

An Exploratory Drug Trial For ME/CFS
In the next month or so, Cortene, a small drug development company, will do a very unusual thing – trial a new drug in chronic fatigue syndrome (ME/CFS). The first two parts of the series on this new drug covered how ME/CFS came to the attention of Cortene, why Cortene believes ME/CFS is a possible match for its drug, and introduced Cortene’s novel hypothesis regarding ME/CFS.
- Part I – The Cortene Way: New Drug to Be Trialed in Chronic Fatigue Syndrome (ME/CFS) Soon – Pt. I
- Part II – Cortene II: A New Drug & A New Hypothesis For Chronic Fatigue Syndrome (ME/CFS)
Treatment Approach
The novel hypothesis proposes that a receptor called CRF2, which triggers neurons to release serotonin, has become unusually prevalent in parts of ME/CFS patients’ brains. Cortene believes that the elevated release of serotonin – in response to even small levels of stress – in turn causes ME/CFS.
In part three, we look at why Cortene thinks its CT38 drug may be able to reduce CRF2 levels, the possible side-effects of the drug, what past studies have told us about the drug, where Cortene is in the process of getting FDA approval for the trial, and the basic makeup of the trial.
A little recap…
- Stress releases CRF in the raphe nuclei, which modulates serotonin in the limbic system.
- Normally, low stress (low levels of CRF) acts on CRF1 receptors to inhibit serotonin; high stress (high CRF) acts on CRF2 receptors to increase serotonin.
- In ME/CFS, Cortene postulates that CRF2 receptors have become stuck on the surfaces of the serotonin-releasing neurons. Now low stress increases serotonin instead of inhibiting it.
- So… serotonin, released in this manner and in the limbic system is the bad actor, but only because CRF2 is stuck.
Two Approaches to De-activating CRF2
The Shotgun Approach: Blocking CRF2
If the disease is in fact driven by the presence of CRF2 on neuronal surfaces, the typical approach would be to block it with a CRF2 blocker or antagonist, similar to Humira® blocking the cytokine TNFα (which decreases inflammation, but lowers immune system defense). This might work, but the drug would have to be taken chronically, and it would block CRF2 receptors all over the body – and likely cause major side-effects – not unlike a shotgun approach to a targeted problem.
The Fine-Tuned Approach – Internalizing CRF2
Instead, and this is the really fascinating aspect of their approach, Cortene is attempting to mimic what the body does naturally to end the stress response; i.e, get the CRF2 receptors off the neuronal surfaces and buried back inside the neurons where they belong.
This kind of receptor internalization – a natural process called endocytosis – is normally triggered when the stress ends, by UCN1, an agonist selective for CRF1 and CRF2. However, Cortene believes that this process failed during the event (or events) that triggered ME/CFS (and some similar diseases). The resulting presence of CRF2 on the surfaces of the neurons means that subsequent stress, even at low levels, activates CRF2, causing rampant serotonin release in the brains of ME/CFS patients.
If Cortene’s approach works, it could theoretically restore the system to a pre-disease configuration by preventing the stress response from becoming easily activated. I asked Cortene two questions: (i) Is there any proof of this idea that CRF2 might be stuck; and (ii) if so, how might it be internalized? For that, we must now turn to Cortene’s animal data.
The Drug – CT38
Cortene’s drug, CT38, is a CRF2 agonist, i.e., it stimulates CRF2. It is comprised only of amino acids that occur naturally in the human body. It has been characterized in animal studies and in a Phase 1 clinical trial in healthy human subjects.
Is there any proof that CRF2 is stuck?

Cortene believes that at some point, a breaking point was reached, which destabilized the stress response system in ME/CFS.
There is no animal model for ME/CFS. However, if in fact the disease results from CRF2 being overactive, over-stimulating the CRF2 pathway in healthy animals should cause the symptoms of the disease. Cortene’s animal data shows just that. High doses of CT38, delivered quickly (or by bolus, to give it its technical term), cause symptoms similar to those found in ME/CFS. The animals’ heart rate increases, their blood pressure, body temperature and gut motility are reduced, their urine production is affected, their movement is dramatically reduced, and their stress hormones are released (norepinephrine and cortisone, the rat equivalent of cortisol). These changes are temporary and revert to normal once CT38 wears off.
These findings support the idea that ME/CFS could result from CRF2 being stuck. They also suggest that once the CRF2 pathway becomes overactive, it can produce a raft of involuntary effects that ME/CFS patients experience, including some that have been confused with behavioral or psychological issues such as difficulty with movement.
How might CRF2 be internalized?
Most receptors internalize when subjected to intense stimulation. Cortene’s animal data demonstrates this: while high bolus doses of CT38 cause ME/CFS-like symptoms in healthy animals, very high bolus doses do not — likely because CRF2 has internalized. However, Cortene’s data also shows that instead of using very high bolus doses, which might be unsafe, lower doses maintained over a period of hours (by infusion) appear to internalize CRF2 without causing symptoms. If Cortene’s hypothesis is correct, this could theoretically bring the stress response system back to normal.
Cortene reports that the animal data for CT38 has been submitted to the FDA and complies with the general requirements for testing this acute (short) treatment in humans.
