

It’s always good to see an exercise test in chronic fatigue syndrome (ME/CFS). It’s harder on the patients – kudos to them for participating in the study – but study after study has shown that exercise allows researchers to dig deeper into what’s going on.
Dr. Montoya of Stanford was the senior author and Kegan J. Moneghetti, a Stanford physician researcher focused on cardiovascular medicine, was the lead author. The idea – to explore what exercise did to ME/CFS patients’ immune and cardiovascular systems and metabolism – was a decidedly good one. We need as many exercise studies as we can get.
The paper stated that they used a submaximal exercise regimen to explore energy production (VO2 max, etc.), and the cardiovascular and immune systems. An echocardiograph measured left and/or ventricular mass, volume, wall thickness, pressure, stroke volume, etc. Vascular stiffness was measured before and after exercise using ultrasound. Fifty-one cytokine and growth factors were measured at baseline and 18 hours after exercise.
Value of Circulating Cytokine Profiling During Submaximal Exercise Testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. www.nature.com/scientificreports 2018 Kegan J. Moneghetti1,2,3, Mehdi Skhiri4, Kévin Contrepois1, Yukari Kobayashi1,2, Holden Maecker5, Mark Davis5, Michael Snyder1, Francois Haddad1,2 & Jose G. Montoya.
The Immune System and Exercise
A surprisingly large number of studies have examined immune factors after exercise in ME/CFS. The results, unfortunately, have been surprisingly underwhelming given the role the immune system is believed to play in producing the post-exertional malaise in this disease. On the other hand, the studies have been small and usually examined only a small number of immune factors. Lloyd’s negative 1994 study, for instance, tested only four cytokines.
A 1999 study which exercised 20 ME/CFS patients to exhaustion and found no immune differences (WBC, CD3+ CD8+ cells, CD3+ CD4+ cells, T cells, B cells, natural killer cells IFN-gamma) concluded that the “immune response” of ME/CFS patients in response to exhaustive exercise is “not significantly different” from sedentary healthy controls. A small early study finding only modest differences likewise suggested that “cytokine dysregulation” is not a dominant factor in ME/CFS.
A larger 2003 CDC study (32 patients) which tested complement factors and cytokines at 4 time-points found alterations only in C4a. Nijs found no such changes in either it or Il-1 beta in a 22 patient study. Robinson found no changes in IL-6 or its receptor in his 33 patient 2010 study. White failed to find any differences before and after exercise in nine cytokines in his 24 patient study.
Light’s 2010 study – one of the few to produce really significant results – did so only by contrasting 11 ME/CFS patients with high symptom flares (high pro-inflammatory cytokines) with 9 patients with low symptom flares (reduced pro-inflammatory cytokines) after exercise.
As with other cytokine studies, the results from exercise studies have demonstrated little consistency. The Moneghetti/Montoya 24 person cytokine study is modest in size and only examines them at two time-points (before and 18 hours after exercise) but looks at the most cytokines (51) yet.
Results
CPET
The paper did not mention which exercise protocol was used but reported that, “All participants underwent symptom limited exercise”; i.e. they experienced fatigue, dizziness, shortness of breath that caused them to stop the test.
The title of the paper stated the researchers employed a submaximal exercise test. A controversy has been brewing for quite some time over whether it’s best to use submaximal or maximal testing in ME/CFS. “Submaximal” exercise tests sound like they’re stressing patients less but submaximal tests can have ME/CFS patients exercising at a higher rate for much longer than maximal tests which tend to be briefer.
The RER results (1.14 – patients; 1.18 – healthy controls) – which are an indicator of effort – indicated that this was essentially – despite the title – a maximal exercise test. Some differences in the literature do exist. The American Heart Association and other groups state that a 1.10-1.30 and above result indicates that a maximal effort was given. Some groups do require a 1.15 result. Whatever the interpretation, with both groups of participants above or very near the 1.15 mark and well above the 1.10 mark, this appeared to have been a maximal exercise test.
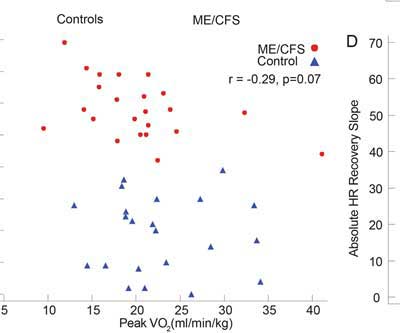
Not surprisingly given the one-day exercise testing regimen, neither maximal heart rate, VE/VCO2, peak VO2, RHI, VE/VCO2, peak VO2 during recovery or 18 hours later were significantly different between patients and healthy controls.
Fatigue scores were not correlated with peak VO2 in this study; i.e. the patients reporting more fatigue did not have lower peak VO2 values. Citing their exercise findings and a heart failure study, the authors warned against associating the degree of fatigue found in ME/CFS with exercise impairments. The heart failure study found only modest correlations between the response to an exercise test and symptoms.
The fact that no difference was detected between CPET parameters between both groups despite marked difference in MFI-20 scores highlights the difference between reported symptoms such as fatigue or dyspnea and exercise performance measured by peak VO2. This underscores the importance of not using these two concepts interchangeably.
That statement opens a can of worms. It’s true that researchers have been unable to correlate multi-functional index (MFI) scores with VO2 max scores (or the peak VO2 levels used in this study.) Davenport’s 2011 study, however, concluded that some of the SF-36 subscales could predict recovery (or not) from exercise. Plus, the p-value in this study (p <.07) for the MFI/peak V02 correlation was not far off from being significant (p <.05) and the authors possibly drawing a different conclusion. Measuring MFI after the exercise, when many of the ME/CFS patients were probably still suffering and the controls were fine, might have gotten better results.
It seems to make sense that a demonstrated inability to exercise would be associated with fatigue. So why is a reduced VO2 peak not correlated with some patient self-assessments of their fatigue levels?
There could be several reasons. It’s possible that ceiling effects in those assessment tools (patients score near the top for fatigue whether they exercise or not) and/or that the tools have difficulty measuring PEM could play a role. Plus, only relatively healthier ME/CFS patients are up for an exercise study.
