast with tiny molecules. It would be far more effective than diffusing oxygen in a waterous solution: peroxide solves vastly better in water then unbound oxygen. Combining all of this it could be an alternative oxidation mechanism that provides energy during prolonged periods of very poor oxygenation as long as some tissue nearby has sufficient access to oxygen (in order to provide the needed peroxide). The increased mytochondria area could be an adaptation to provide low-sufficient aerobic energy production under boundary low blood flow conditions.
This idea deviates a lot from standard ideas but it has some reasonable amount of logic to it. It would help explaining as well why so many of us have often low oxygen saturation (mainly after exerting) and why a large percentage of those that do improve have done something related to better breathing: in order to take up lots of oxygen the lungs seem to depend a lot on good blood flow in the large network of hair vessels. Low blood volumes also weight on capillary blood flow in this critical part of the body.
My gosh, as always dejurgen – such creative thinking! I hope some researchers are reading your comments. You even got the increased mitochondrial area in there 🙂
Do you remember or are you aware of the hypercoagulation work done in ME/CFS about 15 years ago? Dr. Holtorf still uses a blood thinner – the name which escapes me now – with success in his patients. He called it his secret weapon in one talk.
Thanks Cort for the kind words. Also thanks for the link, never heared of it. It looks promising (although I’ll try and find a “natural” approach for it as I had strong side effects of prescription drugs before). It still should be quite valuable.
Saline, on your other recent blog, should combine increasing blood volume and decrease viscosity of blood. Have read up with that blog, I came up with quit a plausible theory as to why blood volumes would be so low. Combined with the post here and the one I made somewhere about Dauer as an alternate immune system I believe I managed to plausible connect quite some number of previously unconnected dots. Should make for an interesting yet unstructured read. Now my brain is utterly exhausted 😉
Interesting. Then there are us with POTS and confirmed low blood volume who also have issue with peroxide buildup – causing vitiligo. Maybe that’s another compensation – too high peroxide. I thought that may be the case with protozoa. Some of us have issues with that and peroxide help modify them.
And the issues with blood viscosity – recently DX with Lupus Anticoagulant indicative of APS and faulty issues with over thick blood. Taking herbal blood thinners a huge help. This also explains – with me – why vasodilation is a better “fix” than constricting. I have to expand my veins for overly thick blood to move. The tachycardia happens because it is trying to assist faulty veins and muscle pumps in the legs to move blood to vital heart and head areas. But this maybe are direct “effect” of high NE produced to speed up the sympathetic system. The body is thrown into a “save me” mode and this needs to happen to help with blood flow issues. It’s a compensation – not a symptom. Then there are the kidney issues – some of us with HyperPOTS also have seemingly – “issues” with that. Too low renin and aldosterone. This again could be a protective mechanism. We may already have too high salt levels. This condition creates it. If we increase our aldosterone, we hang onto even more salt and maybe more fluid with salt. But what’s the consequences to that. Possibly creating more dysfunction in the body. We need to figure out the difference between a true “symptom” of an illness vs. A “compensation”. Then determine if that “compensation/symptom” is the better consequence to what it may be trying to stablize.
And the study with the Lights should prove interesting. They are trying to look at mitochondria and genetics. Also autoimmune issues that may be connected to genetics or epigenetic. Should prove enlightening.
Issie
High Issie,
I hope your resent tests are no pre-sign of having increased risk for Lupus. That would be another nasty disease too much.
However I learned a lot from your post:
I did not know vitiligo was often considered an auto-immune disease. I did find however that that the inability to use tyrosine needed to make melanine is supposed to be a factor. https://nl.wikipedia.org/wiki/Tyrosine
Now tyrosine has strong antixodant properties:
http://www.sciencedirect.com/science/article/pii/S0009279700001794
I just lost the page I just found, but tyrosine acts a lot like gluthathione: it can be re-used by oxidation-reduction many times. Under sever oxidative stress and/or glutathione deficiency it can take complement glutathione. (the other paper I found was easier to read) http://www.jbc.org/content/287/31/26068.full
Another way to lose tyrosine (so not having it to for melatonine) is conversion to L-DOPA. https://en.wikipedia.org/wiki/L-DOPA
It’s needed to make dopamine, norepinephrine and epinephrine. We needs lots of the later (for example with POTS as you meention). That takes tyrosine away. Interesting remark: if we would “burn” lots and lots of (nor)epinephrine then L-DOPA circulation should be higher. However this could result in both reduced as increased L-DOPA quantities depending on it being a push or pull based system. See https://en.wikipedia.org/wiki/L-DOPA for the side effects of L-Dopa. With some imagination one could call it a partial ME symptom list.