The Prior Human Trial
CT38 has been tested in a Phase 1 clinical trial in humans. This trial exposed healthy subjects to a single bolus dose, which was successively raised in subsequent groups of subjects. The purpose of the trial was to determine the point beyond which side-effects become unacceptable (known as the maximum tolerated dose) and whether these side-effects were similar to those seen in animals. (As often happens with these trials, the results contain proprietary, unpublished data, but Cortene reports that the full 4-volume study report has been submitted to the FDA.)
Encouragingly, at the bolus doses tested, the side-effects in rats and humans were the same and consisted of two easily monitored effects: temporary increases in heart rate and decreases in blood pressure.
Similar Approaches
Cortene is not the first drug company to attempt to internalize receptors on a cell. An FDA approved drug, Leuprolide™, prevents prostate cancer progression by causing a receptor in the reproductive system to internalize. With the receptor gone, the cell can’t be triggered to release the hormones that spur further tumor growth.
Without continuous Leuprolide™ treatment, however, the receptor re-emerges. Cortene doesn’t believe this will happen with CT38 because, unlike the reproductive system, the limbic system (that CT38 affects) is dynamic in nature; i.e. it’s designed to have CRF2 internalize, come up to the surface, internalize, and so on, in response to intermittent stress.
The Upcoming Clinical Trial
In 2017, Cortene pulled together a small but experienced drug development team (Hunter Gillies, Sanjay Chanda, Michael Corbett) that has been involved in prior drug approvals.
Hunter Gillies, who is an MD and an exercise physiologist, was instrumental in helping a team obtain drug approval in a disease that faced some of the same roadblocks in the drug development world that ME/CFS faces. Not much was known about that disease (it was rare), its cause was unclear, and it lacked widely accepted clinical endpoints to test. The team used cardio-pulmonary exercise testing (CPET) to get regulatory approval for that drug.
Sanjay Chanda ran drug development for a company – later acquired by big pharma – which pioneered a new boron-based, as opposed to carbon-based, approach to drugs. Chanda is a toxicologist by training and is highly knowledgeable in the preclinical side of the drug development process (animal studies, chemistry and formulation, etc.). Michael Corbett is experienced at outreach in online communities, which could be helpful in mobilizing the ME/CFS community if the drug works.
Cortene is currently working with different vendors to make CT38 and to produce the sterile formulation for patients. They are also working with several labs to ensure appropriate medicinal chemistry (e.g., confirm CT38 selectivity and potency for CRF2, test material biocompatibility, enable measurements of the levels of CT38 in patients’ blood during treatment, etc.).
Most importantly, they have convinced one of the most respected physicians in the ME/CFS community to conduct the trial. If all goes well, the trial will start in the second quarter of 2018.
The FDA
Cortene is gearing up to seek FDA and institutional review board (IRB) approval for a Phase 1/2 trial. The trial is Phase 1 because Cortene is testing tolerability and dosing; and it is Phase 2 because efficacy and tolerability can only be tested in patients.
This proof-of-concept trial will be filed under what is known as a physician-sponsored investigational new drug (IND) application. FDA/IRB approvals ensure that all the boxes are checked; i.e. that the drug has been through appropriate animal testing and meets drug manufacturing standards, and that the trial design is safe for patients (or as safe as can be in early-stage research).
Such approval typically takes one month – only after FDA/IRB approval can the study commence. Cortene is beginning the approval process and hopes that the trial can start in late April.
The Trial Basics

Suzanne Vernon, the research director for the Bateman Horne Center, is the former research director for the Solve ME/CFS Initiative and a CDC researcher. At the SMCI she conceived the first ME/CFS Biobank and “built” the Research Institute Without Walls (RIWW) program which emphasized collaboration and data sharing and brought many new researchers into the field.
It has been fun to get to know the Cortene team and work with them. They all bring different expertise to the table, they are all crazy smart and (most important) they push the envelope. I am super excited that Dr. Lucinda Bateman is the Principal Investigator and the Bateman Horne Center is sponsoring this trial. The team at the Bateman Horne Center has extensive experience running clinical trials and we are super excited to apply our clinical research expertise to CT38 for ME/CFS. I am personally excited about this trial because as you noted in the first blog of this series the CT38 mechanism of action is in line with my HPA axis research. – Suzanne Vernon
(In 2009, Vernon and Broderick published a modeling paper which suggested the HPA axis could be quickly reset in ME/CFS.)
The trial will test 3 dose-levels, each in a different group of patients. The initial group will receive the maximum tolerated dose determined in the prior human trial (i.e., the dose at which only acceptable side-effects were reported). However, instead of using a bolus dose of the drug, the drug will be slowly infused over time. The dose-level will be increased in subsequent patient groups, but only if there are no clinically meaningful side-effects at the previous dose-level.
Drug Testing
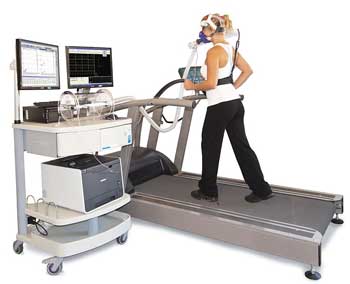
Cortene will use the most rigorous test of their drug imaginable in this disease – a cardio-pulmonary exercise test (CPET) using a stationary bicycle. (Most trials in ME/CFS have employed self-report measures to assess treatment effectiveness.) The exercise test will be done before and after treatment and will measure oxygen consumption, ventilatory efficiency, work done, time to reach anaerobic threshold, and other metrics.