A look at the VO2 peak graph indicates that some of the upper functioning ME/CFS patients had higher VO2 peaks than the lower sedentary controls. One ME/CFS patient – a distinct outlier – had a VO2 peak of 40 – double that of the rest of the ME/CFS patients – and far above that of the sedentary controls. High VO2 max scores are rare in ME/CFS but can occur in the young and/or particularly athletic (prior to getting ME/CFS). It was surprising to see such a distinct outlier included in the results.
Another ME/CFS patient had a higher VO2 max than all but three of the healthy controls. Some healthy controls on the other had very low VO2 maxes. Such variability underscores why two-day tests, which compare changes in individual results over both days rather than raw group scores on one day, are so much more valuable.
Snell’s 2013 exercise study found that significant differences in functional impairment and fatigue in ME/CFS showed up only after the second exercise test:
It might be concluded that a single exercise test is insufficient to reliably demonstrate functional impairment in people with CFS. A second test might be necessary to document the atypical recovery response and protracted fatigue possibly unique to CFS, which can severely limit productivity in the home and workplace.
Keller’s 2014 exercise study concluded the same:
… if based on only one CPET, functional impairment classification will be mis-identified in many ME/CFS participants.
Unfortunately, the authors of the Stanford paper did not make clear the possible confounding issues. Thankfully, they did note that two-day CPETS do have their place in ME/CFS research. The authors also stated that the next step was replicating the study with a two-day exercise study.
However, in 2007 a seminal study by Snell et al. demonstrated the value in using a two-day CPET protocol, through diminished CPET performed a day after the first.
That was helpful, but it was strange to see the authors state some ME/CFS patients have post-exertional malaise and some don’t given the central role it’s now acknowledged to play in ME/CFS.
Contemporary studies have confirmed these findings and suggested the use of a two-day CPET challenge protocol when assessing patients with ME/CFS in particular those with post exercise malaise.
That little boo-boo – that PEM is not found in everybody with ME/CFS – was compounded a bit by the researchers’ statement that all the patients completed the exercise protocol with “no adverse effects”.
All patients successfully completed a symptom limited one–day exercise protocol with no adverse events.
The authors probably meant that no serious adverse events (patient collapsed) occurred during the exercise test but didn’t make that clear and the statement could be read differently.
No Small Hearts or Stiff Arteries Found
Several studies, some of which were larger than this one, have found small hearts in ME/CFS. (One of those studies found smaller hearts only in ME/CFS patients with orthostatic intolerance.) The small hearts are presumably caused by reduced blood flow to the heart (preload) because of blood vessel problems in the pelvis and lower body. Other studies have found increased arterial stiffness. Neither, however, were found in this one.
Cytokines
The direct relationship between cytokines however, differed between case and controls networks supportive of a distinct cytokine inflammatory signature in ME/CFS. The authors
Cytokine testing did, however, reveal significant differences in immune activation 18 hours after the exercise test in both patient and control groups. Ten cytokines were altered in both groups but the seven cytokines that changed only in the controls (IL-2, IL-12p40, IL-17F, LIF, TNF-α and GM-CSF) and the five cytokines (CXCL10, IL-8, CCL4, TNF-β and ICAM-1) that changed only in the ME/CFS group indicated a very different immune response between patients and controls.
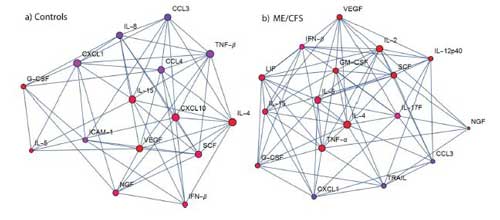
Exercise provoked different immune networks in the two groups. Note VEGF at the top in the ME/CFS group at the right.
Pro-inflammatory cytokine production was still up in both groups 18 hours after exercise but in different ways. TNF-a, – a pro-inflammatory cytokine that is commonly increased in healthy people after exercise, was increased in the healthy controls but not, surprisingly, in the ME/CFS patients.
A network analysis revealed two dissimilar immune networks. IL-4 was a key player in both networks but after that the similarities ended. While IL-5, TNF-a and IL-2 played major roles in the healthy controls immune response, CXCL10, vascular endothelial growth factor (VEGF) and IL-15 played major roles in the immune response of the ME/CFS patients.
VEGF was not mentioned in the discussion section but it’s popped up in several ME/CFS research studies and plays a major role in Dr. Shoemaker’s conception of mold illness.
Discriminating Factors?
The authors stated that “Compared to resting cytokine profiles, our study highlights that post-exercise profiling could have greater value in discriminating case status than resting parameters.” Another statement in the paper, however, suggested that post-exercise cytokine levels were not particularly helpful in discriminating ME/CFS patients from healthy controls.
In the results section, the authors’ statement that, “Cytokines following exercise had nominally better discrimination (greater kappa value) than resting parameters and absolute dynamic change”, suggested that exercise may not have enhanced the ability of cytokines to separate healthy controls from ME/CFS patients much.
Two immune factors, CD40L and CXCL10, best distinguished the healthy controls from the ME/CFS patients. I couldn’t find similar evidence of CXCL10 in past ME/CFS research but this is the second time it has shown up in Montoya’s studies.
Decreased CD40L levels were also found in Light’s 2010 exercise study, but increased, not decreased levels were found in ME/CFS patients’ mast cells in Gradisnuk’s 2017 study. The soluble CD40L receptor was also increased in ME/CFS patients three years after a Giardia infection.
CD40L is primarily found on activated T-cells. (Defective CD40L receptors have been associated with something called hyper IGM syndrome which is characterized by defective CD40 signaling. People with hyper IGM syndrome “have decreased concentrations of serum IgG and IgA and normal or elevated IgM, leading to increased susceptibility to infections.”)
CXCL1 could end up being an important chemokine; its levels decreased with exercise in this study in both the ME/CFS patients and the healthy controls, and the study authors reported that it demonstrated “high relative centrality with the network participants with ME/CFS”. Increased CXCL1 levels were associated with more severe ME/CFS patients in Montoya’s recent cytokine study.