“Then there are the kidney issues – some of us with HyperPOTS also have seemingly – “issues” with that. Too low renin and aldosterone. This again could be a protective mechanism.”
That may connect well to the resent comment I made on https://www.healthrising.org/blog/2017/04/15/saline-pots-chronic-fatigue-syndrome/ about a potential explanation on too low blood volume.
Thanks to your post, I learned that low renin has a strong effect on vascodilation in the lungs, strengthening the link I mad between low blood volume and lung dysfunction.
I too agree the difference between symptom and compensatory mechanism is often mistaken.
@dejurgen – not sure why it didn’t put reply buttons on your last post to me. But, take a look at the connection with D2 – dopamine, glutamate- NMDA receptors and N and P/Q type calcium channel receptors and choline. You may find the connections just as interesting. If you go back and look up my post you will find some of my discoveries. With some of us POTSies, they are finding antibodies to them and issues with TRPM3. Here’s a thread we have going on choline:
://www.healthrising.org/forums/threads/who-doesnt-benefit-from-acetylcholine-supplements.5216/page-2#post-28528
FYI- I appreciated your comment on saline. I read the article and dismissed it as I’m not of the opinion that it gives any long term benefit. It’s a day bandaid at best. And I’m also not in agreement with some of the med used in POTS. I don’t think increasing salt to the levels they suggest is a good thing. In fact, I think long term you, potentially, could mess the kidneys up and possible damage veins more if it’s an IV regularly. I didn’t even comment on that one – cause I’m very strongly opinionated on that one. I did a lot of research on this if you are interested it’s on the DINET forum site.
Issie
@deJurgen – I’m finding thinning blood with herbals and upping choline levels very beneficial.
I use Supreme Nutrition products mostly.
Issie
http://www.wellnessresources.com/health/articles/choline_helps_pots_chronic_fatigue_autoimmune_disorders/
http://www.dartmouth.edu/~rpsmith/Cholinergic_Transmission.html
Check this out. Using Huperzine A, pregnenone and some herbs to thin my blood is Making a world of difference for me. I also use some med for MCAS and POTS that are mild calcium channel blockers.
Issie
Issie
Hi Issie,
the forum structure only allows comments two levels deep it seems, in order to avoid those long and thin stripes of text I suppose.
I’ll look into your info in the coming week, I kinda surpassed my energy envelope enough now. What type of herbal mix do you take right now? There is a whole range of different products from Supreme Nutrition.
Currently I eat daily celery. It’s supposed to thin blood but above all it is low calorie vitamin and mineral alcalic rich food that is considered to be the best vegetable against rheumatics. Caution: it is a strong allergenic.
I’d like to draw attention to an apparent paradox that didn’t seem to get addressed: They found that the cristae are highly folded to provide more surface area for oxidative phosphorylation, BUT mitochondrial ATP production *only* reached normal levels. Assuming we’re not talking about extra empty membrane space, but indeed more electron transport chain complexes for a given mitochondrion, then what’s the deal? If we require extra levels of molecular machinery just to get up to the normal levels of that machinery’s product, what does that tell us? The possibilities I can think of are that either the machinery is structurally inefficient (mutations or allostatic modulation), or that it’s not receiving enough precursor material. And that this continues to be the case even outside of whatever problematic elements exist in the serum.
Two clarifications–
I meant allosteric, not allostatic 🙂
By insufficient precursor, I mean to either meet energy demands or to saturate the ETC (though in the long term these ought to be the same, I think), and not necessarily the normal absolute amount of precursor a healthy person would have.
Great point KV! (One that I missed entirely :))
It sure sounds like that could be a compensatory response to problem with the mitochondria. As I remember the Lights have evidence suggesting that something is going with the mitochondria and I think Armstrong has left that door open as well.
I hope the Stanford group gets more funding….
Cort, it turns out that ME/CFS researchers have only been explaining part of how ATP production works. They’ve not explained how adipose tissue stores ATP cells then the body calls on them. I was confused too till I read about this. Could you question a researcher about the possibility that we may have connective tissue problems (genetics, perhaps such as Ehlers Danlos, etc.) and perhaps the ATP cells are not being stored properly in faulty adipose tissue or leaking out before they can be used?