If I understood it correctly, because exercise capacity is affected by altitude, some altitude requirements may be necessary. The trial will include 18 patients from the Salt Lake City/Utah or similar areas. If this proof-of-concept trial is successful, it will probably warrant larger studies in other locations (and altitudes).
Cortene will also use self-report measures (i.e., daily visual analog scores for fatigue, pain, sleep, cognition, flu-like symptoms, headaches/sensitivities, shortness of breath, gastrointestinal symptoms, anxiety and measures of post-exertional malaise or PEM), as well as continuous monitoring via Fitbit™ and a daily online cognitive test, but their inclusion of an exercise stressor raises the bar on the trial significantly.
If Cortene can move the needle on ME/CFS patients’ ability to exercise, and/or reduce the burden of PEM, it will have proved it can crack probably the toughest nut in this disease. I asked Cortene why they chose such a difficult test as an endpoint?
It all comes down to serotonin. As explained in Part 2 of this series, serotonin increases motor neuron excitability, but too much serotonin (emanating from the raphe nuclei at the top of the brain stem) inhibits motor neuron signals (via 5HT1A receptors on the motor neuron, where it emerges from the cord). During CPETs, ME/CFS patients’ muscles show reduced oxygen uptake relative to healthy subjects (Vermeulen 2014, Vermeulen 2010). Most researchers have taken that to be evidence of a hypometabolism but Cortene believes it may result from inhibited motor neuron signals.
In other words, it’s not that the muscles cannot function because they aren’t getting enough oxygen, glucose, etc.; it’s that the muscles aren’t being triggered to function, so they don’t take up oxygen, glucose, etc., which in the longer term may lead to hypometabolism and muscle atrophy. Removing the excess of serotonin in the cord by causing CRF2 to internalize should, theoretically, resolve the cause of this problem.
Questions
Permanent System Reset?
I asked Cortene why they believe the receptors are getting stuck on the surface of the stress response neurons in the first place, and why they wouldn’t return to their maladapted position once the drug has been stopped?
Cortene stated that they believe that at least one of two conditions are necessary for the CRF2 receptors to become stuck. Either the patients have been exposed to a great deal of prior stress, which has eventually caused the receptors to remain at the neuronal surfaces, and/or they have a genetic predisposition to a more intense than usual stress response.
Several ME/CFS studies (see Pt 2 of this series) suggest a genetic predisposition to an unusually strong stress response may be present, which could manifest in two different ways. Higher than normal levels of stress mediators (e.g. CRF2, CRF1, serotonin, the cortisol receptor, ACTH) may be produced in response to stress, and/or the ability to break down stress mediators (such as serotonin, norepinephrine, dopamine) to return to baseline may be inhibited.
If CT38 successfully internalizes the CRF2 receptors, Cortene believes it should eliminate the maladaptive dynamic that has caused the system to become “stuck”. Because the drug will not change the patients’ genetic predispositions, ME/CFS patients could be at risk from another stressor that causes these receptors to become stuck again.
Since most ME/CFS patients were normal prior to the stress episode that caused the maladaptation, however, Cortene believes it was the intensity of that stress that brought about the dysfunction. That would suggest that avoiding similar stresses might allow one’s stress response system to avoid “getting stuck” again. Plus, if the drug works and an intense stress caused another maladaptation, the patient could, of course, be treated again — although additional research with CT38 would be required.
Targeting Neurotransmitters?
Thinking about their hypothesis and its prediction of increased norepinephrine in the limbic system, I asked Cortene about the failure of the ME/CFS Clonidine trial. Clonidine, after all, reduced norepinephrine levels in adolescent ME/CFS patients, but it not only failed to improve symptoms, it actually made the adolescents worse.
Cortene acknowledged that clear evidence of norepinephrine dysfunction in ME/CFS (e.g. antibodies for adrenergic and muscarinic cholinergic receptors, dysautonomia, elevated plasma norepinephrine) exists, but laid the failure of the Clonidine trial on a lack of specificity.
Cortene believes its more targeted approach will allow it to escape the fate of the Clonidine trial.
Clonidine, they reported, is a dirty drug that binds to multiple receptors. It was developed to reduce blood pressure, which it is thought to do by acting on the I1 receptor. It was used in the trial because it is known to reduce norepinephrine in the blood, but because Clonidine also activates the alpha2 receptor, it should theoretically increase norepinephrine in the limbic system. This could explain the trial results.
It is important to understand that, unlike the blood, the brain is not a transport system, and the neurotransmitters (like norepinephrine or serotonin) vary from one neuron to the next. This is why an SSRI, which indiscriminately blocks the serotonin transporter on all neurons, cannot possibly modulate the serotonin system and its 15 sub-receptors without inducing numerous side-effects.
So why is CT38 different? First, Cortene reports that it was tested against a large panel of receptors, transporters and ion channels, and was found only to bind to CRF2; i.e. it is highly selective for CRF2. Second, while CRF2 receptors are found all over the body, Cortene’s hypothesis predicts that these receptors are in excess only in the limbic system, which CT38 will target and hopefully internalize. CT38 will also reach CRF2 receptors elsewhere, but any receptor internalization, in non-adaptive systems, is likely to reverse (like the receptors Leuprolide™ targets). This is supported by the animal data, which showed no obvious loss of effect even over months of continuous dosing. Finally, if receptor internalization occurs and persists, CT38 would not have to be taken chronically.