Another chemokine, CXCL10, (chemokines have been up in ME/CFS recently) which plays a role in autoimmune diseases, diabetes and inflammatory bowel disease, figured prominently in the ME/CFS immune network. CXCL10 also clears the way for the entry of natural killer cells and T lymphocytes into the brain. Increased levels of CXCL10 have been found in the spinal fluid of ME/CFS patients. CXCL10 was also increased in the more severely ill in Montoya’s recent cytokine study.
Researchers often produce convoluted, hard-to-understand sentences and are hardly expected to be masters of the English language, but grammatical errors abounded in this paper.
- “This is consistent with previous findings indicating that a failure to reduced levels of CD40L post exercise is associated with increased symptom flare post a bout of moderate exercise.”
- “Unlikely CD40L, in univariate analysis separately by cytokine, CXCL1 decreased with exercise in controls and cases.”
- “Further supportive of an immune mediate pathway in ME/CFS, we found CXCL10 played a central role in the cytokine network and contributed to case discrimination when combine with delta change in IL-4, G-CSF, IL-1β, IL-7 and CD40L.”
Conclusion
Despite some of the issues it was good to see a relatively large – for this field anyway – study examining the effects of exercise on immune parameters. The study demonstrated that exercise provoked markedly different immune responses in ME/CFS patients and healthy controls and did highlight some interesting immune factors. It did not find evidence of vascular stiffness or small hearts.
It’s not clear what to do with yet another short-term cytokine study, though. It does suggest the immune system is involved but we’re continuing to see a lot of variability in cytokine studies. Since cytokines don’t necessarily point to cause because they can be provoked in so many ways, their main value is in potential treatments. Cytokine-busting drugs are now plentiful and will only get more plentiful in the future but we need clear, consistent results stating that X or Y cytokine plays a major role in ME/CFS. TGF-B is the only cytokine thus far to be somewhat consistently abnormal in ME/CFS.
More intensive studies do seem to be getting better results. Montoya’s recent large cytokine study showed a strong correlation between severity and increasing cytokine levels. Younger’s “good day/bad day” study got excellent results and was refunded. With regards to exercise, this study’s results will probably be quickly superseded by more intensive efforts by Nancy Klimas and David Systrom.
Dr. Klimas has created a very large database of cytokine and other results taken from testing done at 8 time points, as I remember, before, during and after exercise. She has enough data to use those results to devise treatment options that she hopes will knock out post-exertional malaise. Dr. Systrom, funded through a donation made to the Solve ME/CFS Initiative (SMCI), is using invasive (catheter) testing to measure many different factors at different places in the body as ME/CFS patients exercise.
Hopefully these studies will provide a clearer path.







I just recently read an interesting blog online on ‘Patient’ it was titled ‘Silent Migraine’ one Comment on there a Guy 5 years out from CFS was diagnosed with (SM) he claims to have fully recovered, also I read somewhere else (SM) can come with a chocolate Allergy found in Allergist Office skin testing…I have heard all Coffee Caffeine Nuts Cheese Chocolate & other food are to be eliminated & some respond to Migraine medicines. Also avoid household sprays colognes etc. I know I trip off from Cologne sprays every time & some can have headaches some do not with (SM)
I wonder if the post-exercise cytokine signature can help distinguish which subgroups PWME are in.
Also, I wonder about the ‘Cytokine busting drugs’, what are they? Do they need to be tailored based on our cytokine signature? Is prednisone a cytokine buster? Some of us (myself included) find it impossible to cause PEM while we are on it.
Cytokine blockers include etanercept (TNF-a), infliximab (TNF-a), Adalimumab (TNF-a), certolizumab (TNF-a) and golimumab (TNF-a). Anakinra (Il-1), Rilonacept (Il-1), tocilizumab (Il-6), Siltuximab (Il-6) and some others.
Using them would require, I assume, finding a subset of patients with high levels or if Dr. Klimas or somebody else can show that they are having an impact during exercise.
Exercise subsets in ME/CFS definitely exist – and identifying them will be important as well – and will take much larger studies.
Basically I think we need larger, more comprehensive studies.
“five cytokines (CXCL10, IL-8, CCL4, TNF-β and ICAM-1) that changed only in the ME/CFS group indicated a very different immune response between patients and controls.”
-> I did give it a try and Googled those five. Not all cytokines are made everywhere. Looking into what can produce these may tell more about the main origin. A selective reading gave these data:
A) https://en.wikipedia.org/wiki/CXCL10
These cell types include monocytes, endothelial cells and fibroblasts.
B) https://en.wikipedia.org/wiki/CCL4
CCl4 is produced by: monocytes, B Cells, T Cells, Fibroblasts, endothelial cells, and epithelial cells.
C) https://en.wikipedia.org/wiki/Interleukin_8
produced by macrophages and other cell types such as epithelial cells, airway smooth muscle cells[3] and endothelial cells.
D) https://en.wikipedia.org/wiki/Tumor_necrosis_factor_superfamily
TNF is a monocyte-derived cytotoxin that has been implicated in tumor regression, septic shock, and cachexia.
Lymphotoxin-alpha, formerly known as Tumor necrosis factor-beta (TNF-β)
https://en.wikipedia.org/wiki/Lymphotoxin_alpha
is a cytokine produced by lymphocytes
E) https://en.wikipedia.org/wiki/ICAM-1
typically expressed on endothelial cells and cells of the immune system
=> Looking into what can produce these may tell more about the main origin.
A, B, D and E can be produced by some (I believe not always overlapping) immune cells
A, B, C and E can all be produced by endothelial cells
=> So immune cells and endothelial cells pop up a lot. Neither immune cells nor endothelial cells alone can explain 1 common origin. The immune cell origin seems to need simultaneously different types of immune cells to be involved and still lacks C) according to wikipedia.
=> D) also links to septic shock, linked sometimes to ME and clearly linked to the blood stream. So do endothelial cells (cells on the inside of the blood vessels). IMO the blood-vessel origin could hence be a common origin.
Note: selective reading of mine.
“While IL-5, TNF-a and IL-2 played major roles in the healthy controls immune response, CXCL10, vascular endothelial growth factor (VEGF) and IL-15 played major roles in the immune response of the ME/CFS patients.”