Fuller explanation of energy production:
http://www.ideafit.com/fitness-library/the-three-metabolic-energy-systems
Nice catch! Read over it as well.
This makes a lot of sense something is very wrong with the metabolism of ME/CFS patients. Just a quick look at how much food a bedridden patient needs to eat would demonstrate that something is way off base. Look at how skinny the profoundly ill are yet their calorie intake is often enormous. For me something changed as my symptoms reduced over 3-4 days my food requirements reduced by a factor of 4!!! Very significant. A recent crash and I need to eat more??
I wonder if this elevated energy production/hypometabolic state is what causes the wired and tired feeling? I’m wired/tired right now and it feels like my body is confused, like it’s stuck in a feedback loop.
Truly a “lulu” of a finding! Could not agree more. Keep digging folks, maybe an answer will come for some!
The paragraph beside the test tube illustration contains an error. It states the the common view is hypermetabolic, and this study found hypometabolism. It is in fact the other way around!
This study is also only partilly differening from one of Sarah Myhill et als papers. That found much lower Mitochondrial production of ATP (as I know for certain I have) but in about half of patients higer Cystolosic production of ATP as a compensatory mechanism.
It would be interesting to know how the rate of ATP production in these doubly folded mitos per unit of surface area compares with normal healthy mitos.
The sentence I think is correct but it states confusing. It states that other studies suggest that ME/CFS is a hypometabolic disease.
Interesting re the Myhill studies – I didn’t know they found increased cytosolic which I assume means glycolysis – ATP…That would fit right in with this study.
I stand correct, yes it is correct, but as you state a it confusing unless very carefully read (in my case PEM following a shopping trip).
One furhther point on immune activitation and glycolsis. Professor Anne McArcle of the University of liverpool produce a working hypotheis for ME some years ago and was resarching to confirm it. This relvoved around a well known aspect of immune function.
Infection down-regulates mitchondrial preformance and upregulates the compensatory process of ATP generation in Cytosol that produces far more lactic acid. It is this process that produes ‘flu like’ pain when one has an infection.
That lacate is utilised in immune system signalling in some way (that I do not understand).
Once these cells were taken out of serum in sounds like that cystosolic process continued where it should switch off (confirminng Professor McArdle’s hyptothesis at least in part). But did not continue the mitochondiral down-regulgation.
This may relate to the manner in which the hyptothalamus is signalled by macrophages and should an infection persist downregulates the production of thyroid hormones.
If these experiemnts were conducted for long enough to be useful, it would be interesting to know how the levels of Catechols, Cortisol and Tri-iodothyronine compare with the serium of real ME patients. Glycoslis after all is controled in part by the stress hormones. above, and mitochondiral operation regulated by T3.
This paper might have hit on the problem and offers a solution;
A selective phosphodiesterase 3 inhibitor rescues low PO2-induced ATP release from erythrocytes of humans with type 2 diabetes: implication for vascular control.
https://www.ncbi.nlm.nih.gov/pubmed/21963837
https://www.healthrising.org/forums/threads/who-doesnt-benefit-from-acetylcholine-supplements.5216/page-2#post-28533
I put this link above and it didn’t transfer properly to make it easy to go to. Here it is again.
Issie
Where is a link to the Wang paper?
Hi, still looking for a doctor in central Florida that knows CFS. Any suggestions? Age old question, it seems we are still in the dark ages in Ocala.
I only know of Dr. Klimas, Dr. Vera and Dr. Reye in Miami/Ft Lauderdale. Any chance you could get down there?
There are also some doctor databases here – https://www.healthrising.org/forums/resources/categories/doctors-finding-a-doctor-your-doctor-visit.201/ – maybe there is someone in there in your area.
Hey Cort,
I took your very informative article and ran with it (possibly off a cliff, but we will see).
http://forums.phoenixrising.me/index.php?entries/my-current-understanding-of-me-part-4-lactic-acid.2186/
I would love it if you could ask Dr. Wang or Prof. Hanson about any of the possibilities (summed up at the end of the blog), although I know you have excellent questions of your own to ask them when you interview them.
Anyway, thanks for the very informative articles.
* sorry for the reduplication, I put my comment in the wrong place before.
This is so interesting, thank you for yet another excellent dissemination!
Something i didn’t see mentioned at all was the cell danger response and how that could fit into this ATP production puzzle.
Insufficient oxygen, especially in the brain, has been mentioned in a number of other places, and to me that makes the most sense as an explanation for seeing the extra mitochondrial folds and double ATP production.