An Experimental Drug
Cortene cautions that CT38 and their approach are highly experimental with all that that implies. Animal and human data suggests that the drug is safe, but because no animal model for ME/CFS exists, it’s impossible to predict how it will fare in ME/CFS patients – who can be notoriously hypersensitive to drugs.

We should know in a couple of months if Cortene is right about its hypothesis and its experimental drug for ME/CFS.
This Phase 1/2 study should help us understand whether the drug can improve function or symptoms in ME/CFS patients. Cortene’s hypothesis, that the CRF2 receptor activity is causing or contributing to ME/CFS is, however, just that – a hypothesis – as it’s impossible to measure CRF2 levels without doing a biopsy. Cortene’s hope that a slow infusion of the drug will more or less permanently reset the stress response system in ME/CFS is untested as well, and Cortene is unaware of a drug that has produced the kind of system reset that they hope will occur.
FDA and IRB approval will hopefully come in the next month or so.
- Please note that the trial will not be open for enrollment until after FDA and IRB approval.
- The 4th Cortene blog will announce the opening of the trial and provide the next steps if the trial is a success. The 5th blog will provide the results of the trial.
- Stay tuned for a focus on the Bateman-Horne Center over the next couple of weeks.
- The Cortene Chronic Fatigue Syndrome (ME/CFS) Drug Trial Begins








Very exciting! Thanks for explaining it all so well. Can’t wait to hear some results.
Sigh. Another bogus study that ignores the findings of Stanford, and others. But, I’m sure it will get funding since it suggests psychological factors cause ME/CFS. The biases and design of this study will yield faulty results which I’m sure will appease the likes of Vernon and others who want to promote psychological factors as causing this disease even in the face of strong (much stronger research than this puny study) research suggesting other factors at play. Not sure why this bs even gets promoted in blogs. Not to mention the risks associated with this. Just unethical and a huge stretch.
Sigh indeed! Big sigh!
Dr. Matt – I request that you check out Suzanne Vernon’s track record in ME/CFS. Here’s a link – https://www.ncbi.nlm.nih.gov/pubmed/?term=suzanne+vernon+chronic+fatigue+syndrome – to the research studies she’s participated in in this field. I think you may be surprised at what you will find.
There are over forty of them. (I posted the last five below). There are genetics, cognition and brain scan, immune system, gut studies..I have looked through all 41 of hem and I don’t see any that are psychological in nature. Nor when she was the Research Director of the CAA did she fund any studies of that kind. Instead she brought researchers in like McGowan (epigenetics) and Unutmaz (immunology) and others into this field.
Perhaps you believe that anything that effects the stress response is “pyschological”. The stress response, though, regulates the immune system, the metabolism, the sympathetic nervous system and the hormones. It makes sense does it not that a stress response – a response that allows the body to respond to a stressful event – would be all encompassing and would need to get all the body’s systems on board? It’s a very physiological response.
Yes, serotonin enhancing drugs are used in depression but Cortene’s thesis is that too much serotonin is being produced and that serotonin is, among other things, whacking the motor cortex and impeding signals telling the muscles to start working. I don’t see anything psychological about that.
If Cortene was proposing something like CBT/GET or was asking you to change your thoughts or behave differently, I would agree with you but the only behavioral activities their approach involves that I can see would be going to a doctors office and lying in a chair and getting an infusion. No thought changes needed. I’m afraid I see nothing psychological in taking drug that might allow patients to exercise again.
Respectfully yours, Cort
Epigenetic modifications and glucocorticoid sensitivity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).
de Vega WC, Herrera S, Vernon SD, McGowan PO. BMC Med Genomics. 2017 Feb 23;10(1):11. doi: 10.1186/s12920-017-0248-3.
Neural consequences of post-exertion malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Cook DB, Light AR, Light KC, Broderick G, Shields MR, Dougherty RJ, Meyer JD, VanRiper S, Stegner AJ, Ellingson LD, Vernon SD. Brain Behav Immun. 2017 May;62:87-99. doi: 10.1016/j.bbi.2017.02.009. Epub 2017 Feb 17.
The utility of patient-reported outcome measures among patients with myalgic encephalomyelitis/chronic fatigue syndrome. Murdock KW, Wang XS, Shi Q, Cleeland CS, Fagundes CP, Vernon SD.Qual Life Res. 2017 Apr;26(4):913-921. doi: 10.1007/s11136-016-1406-3. Epub 2016 Sep 6.
Tracking post-infectious fatigue in clinic using routine Lab tests. Harvey JM, Broderick G, Bowie A, Barnes ZM, Katz BZ, O’Gorman MRG, Vernon SD, Fletcher MA, Klimas NG, Taylor R. BMC Pediatr. 2016 Apr 26;16:54. doi: 10.1186/s12887-016-0596-8.
Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, Robertson CE, Schrodi SJ, Yale S, Frank DN. PLoS One. 2015 Dec 18;10(12):e0145453. doi: 10.1371/journal.pone.0145453. eCollection 2015.