CXCL10: see above;
vascular endothelial growth factor (VEGF): name says it: linked to blood vessels.
“It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate such as in hypoxic conditions… …VEGF’s normal function is to create new blood vessels during … new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels.”
=> relates to hypoxic conditions and amongst others grows new vessels to bypass blocked vessels (uric acid crystals?)
IL-15 is secreted by mononuclear phagocytes (and some other cells) following infection by virus(es).
=> mainly secreted by immune related cells, not or far less by endothelial cells.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC508680/
“mouse-human rheumatoid arthritis model In vivo”
=> clear link to FM; Also link to uric acid (rheumatoid arthritis is a uric acid related disease) that according to my recent ideas can damage capillaries = blood vessels a lot by crystallizing in them.
My conclusion so far:
I (selectively) see growing relations between constricted capillaries during exercise, hypoxia, following anaerobe functioning, rapid depletion of local glucose stocks due to anaerobic functioning combined with poor blood flow failing to deliver very high rates of glucose (glucose per ATP goes up > ten times) during anaerobic functioning, very rapid conversion of protein to strip its glucose as desperate way to deliver enough local glucose (and really low protein levels in ME patients blood), very rapid production of local purines, uric acid and ammonia being byproducts of protein consumptions and being toxic and finally the low solubility of uric acid in combination with far to vast production of it during anaerobic exercise and slow removal due to poor blood flow, resulting in the local formation of uric acid crystals in the capillaries that can and will block the already too constricted blood vessels.
If true, it is a very bad combination that warrants extreme defensive measures, such as switching local cells massively from ATP producing engines to peroxide producing engines. That is an extreme action with massive CDR seen by Naviaux. Extreme as it stops the process of producing much needed energy (ATP) and converts the already far too short oxygen supply into toxic peroxide/ROS. Potential benefit: uric acid is an anti-oxidant, something that is normally a good thing and can neutralize toxic ROS. Here however it seems toxic ROS is likely produced to neutralize uric acid as massively blocking local capillaries is very close to irreversible complete organ failure and quick death.
Weapon number two in removing uric acid crystals once they block these capillaries? Produce a massive immune reaction near these crystals, “precision” bombing these crystals with massive amounts of even more toxic ROS components, hitting nearby endothelial cells as collateral damage in the process. That’s very close to blood-vessel auto immunity. When it’s too bad, the immune system can also target the endothelial cells close to these uric acid crystals, turning the inner blood vessels into a softer yellow paste (like in zits) so that the crystals can easier flow away hopefully no longer blocking the capillaries. That would be full blood-vessel auto-immunity. And with it, plenty of nasty pathogens join the party leaving the immune system both occupied and exhausted.
Could that be? Would it make sense and link so much of our disease together?
Well done again! 🙂 I love this hypothesis. We need to get it published in our “new hypotheses series”…Who knows? It might turn out to be the right one?
Hypothesis it still is. As with so many ideas the proof is in eating the pudding.
This is completely impossible for any person on itself to do even if it would be a partly valid hypothesis, I’d be more then happy to settle with being able to reach a firm and clear enough “model based” health improvement I aim for now.
Even if it’d worked out, it would not prove anything but add value to it. At the very least it could provide a “personal witness story” that can be published.
It would be great if this hypothesis was close enough to at least be able to improve the “pacing protocol” a lot. That’s my personal holy grail now: combining existing info from other people who succeed in very varying ways to improve their health and recent scientific discoveries in a way that we could better understand why they might work, under what circumstances and what could improve the results.
So far, a helpful strategy for one patient means very few for another patient. If only we could improve this…
As said/mailed before I am very reluctant to convert it into a “new hypotheses blog”. It would “fix” many of my ideas. That’s too soon. I change ideas still very often.
I’ve seen in mainly harder-to-verify sciences many times scientist defending obsolete ideas until the very end because admitting error equals total failure and risks discrediting all the other work that that person has done. It’s not hard to think of a few ME “scientists” who work in that way.
We do not need more scientists who stick to all of their original published ideas till the very end: too much of these ideas WILL be wrong. We need scientist who are willing to drop wrong ideas on the spot after thinking it trough! Time and again!
More importantly, I am a scientist but have no medical education other than from doctor Google. Many doctors cringe at doctor Google, even when consulted for a mere cold. If any non-doctor WOULD get a key mechanism wright of a disease many doctors today can’t even believe it can exist, it would be totally unacceptable to far too many in the medical profession. It would stigmatize any doctor even willing to consider these ideas IMO. That wouldn’t help us patients at all.
In the more likely event it’s utter BS, no need to publish it either.
So if enough people would see value in this idea (that combines reasonably well with some other parts of the disease as discussed earlier), we still would need good ideas on how to solve this “Catch-21”.
dejurgen
I love the idea of a hypothesis series. This is very exciting as we are all so very motivated. Yet, as in my case, we are not all well educated in the field. However, I am learning so much from all of you!
I love this hypothesis too – and again, am thinking can HBOT benefit a hypoxic environment?
I would think….
Would that capillary explanation be the reason switching from using glucose as fuel in a normal diet to fat in a ketogenic diet work so well? I was getting heart pain and the feeling of a heavy heart because since the holidays I have indulged occassionally in “normal” food, you know, good stuff like croissants, a piece of chocolate cake, mashed potatoes. When my Dr wanted to send me to a cardiologist for the works about 10 days ago to investigate the possibility of Kounis/Takotsubo, which is a small vessel problem, I am pretty sure all my tests would most likely be fine so I sucked it up and went back on a Ketogenic diet to fuel my heart muscles differently. . Within 3 days I felt like 50 pounds lifted from my chest and my heart and energy was back to normal! Today I am in bed, exhausted (that’s why I’m here ?) because yesterday, feeling great, I spent the day doing much needed yard work (I’m in Florida). Sigh…one day forward, one day back but it’s better than spending every day on one’s back so for me, the keto diet remains an important part of leading a somewhat normal life. As always, thanks for the enlightened and hopeful info!