Hi there! I am cautiously optimistic about CORTENE. I’ve been sick do four years and have come to know ME asa rapacious creditor. Though I live in VA, about a year and a half ago—after much research from my bed, I called Stanford to go to Montoya’s clinic. I waited almost a year for an appointment. I’ve been back twice and am Valcyte, LDN and something new he just added. Anyway, I was just there and saw Dr. Bonilla and asked him about CORTENE. He said, in not so many words, that they were totally on the wrong track and that he believes this s%it disease is largely viral. After reading your post, I’m not seeing a viral relationship. I’m so tired of years of my ticking by and would pretty much take anything at this point. What are your thoughts on this CORTENE/Stanford disconnect? Thank you!
I don’t believe anyone can say with certainty that this disease is viral. I believe that it is viral for some people but many people have tried antivirals. THey’ve been very helpful, even curative for a few, very helpful for some, moderately helpful for others and not helpful at all for others….
The last couple of studies that have intensely looked for evidence of viruses in the blood have not found them. It is possible that a localized infection – aka Van Elzakker’s infection hypothesis of the vagus nerve might not show up well in the blood – although as the testing gets better that less and less likely from what I hear. If I understand Kristin Loomis of the HHV-6 Research Foundation correctly its possible that the right kind of testing has not been done for some herpesvirus infections
Plus Skio Pridgen has had success with his antiviral approach in fibromyalgia….So there’s that in the mix. I think the issue is still in flux. Basically I’m wary of anyone saying anything conclusive at this point about ME/CFS.
Because the Cortene hypothesis proposes that immune dysregulation by the HPA axis occurs which could, if I understand it right, lead to difficulty fighting off intracellular infections, I don’t understand why the doctor would dismiss it.
Great reply, personally thing this illness is triggered by viruses & for some it maybe active viruses causing the illness, but by saying we have tried antivirals & we are still ill, there have been no new antivirals for decades & non are really successful at killing EBV or CMV. But atleast with this new trial we are month away from starting & hopefully getting the results within 6 months?
So for once we have a small amount of time to find out if this shows that it works or not, unlike a new drug that would take years to even get to the phase 1 trial.
Between this serotonin drug trial & the Straford implementation markers research we May have some hope. Well done
RE: Cort Johnson on March 18, 2018 at 7:23 am
“I don’t believe anyone can say with certainty that this disease is viral.”
I have had ME/CFS 4 24 years. I have noticed that if I get virus based bug, like the flu, or anything that triggers a fever as an immune response, afterward the ME/CFS symptoms go into recession. I feel almost normal (98%) with much more energy and stamina. However if I over-do it because I feel normal I can find myself back in grips of the ME/CFS for years. This has happened maybe 4 or 5 times. The last time it happened and ended was there were 2 events – I had gotten the flu or something like it and ran a fever for a day. or 2 then a month later there was a very stressful event – the death of my ex-wife which not only hit me emotionally but also involved a lot of driving to be with my daughter. I pushed myself to get back home for a music job and that was the end of my remission.
So my thinking points to perhaps I have a virus that my body doesn’t respond to on its own but when the immune response to a cold or flu kicks in it the body also attacks that virus too.
When I contracted this in 1994 I was on a trip to Mozambique and had gotten a bunch of inoculations for the trip. I caught what I thought was a cold on the first leg of the trip to London and went sight seeing anyway with the cold then on to Mozambique. However when I thought I was over the cold and went to a gym to exercise I was knocked out. At that time CFS was just getting press and my experience was similar to those who also found themselves with CFS.
I don’t know how all the shots are related to this disease but they stand out as substances that were directly injected in my body just before the 1994 trip.
I would say the severity of the symptoms vary day to day but for the most part I am still functional but require more rest than most people.
I would love to participate in this study when there is enrollment for trials.
I appreciate the news and explanation of the study and missed a psych connection. It seems to me that after so many years, since my dx.in 1997, it makes sense that no SSRIs have helped and in fact always made me wors e and why the use of Clonidine for headaches has me totally bed bound as I am right now. My local Pres. of ME/CFS support group spent over a year in Nevada chasing Providgal and she is no better. I have tried so much and have resigned myself to my bed this year but this study makes sense and again I thank you for this post.I firmly believe that ME has nothing to do with Fibro. Sympathetically mediated pain, like diabetic neuropathy, is helped by Lyrica. It did nothing for me but add 35 #! I tried telling the doc I had no fibro but it was new and he was trying to help. This disease is so awful,but this gives me hope. I think the anti vitals have been given a chance. No more. I wish all of us success.
I absolutely agree Cort. There seems to be nothing psychological about this at all. I think perhaps he saw that serotonin was involved and assumed psych just because of that. On the contrary it’s all biochemistry based here.
It surely is all biochemistry….lots and lots of biochemistry 🙂
The limbic system is the alarm centre for the mind/body, and as such can be stressed by things other than psychological stress/trauma, such as toxins, infection, viruses, mould, chemicals, temperature etc..
I don’t see any reason why these stressors would not cause exactly the same limbic serotonin problems as psychological stress.
In fact, this seems to me a most likely explanation for the variety of subtypes, and the prevalence of psychological explanations.