Hi Patrice,
Trying to understand why near opposite keto diet and vegan diet seem to be the best available for many patients was part of how I came to the idea that uric acid *may* play an important role in the small vessels and our disease. Another part came from discussions with Izzie and Phil on calcification and what could be in deposits ending up in fascia.
The keto diet is close to fasting, so hormones try to save glucose as much as possible. IMO it makes a lot of sense to let hormones turn down anaerobic functioning as much as possible in a fasting state as anaerobic metabolism is very inefficient and eats through glucose reserves. When anaerobic functioning is very much restricted, this hypothetical very fast conversion of protein in order to strip glucose is blocked too. So if this idea would be wright, keto diet might work by preventing extremely fast rates of local protein consumption avoiding too big problems with local uric acid.
The vegan diet on the other hand is an alkali diet. Uric acid solves poorly in water/blood. When the max is reached, it starts to deposit leading to problems. Many uric acid salts like calcium-uric-acid and magnesium-uric-acid salts solve a lot better in blood. So they can buffer a lot more uric acid per milliliter blood before uric acid crystals are formed. Unfortunately uric acid is among the weakest acids available. Chemistry prefers formation of salts from stronger acids so formation of calcium or magnesium uric acid salts is limited. Having a more alkali environment should solve most of that hurdle however. So a diet that makes the blood slightly alkali could reduce this hypothetical uric acid problem as well.
At least that is the idea ;-). Don’t know if biochemistry will follow suit ;-).
Dejurgen, your uric acid hypothesis sounds a bit like (body wide) ‘gout’ malfunction. If there is a link, perhaps CFS/ME patients might try gout treatments such as Probenecid, Pegloticase, Allopurinol or Febuxostat, and see if there is any improvement in exercise recovery.
Just a thought…
It might be interesting to see how many of us have gout symptoms.
I was just thinking the same thing.. sounds like a whole lotta gout. I am not able to talk intelligently anymore, and have forgotten many things I have learned over time, so I can’t remember the specifics of gout. Uric Acid being referred to so often in this conversation rang the “gout” bell in my head. I know it involves swelling, but I can’t remember enough about it for contemplation of a very constant symptom I experience, so I’m throwing it out to you guys.
I have a symptom that I’ve never heard anybody else discuss At length or in passing to be honest. so I don’t know if it is part of ME/CFS, or simply a condition that I’ve developed that sits apart. every time I do what I call too many reps of flexing the same joint, say for instance the knee by going up the porch steps, the tissue around that joint swells up becomes extremely painful and I usually develop tendonitis around that joint where the tendons are connecting. I experience this in my hands, knees, elbows ankles, feet and wrists. I also have been diagnosed with Hyper lymph edema. Physical movement will cause the lymph gland nearest The Joint used to become swollen and inflamed. It was explained to me that my lymph nodes can’t circulate The increased blood flow as fast as they need to when I start to use flexion in my joints and muscles become involved as well as connective tissue. I have this symptom as dependably as I have p e m. blame speech-to-text for it messing up things in this reply. I can’t use my fingers for even swiping on a touch screen keyboard. they become extremely painful and swell very rapidly. Do a lot of patients have swelling with extreme pain and inflammation that last for days until it is healed after physical activity? I have to wear braces and things that stabilize the joints if I’m going to be using them. otherwise they are going to swell up humongously. Constriction socks for diabetes has helped with my ankles and my feet. I no longer developed cankles that drag the floor when I walk to the kitchen for a glass of water, unless I’m having the nerve to be in a nightgown and barefoot. wearing socks to bed is just too much. Part of what keeps me so bed bound is the physical injury to my body from moving around. the swelling is so severe it cuts off circulation. I had circulation cut off to my hand from injuring my wrist when filling the coffee carafe with water because I’d forgotten to set it down in the sink to fill. instead I instinctively held it in my hand by the handle. my wrist gave out before it was even full, I was not wearing my wrist brace, and I heard a loud snap and my hand bent at a weird angle on my wrist and I hollered like holy you-know-what because it really hurt. I never went to the doctor because I figured it was just tendonitis again and I iced it kept it elevated and then braced it. it wasn’t sufficient to keep the swelling down and my hand was totally dead feeling for 48 hours before I started feeling that those little prickles before I started feeling those little painful prickles that herald the return of circulation. I never told my doctor. 4 years later and still not having recovered my full range of motion in that wrist and I’m wondering if perhaps I fractured something and it healed wrong? I don’t know. the point is I am so used to injury as a common Day event if I do things that I don’t recognize an injury that requires a doctor’s assistance. My pain levels from everyday movement involved in things like putting on makeup, cooking, walking, washing my hair or even rubbing lotion on my skin ( it hurts my hands and my wrist to press hard enough to rub lotion in.) but the amount of activity it takes two cause this swelling I’m talking about is also the amount of activity that causes post exertional malaise for me. I also reach the point of exhaustion and profuse sweating and Flushing.
I also feel severe pain in my connective tissue and muscles that is chronic. it’s accompanied by muscle spasms your usual ticks and twitches involuntary limb movements. I need to be in a reclined position with my legs raised and my knees slightly bent in order to prevent muscle spasming and cramping and torso pain caused by muscles weakening if I’m up right. I was told it was structural fatigue. I just know that everything starts cramping up from hip to shoulder front to back. and again there’s the p e m. I think that my battery lifespan is at 5% and it became that way from my doing too much on the days I felt better. I became more limited all of a sudden when an activity I had already been able to do while ill suddenly became too much to do with no forewarning. so be careful about doing things when you feel better. I can’t emphasize that enough.
Does anybody out there experience this connective tissue and lymph node swelling with inflammation? is that gout? I should say is that possibly gout? well I’m totally done here because I’m having trouble talking. But seriously is this something that could maybe be treated by my doctor or is it part of me CFS based on everybody’s experience in here? this disease is so hard to just figure out and manage. I love the conversations you guys have. sometimes the words are too big and I fall asleep trying to read. but then I wake up and I just start over. sometimes it takes many many tries but I’m a bit stubborn and it’s to my detriment in this situation. this feels more like I wrote a letter and I need to sign it now. Or more accurately a novel and it’s time to write the epilogue. My apologies and thanks that nobody’s yelled at me for how long it takes me to say things. it’s a kindness that I’m not use to.
Hi Nancy,
I agree it does sound a bit like body-wide gout malfunctioning. I took a likewise angle on it as you did. Yet it is not exactly body-wide gout.
Body-wide gout is lethal IMO: just consider the effect you have when a toe nail grows in your skin: purple and swollen a lot. Now imagine that happening to the entire body. In my imagination it does make look sepsis even mild. But sepsis is not, it’s very dangerous short time and impossible to support long time. So if it is like both body-wide gout and mild sepsis as some researchers believe it has to have an almost equally big anti-inflammatory component as a very big inflammatory component.
There’s more then one indication that that may be happening and it could create part of the strong variation in symptoms and reaction to treatments: too big inflammatory reaction and too big anti-inflammatory are close to cancel each other out. Small variations like day-to-night may be sufficient to upset the delicate balance, let alone “fixed” medications not taking into account this balance.
Another difference is that while I see it body-wide, I see it vary *a lot* both from place to place (organ to organ) and time instance to time instance. Exercise upsets this dynamics a lot IMO, just like day and night changes. So again I see simultaneously dangerously high uric acid concentrations at one place and low ones at another. Problems could happen at lower uric-acid blood levels than normally considered problematic as variation is very high in this idea.
I dislike the idea to “force” uric acid low. If meds work on the precursor, purines, it can become nasty. Purines are a key component in life itself. They are a very important messenger and more. Don’t want to reduce it “fixed” yet. Getting uric acid levels very low seems safer. But life is complex, uric acid may play other beneficial roles and require it to be sufficient. Also, low local uric acid levels may drive conversion of purines to uric acid and deplete purines. Who knows?
I’m trying out some ideas. I prefer not to block one single component in a “run-away system” but to gently reduce all components. Blocking one component can have good effects but also very nasty side effects. A bit like breaking a bike by putting an iron bar in the wheels IMO.
Thanks for jumping in on the discussion and the good ideas! I’ll look into those meds for sure to see if I can learn something more about mechanisms.
Yes, to tinker with one system ultimately tinkers with all–but by throwing out that idea I didn’t necessarily mean to entirely block all uric acid–just to adjust levels. But who knows if that theory is even correct?
Cort’s article was reviewing exercise intolerance. For myself, I find intense mental days, not necessarily involving exercise stress, will still cause a significant functional decline for a day or two afterwards. How do researchers explain that? I’m sure I’m not the only one…
“But who knows if that theory is even correct?”
Any theory about ME is having a small chance at being correct, just because the problem at hand is so difficult. Doctors have a clear advantage of good medical education. They have the disadvantage that they can not launch a wild idea without testing it first. Testing even a single idea taxes budgets of entire research groups so that is quite a hurdle.
Patients have a clear “advantage” of knowing how the disease feels like and can better judge if a theory fits their experience. Plus they can better risk being totally wrong.
Some advances with ME/FM came from doctors, others from patients (pacing, using keto diets for some cases…). It would be good to see a better cooperation between both groups like it happens with most other diseases.
“I didn’t necessarily mean to entirely block all uric acid–just to adjust levels.”
Didn’t mean to say you suggested this. I just have a few really bad experiences with meds firing back big way so it kinda triggers me ;-).
Note: when I wrote “breaking a bike” I meant stopping it, thus braking.
“I find intense mental days, not necessarily involving exercise stress, will still cause a significant functional decline for a day or two afterwards.”
I have that too. Once I was only able to crawl rather than walk directly after peeling three apples. That’s not that hard physically but really taxes my brain (eye-hand coordination). It feels often like “it” (the problem) sinks to my legs. My best guesses are:
* too much mental activity derails blood flow body wide and legs are prone to derailed blood flow due to difficulties returning used blood to the hart.
* once part of the brain starts an inflammatory reaction, the whole body gets flooded with increased inflammatory stuff. Some part of the inflammatory reaction may cause debris like small blood clots or uric acid crystals covert with other junk. Debris mainly sinks to the bottom and impedes the return of blood to the hart.
I forgot something important! For several years now I keep developing crusting oozing sores on my forearms and lower legs. my skin on my arms is like leather now and I have a profuse amount of rubbery types of cysts growing all over me. I had many cysts that weren’t rubbery that were filled with a Cheesy like substance my doctor said over my entire adult life prior to becoming ill. they all grew to the point of causing pain from their size and my doctor needed to remove them. Now they’re everywhere and I also have some that are blue in color. as if there’s a collection of blood that has formed the cyst. it’s not a cyanotic color, it’s more the blue of a bruise. with this tie in with gout if it was widespread?
I believe Cheney believed uric acid played a role (???)
Sorry Cort, I didn’t see that on these thread comments… (brain fog?)
The mention of uric acid is interesting. I believe I have had CFS and maybe Fibro since 7 yrs old. Only diagnosed with CFS/Fibro 2 yrs ago. I am 68. 25 yrs ago I had a hand operation and the surgeon informed me that I had uric acid crystals in the knuckles. I was told to eat a Gout friendly diet by my GP. However have never had any Gout episodes. Have had negative tests for inflammation and no evidence of arthritis. I do have almost constant severe pain mostly in legs plus many other symptoms. I had 2 HTMA last year and one of the recommendations was to eat more purines? I haven’t. I don’t understand any of this but thought I would mention it?
When I was in my early 50s I hopped to the Doctor’s on borrowed crutches and was amazed to be told that gout was the probable cause. I thought it was a disease of elderly, overweight alcoholic gents and i was a thin sober female. Fortunately it only occured once more. I have had CFS and it’s pain all over for decades. Interesting theory.
Thanks for mentioning this! Part of my idea is that uric acid problems do happen with some patients below “official” blood test values.
“25 yrs ago I had a hand operation and the surgeon informed me that I had uric acid crystals in the knuckles… …Have had negative tests for inflammation and no evidence of arthritis.”
=> Does that mean that you had multiple blood tests spread over the years that include uric acid measurements and never had too high values? If so, I’d be really glad to hear this!