The mind and body are the same thing, and a stuck transmitter problem is a physical problem.
In the absence of explanation or cure open mindedness is crucial.
if this drug can get ME my life back, I coulndt care less wether its the biological approach or the psychological apporach that score. I Think ME is a biological deseaae but will be first om Line to try this drug
I find the thpretical base solid.
I agree with your assessment of Vernon. She was Bill Reeves sidekick for decades and has her name on a lot his disgusting “research.”
That’s fine Organix but if you’re going to say something like that please back it up with facts. If you’re right fair enough, if you’re wrong then you’re unfairly besmirching someone’s reputation. I’m sure you don’t want to do that. You can find Suzanne Vernon’s publications on ME/CFS here – https://www.ncbi.nlm.nih.gov/pubmed/?term=Vernon+SD%5BAuthor%5D+chronic+fatigue+syndrome
Baugh humbaugh! Let them eat cake! Foolish little ME patients, foolish little doctors, and yet another incompetent study.. that can upset a guy like me who knows everything. Baugh humbaugh!
I’m trying to understand this. Does this mean that if SSRIs are helpful, which they are for me, that then this new drug would not be a good fit?
I don’t know. Cortene could better answer this. The drugs are very different and its a very complex system. Cortene is trying to knock off a receptor in one specific part of the brain while I believe SSRI’s effect many receptors across the brain.
I am currently on a tricyclic. Amitiptylene is working somewhat but I still need 11-12 hours of sleep every day/night and I have to increase my dosage when I exercise due to changes in metabolism (?). Even when I get the dose right I typically experience a crappy headache & muscle pain on waking and throughout the day. On occasion I do get some days of relief and those are the days that keep me going. I’ve had this for ~45 yrs Yuck.
Thanks Cort. Wouldn’t it just be wonderful…a big wish and a prayer for success.
I agree Vicki , Prayers Love and Hope xxxx Thank you Cort
Yes pray to your god which gave us this affliction in the first place to heal us, that’s just dandy, and let’s pray to this omnipotent being to cure the cancer that he has also burdened us with, praise be to simple minded uneducated morons.
holding my breath for this one and am not even in the trial! after my brief encounter with an ssri I disagree that it ‘all comes down to serotonin’….maybe half, butwhatdoiknow
:)… We shall see. I feel the same way – the body is so darn complex – I don’t think anybody knows what tricks it has in store for us. For me I’m very glad that a drug company got interested in this illness! We’ve had almost no interest – maybe no interest from the drug companies and up pops Cortene – they’ve done a lot of work to raise money for this trial, they’ve immersed themselves in the ins and outs of this illness, they’ve devoted I don’t know much time – at least a year, probably two of their lives to making this happen.
That suggests to me that we’re not necessarily this unsolvable disease that drug companies won’t take a chance on. Whatever happens with this trial it’s great to see someone in the drug industry devoting a lot of time and effort not because ME/CFS community needs help – but because they think their drug will actually work. However the trial turns out I find that very promising…:)
Cort-
Thanks as always for your comprehensive blog and thoughtful responses to comments. Based on my experience (50 years??) , this sounds very promising. Fingers crossed!
Thanks so much for the work you put in here Cort explaining things so well. And whatever happens as you say finally there is some interest from scientists and researchers in actively looking at both developing animal models of what is happening in our system(s) that causes all of our symptoms. This means we are at least on a more hopeful road that we have been as a community. Again really appreciate your communicating all of this complex science to us.
Huhman super excited about this drug. If it does what it’s supposed to with CRF…I think they’re onto something at least for a subset or two of us.
“The exercise test will be done before and after treatment and will measure oxygen consumption, ventilatory efficiency, work done, time to reach anaerobic threshold and other metrics.”
=> After treatment, would that be shortly after treatment or in a longer follow-up manner? If it would be only in the following day(s) I could easily see a certain effect (good or bad) and a few days after a near opposite effect on the patients. Initial improvement and delayed backlash is quite common in our disease.
“ME/CFS patients could be at risk from another stressor that causes these receptors to become stuck again”
=> In https://en.wikipedia.org/wiki/Serotonin I find:
“In high concentrations, serotonin acts as a vasoconstrictor by contracting endothelial smooth muscle directly or by potentiating the effects of other vasoconstrictors (e.g. angiotensin II, norepinephrine). The vasoconstrictive property is mostly seen in pathologic states affecting the endothelium such as atherosclerosis or chronic hypertension. In physiologic states, vasodilation occurs through the serotonin mediated release of nitric oxide from endothelial cells.”
“Serotonin secreted from the enterochromaffin cells eventually finds its way out of tissues into the blood. There, it is actively taken up by blood platelets, which store it. When the platelets bind to a clot, they release serotonin, where it can serve as a vasoconstrictor and/or a vasodilator while regulating hemostasis and blood clotting.”
“Serotonin is also a growth factor for some types of cells, which may give it a role in wound healing.”
I quote those because it could point to serotonin as a needed component in wound healing. I believe in ME/CFS/FM the (capillary) blood vessels are damaged / hit hard, and the quoted info seems to indicate that this is where serotonin could be most effective as a wound healing mediator. While parts of the process are unpleasant/disruptive (like vasoconstriction and increasing norepinephrine) and may lead to ME/CFS/FM symptoms, wound healing *may* be a necessary thing in our disease.