If so it would ad weight to this idea. One is not supposed to find uric acid crystals when blood values are fine. In my theory most crystals are there only for some time and dissolve after the “episode/PEM” so evidence for them potentially playing a role just disappears. But they can remain in the body when it’s really bad.
“I had 2 HTMA last year and one of the recommendations was to eat more purines?”
=> I guess HTMA here is Hair Tissue Mineral Analysis? The recommendation to eat more purines kinda fits my idea too:
* purines are essential components in life so you need sufficient of them
* if concentrations rise far too high (local, during exercise for example) then they get toxic so they need to be broken down as quickly as possible. But doing so may, on average, lead to too few purines and uric acid.
* I compare it to a poor person going to an expensive restaurant the day after he receives his wage. “Burning” so much of it on one day doesn’t mean he’s rich. The more he burns on that one day, the emptier his pockets will be during the rest of the month.
Thanks a lot for mentioning this! This may be the kind of info we need. Please feel free to correct me if I read wrong or add experiences to it!
“to be told that gout was the probable cause. I thought it was a disease of elderly, overweight alcoholic gents”
=> “real” gout may be, but this idea of gout-like problems may be different. In fact, uric acid problems/crystal formation in my idea is “pretty good” at displacing calcium. Osteoporosis, or de-calcification of bones is a primarily female problem (as are ME/FM). If the two would indeed be linked…
De-calcification of bones could even “mask” gout as calcium-uric-acid salts solve far better in liquid then pure uric acid. So it’s easier to transport the uric acid over larger parts of the body. So it doesn’t end up staying near permanent on a single location like the toes like in “real” gout. Downside would be that inflammatory problems would spread easier to body-wide problems if the system gets overwhelmed.
Part of the link is that inflammatory injury in the region of tendons often leads to severe calcification of these tendons.
Yes, you are correct! I was a patient of Dr. Cheney and he told me he sees very low uric acid in all ME CFS patients. He wondered out loud if increasing uric acid would make a difference.
Thank you very much for your comment Elisabeth! That’s prime quality info as Dr. Cheney has access to first grade measurements of ME patients blood.
In this blog and more clear in previous blog I mentioned the idea that this supposed “time and location specific” overload of purines and uric acid could go well hand-in-hand with low-ish uric acid blood values.
If uric acid production over 24 hours would not be a lot higher in ME patients compared to healthy persons then blood values should be lower as (at least I suppose and researchers like Naviaux seem to have measured it) our bodies produce massive amounts of ROS in a massive CDR. If ROS really is very elevated then it should “decimate” uric acid values as uric acid is an anti-oxidant that changes chemical structure when making contact with ROS.
Searching further I did found this. http://www.biomed.cas.cz/physiolres/pdf/66/66_1001.pdf
“On the other hand, uric acid has a potent capacity to scavenge
reactive oxygen species (ROS) and this ability is closely comparable to that of vitamin C (Ames et al. 1981). In addition, uric acid concentration in plasma is approximately 15-450 μM, which is 10
times higher than that of ascorbic acid (Ames et al. 1981), showing that uric acid is a potent radical scavenger in plasma.”
and
“Patients with rheumatism (Grootveld and Halliwell 1987)
, Down’s syndrome (Zitnanova et al. 2004), and Parkinson’s disease
(Schwarzschild et al. 2008) have a significantly lower concentration of serum or plasma uric acid than healthy individuals. This is because uric acid is degraded by ROS, whose levels increase in these conditions.”
and
“When uric acid reacts with ROS, uric acid is
non-enzymatically converted to allantoin (Plank et al. 2011). Patients with rheumatism (Grootveld and Halliwell 1987) and Down’s syndrome (Zitnanova et al. 2004) have significantly increased concentrations of serum or plasma allantoin.”
My note: Parkinson disease is one of the “applications” of a keto diet.
Remark also that rheumatism, which many people would link with increased uric acid, shows significantly lower concentration of serum or plasma uric acid than healthy individuals.
I however do not feel we are short on anti-oxidant or uric acid. Or to be more accurate: we are short of it in most parts of the body to fight ROS, but increasing it would (according to my idea) make the defense against “local uric acid attacks at bad times” broken, and getting things a lot worse.
So decreasing uric acid a lot with strong meds may not be the best option as it depletes anti-oxidant in most parts of the body. Not decreasing it however would maintain the poor local blood flow. Another case where we’re stuck between two bad things?
If I’m wright, it may explain why few patients have stellar results with anti-oxidants even with our high amounts of ROS. To me, it’s useless. At least it did not turn my health worse.
Maybe another point for the vegan diet? Lower in purines (so giving lower uric acid) but higher in vitamine C yielding roughly equal anti-oxidant capacity but less of the nasty uric acid that can form crystals in capillaries and block them?
I just read that decreased uric acid on a blood chemistry panel/test is an indication of molybdenum deficiency – a cause of mcs and other sensitivities.
Good find. Never heard of that one before. So another thing to consider.
So we’ve got three options IMO:
* Too few uric acid, what could be the most straightforward conclusion.
* Too much uric acid when one believes uric acid is problematic like in many inflammatory and immune diseases.
* Too few for some processes and too much for other process as I tend to believe wright now. I must say it’s a bit a gut feeling so no science, but my gut feeling served me relatively well in the past.
Is molybdenum part of the standard Hair Tissue Mineral Tests? If so, many patients on this forum have done such test. Would be interested to hear some results on molybdenum being short, normal or too much.
For now I’ll stick to my current diet relatively rich on food containing molybdenum and not too high on purines, supplemented with daily vitamin C. If lower uric acid would have downsides and uric acid would act a lot like vitamin C, then replacing some of the uric acid by vitamin C may keep most of the advantages while reducing its potential downside I hope.
I tried to get wiser on protein metabolism, but it’s to difficult for me now. But I looked back into https://www.healthrising.org/blog/2016/11/10/metabolomics-chronic-fatigue-syndrome-starvation-australia/ and found:
“Urine
…and increased allantoin and creatinine.”
I didn’t get any wiser on creatinine, but allantoin is a breakdown product of uric acid https://en.wikipedia.org/wiki/Allantoin
“Allantoin is a major metabolic intermediate in most organisms including animals, plants and bacteria. It is produced from uric acid, which is a degradation product of purine nucleobases, by urate oxidase (or uricase).”
So increased allantoin found/?dumped? in urine by this study combined with decreased uric acid found by Dr Cheney may well indicate that our bodies try to reduce uric acid content. That is when allantoin production isn’t out of control at least.
I don’t think it was appropriate to draw attention to the grammatical errors in this paper and blame them on the authors. Dr Montoya’s first language is not English. Sure, he should have asked someone to check the manuscript for these kinds of errors, but singling them out for criticism doesn’t contribute to your analysis of the paper.
I reread the paper again and I stand by my comments. I have never seen so many errors – or close to it actually – in a paper. It was because they became so glaring for me that I noted them.
Dr. Montoya was not the issue. I’ve never found this problem with his papers. He is the senior author of the study and helped conceive the study but the end of the paper indicates that while he did review the paper he didn’t write any of it.
It’s not just the authors either. That journal published a paper with a bunch of grammatical errors-something that their editor missed. This paper, by the way, shot through the review process very quickly for some reason.
With a growing focus on cytokines in CFS/ME, surely it is time to look deeper at HBOT (hyperbaric oxygen therapy) as a potential treatment? HBOT known to have an effect on moderating cytokines, let alone a considerable effect on viruses/pathogens? Considering the lack of side effects compared to drugs, I would love to see more opinion on this and my belief in HBOT (as in hard chamber not mild) is certainly increasing. As always, thank you for this amazing blog Cort.
Not convinced that cytokines are central to the cause of CFS. I think they are secondary aspects.
What is the point on the grammar comment? Throwaway comment or suggestive that we shoukd be wary of results from an institution that lacks quity control?
Really appreciate your work, but not sure on this one ?
I often come across difficult to understand, convoluted language in scientific papers but I almost never see these kinds of grammatical errors and there were quite a few in this paper.
Believe me I know how hard it is to produce a grammatically correct article. I need editors to do it and even they miss things sometimes. The editing process clearly broke down.
I don’t know about being wary of the results….I think the results are clear. The grammar is a small issue relative to the results but communication is important and everything in the end reflects; how the study was done, what it was done on, and that includes how it was written up…It’s all of a piece.
You want the whole thing to work and if you lose out on that last one then you lose something…
One word wrongly used or misplaced can change the entire meaning of a sentence.? I was a (hobby) short story writer can hardly get a sentence straight these days or concentrate on anything.
Hi Déj, sorry, but have not much to add (no tests) except that on observation of X-rays a GP told me 2 yrs ago, that my bones are as strong as a hardworking 45 yr old male. In the past I have done a lot of weight bearing work until I got too ill. Much to my detriment I find, as now basically have to pace myself and only am about 30 % active/slow movement around the house, with short bursts of cooking etc am quite ill these days if only I’d known,but acceptance is the only way forward now and hope that these wonderful researchers may find a solution.
Looks like allendronic acid is a bisphosphonate like Fosamax, which seemed to increase my ME/CFS symptoms. Glad you”ve been able to tolerate it and seen improvement! write custom essays
VO2, which is essentially cal/min, is the energy spent at time t, not the stress at time t. (1 cal/min will do more damage when you are already stressed, than it would when you are rested). So, it’s no surprise that VO2 Max did not predict the post-exercise fatigue for CFS patients, though it still can predict in certain circumstances (like in healthy people).
q is a better measure of the stress and I found max(q) correlates to fatigue better than max(cal/min) or sum(cal/min), especially in early stages of CFS. q is defined as each cal/min exponentially reduced over time and summed up at time t. It’s my proxy for the stress effect of all exercise calories at t. In R language, it is written as:
q = Reduce(function(a,b)a*(1-k)+ifelse(b-C<0,0,b-C),as.vector(calories),0,accumulate=T)
There is also multi-day accumulation of the stress because the effect of exercise lasts several days. That could be why one-day sub-maximal CPET protocol did not induce PEM, while two-day protocol does. In my research, I found that 4 day sum of max(q) correlates to fatigue the best. (I'm still fine-tuning it to figure out the best accumulation weights within the 4-day window).
As for the post-exercise cytokine profile as a CFS biomarker, it seems to me that someone would've found it already if it was that easy. I have my doubt, so I'll have to wait for someone to replicate it and confirm.
Hi DeJ yes my HTMA of molybdenum is well into the low section on the graph,sulphur is high in the accepted reference range. I do eat a lot of legumes and other food high in molybdenum so is a bit puzzling doc hasn’t commented I will ask next time.
Hi Peach, thx for the info. As I read that molybdenum deficiency was very rare I hoped a diet rich in it would suffice. Maybe not so…
If I recall well I read that molybdenum plays a role in both good energy production as well as detoxing the liver. If so it could be a sign of the toxic state our bodies are in. Plenty of oxidative stress needs plenty of detoxing too. That could consume molybdenum and would align with our general low/depleted glutathione levels.
If it were oxidative stress, selenium and zinc could be low to. These are often regarded as important in anti-oxidant function too. Selenium intake however is greatly influenced by diet: people who eat many nuts would have a far larger intake of it then those who don’t. I ever read that there is one particular type of big nut that reached maximum recommended intake by only eating two of them daily.
Dej… Brazil Nuts are high in selenium. My doc increased my zinc and selenium supplements. In my area of coastal New South Wales Aus. acid soils are low in selenium (need to add lime to soil) hence food can be low in selenium. Just an interesting point.
More from an old article on cytokines—reads long like a book but some gems imo
(from my skimming only, too much to absorb, but still:
“Lactate levels were reported to be increased by over 380% compared to levels in healthy volunteers”
“the choline / creatine ratio was significantly elevated in patients compared to controls. “ (using MRS)
“afferent sensory fibres of the vagus nerve are able to detect minimally elevated levels of cytokines and relay the information indicating the presence of peripheral immune activation to the brain via ascending fibres [147,148].”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3964747/#!po=0.170068
And more on the same:
https://www.google.ca/amp/s/www.researchgate.net/publication/280641339_R_E_V_I_E_W_Leaky_gut_in_chronic_fatigue_syndrome_A_review/amp