That’s why I would be quite interested in knowing how long after the drug administration the patients are followed up. If this potential dirty wound healing would be needed, suppressing it might trigger less symptoms in the short run but more damage and disease in the long run. That could then be a “perfect” “stressor that causes these receptors to become stuck again”.
I hope it won’t, but even if it would then vigorous pacing after administration may make a difference. Give a ME patient a drug that allows him to do 3 times as much the day after and he may well be tempted to do 5 times as much, that’s part of this disease.
But, things are very difficult like always in this disease. So I am grateful to the researchers that they do dive into this disease deeply and I fondly hope their hypothesis is wright and will lead to better health for many! Good luck with the trial!
What???? Please don’t be so negative!!!! At least someone is trying!!!! I have been bed ridden for 32 years!!!!! For Gods sake be happy someone is trying something that may just may be available in our lifetimes!!! And if it doesn’t work??? At least someone is trying!!!!!!for the next generation. AIDS patients got all of the help! Why have we been left to linger and lose our-lives, why are special interest groups more important than we are. I had hopes and dreams…..I have tried every supplement on the market, antiviral….etc, etc, etc. 32 years of continuous trying. I am full of anger and despair. I was a kind sweet young woman with a bright future at age twenty three I will be fifty five in a few days. No children or husband. By the way I have a double SNP in the stress gene and I can not take any form of stress. No noise, loud voices, unexpected surprises and even expected surprises. Whatever this disease is it certainly involves the stress response for me.
I respond to adrenaline and stress wipe me out. As for aids it affected a lot more politicians and celebrities so all the money went there an we were left to languish in the wilderness.
Have u ever tried ozone therapy?
Whew!!!
Wow!!! Adding to my prayer list. Big win that a drug company is interested in ME/CFS. Very interesting science. Keep up the great work. Can’t wait for more updates. Very happy the Bateman Horne Center is involved. My only tweak would be to use a wearable that incorporates FirstBeat technology to give 24-hour HRV and stress/recovery data.
Just wondering if the PTSD community is interested in Cortene as well? PTSD also involves a faulty HPA axis…
Hypothyroidism Hashimotos community has HPA axis issues as well.
Thanks, Cort.
If you talk to these folks again, could you ask if they might consider Cortene as suitable for FM someday, seemingly similar pathophysiology.
Absolutely they do. Similar HPA axis and sympathetic nervous system problems. Lyme disease as well.
Cort, when/how is it possible to sign up as a participant for this study?
Thanks!
When the FDA and IRB approval is done – hopefully in the next month. A blog will announce that.
Thanks for another great write up Cort and hope your cold is gone. Very intrigued by Cortene’s hypothesis. Good luck to all those involved in the trial and I really hope this works out in our favour.
Thanks Cort for more great news on very promising drug targeting a theory directly.
One marvellous thing about this, is that the theory is so neat, and the drug potentially so perfect that we are talking about an actual cure. Not something to manage symptoms, or provide some relief, which is what we might have hoped for at best.
A cure! (And maybe a cup of tea – during transfusion, and some advice about going away and looking after ourselves better next time…)
I’m so glad we have further blogs on this to look forward to.
The very best of luck with the FDA Cortene!
🙂
Dear Cort,
What is CT38? Is it Urocortin 2?
Thank you
If excessive serotonin in the brain plays a role, I wonder what the effects of a Serotonin Re-uptake ENHANCER (SSRE) might be for CFS/ME/Fibro patients? An SSRE may reduce serotonin instead of enhancing it as an SSRI (re-uptake inhibitor) does.
If this interests you, you might consider doing a search for Tianeptine. This drug has been approved in Europe for decades. Purportedly, it has equal or greater efficacy to SSRI’s for depression – particularly for treatment resistant cases. It may also have fewer side effects than typical SSRI’s. A website that presents several research articles on Tianeptine is;
http://www.functionalps.com/blog/2012/10/30/serotonin-reuptake-enhancer-as-an-anti-depressant/
Personally, I have had fibromyalgia for 32 years – that includes low level chronic depression. However, every trial that I took of (many) SSRI’s – always made my symptoms worse instead of better. When this article suggested that an (effective) serotonin excess in the brain may be implicated – it related to my personal experience and caught my interest.
In addition, my current health challenges commenced during an exceptionally high stress time in my life (physically and mentally). My symptoms are still exacerbated by stress, although my sensitivity to stimulation has increased over the years, such that now – even the stress/stimulation of normal light in my eyes,digesting a large meal, or a slight over-exertion of energy may exacerbate symptoms. If I understood this article correctly, it affirms my experience by relating the commencement of the disease to high stress (for many), and by suggesting that our major common symptoms may be secondary to chronic stress (via chronically elevated serotonin).
I am interested, although I remain cautiously optimistic. TY for the great info Cort. I look forward to future posts on this research.
Dave,
Did you try Tianepine? My son ( FM/ME) was on a SSRI for several years and his symptoms showed a same evolutian as yours.
As for me (FM) I never took a drug ( except for the usual supplements and hormones) and improved moderately over the years ( 30)
Chris,
Many years ago when I first learned of Tianeptine, it was not available in the U.S. or Canada. I commenced a process to have it approved as a “special needed drug” for me, here in Canada so I could import it,but I abandoned the application when the bureaucracy was overwhelming. I would consider trying it again – even just as a trial, if I could acquire it without excessive hassle.
I would love to hear the experiences of any who have tried it. I doubt that it would be highly effective for Fibro, CFS or ME, because if it were – I suspect we’d have heard its praises shouted from European patients. I hope your son finds some respite, until a cure might be found. Please let me know if you find a source of Tianeptine, and your/his experience if you try it.
Like you, my first onset of ME/CFS symtoms occured when I was experiencing off-the-charts stress. Over the course of 25 years I saw improvement in my symptoms; however when I experienced stress the symptoms worsened. Two years ago, I once again found myself in an extremely stressful work environment. Shortly after I resigned my body “crashed” and my ME/CFS came back with a vengence and my symptoms are now extremely severe. In short, experiences with this illness supports the hypothesis that stress, in my case, seemed to play a big part in the deterioration in my health.
Ha! I had LOW levels of stress (lol) just prior to getting ME/CFS…I was doing great!
My experience is that high level stress brought this on and makes my function drop significantly with every episode. It can be physical or psychological and neither have to do with mental illness. Cortisol levels rise with eitjer. Thank you for this.
Dear Dave,
Thank you for an interesting opening, being in Europe I got curious and started searching further. I read on another forum of one ME-patient that has been taking Tianeptine. There was no discussion of the effects on her ME-symptoms, but she seems to be ordering it from another country and paying a lot so it must be worth it. I doubt it is curative to ME tho. I can try and ask.
And to Cort: A million thanks for writing this blog, I’ve been crying tears of joy. Having at least a tiny bit of hope means the world to me and this news is so much more than a tiny speckle of hope. Even if the drug didn’t work I think that a negative research result is a research result and in this case, it can be very significant showing the way forward.
Thanks Lia! I too love that a drug company – even if its a very small one! – has chosen ME/CFS.
Where can I sign up??
Enrollment into the trial won’t take place until Cortene has received FDA and IRB approval.
Thank you for the clarification.
What I love the most is how fast they can move to testing. Hopefully this will create a new paradigm for creating and testing new meds.
I’m anxious to see the results.
If this drug trial will be successful then it will be a breakthrough in treatment of fibromyalgia
Speaking as a laymen I would think so.
Very interesting comments Cort. I believe that the stress pathways are affected by many things both biological illness and also mental health issues. If the increase in serotonin is as relevant as this study suggests then we have been looking at treatments for both CFS/FM as well as depression from the wrong angle. My husband a clinical psychologist with 50 years practice in Psyhcotherapy and very concerned about the overuse of of antidepressant medication generally, is studying this idea very closely. He is seeing many implications of seretonin overload in me as well as I have had ME for over 40 years. He is currently working on a paper looking at all the implications of this theory. I will be happy to share his thought with you.
Hello Maggie – when i was first diagnosed I was referred to a “Professor” at St Thomas’ hospital in London. He insisted that I take various anti-depressants as he claimed to have ‘cured’ a nurse with ME using these. When I said that I was not depressed but exhausted, he was adamant. I did not want to take these drugs but thought that if I refused they would think I didn’t want to get well. The effects were devastating, it affected my breathing, my sleep, hallucinations, my weight ballooned… I asked to be taken off them but when he said ‘no’ I stopped them after 3 months. I know this treatment made me worse, so I concur that anti-depressants are the worst medication, for ANYTHING.
Same thing happened to me. I was mildly anxious and absolutely did not feel depressed. My new dr who knew almost nothing about me insisted that I try Prozac! I told her that I wasn’t interested and didn’t think I needed it and that I was also chemically sensitive. She continued to pitch it to me with the enthusiasm of a car salesman. Prozac ruined my body chemistry….many many scary adverse affects. I feel like my nervous system went haywire and honestly it has been worse ever since. I def can not tolerate SSRIs.
Ouch! Sorry to hear that!
Same. My whole nervous system went haywire within 7 days of using an ssri. I definitely had serotonin syndrome. It was a nightmare all the nasty side effects. I have not been the same since. Many lasting issues from that experience.
I also love the one shot use of a med. Its seems so much safer.
A few months ago I took a single half-dose of the smallest Amitriptyline available. I’d heard a CedarSinai Mast Cell Activation Syndrome expert doctor say that Amitriptyline not only had antihistamine effects but also carried anti-heparin effects. Very nice for a mast-cell self test.
Because I had started bleeding all over internally (a rare blood cancer), and it really burned, I thought I would try the anti-heparin blood-clotting effect of Amitriptyline. It worked. That single dose stopped the burning pain permanently so far. Some bleeding returned but I think it’s disappearing by itself.
Just a single dose. I love it.
Agreed. The single dose is a very exciting thought. 🙂
Thanks Cort, such an appropriate name CORTene! Over the past 40 years I have told Drs that my body is on ‘red alert’ all the time. So easy to trigger an unnecessary stress state with high BP, raised erratic pulse, & severe intentional tremor. Volatile chemicals, fragrances cause muscle spasms even convulsions & days lapsing in & out of consciousness. Why?? Prior to having ME I was a nurse, midwife, i/c Casualty at night, I did NOT panic. So what changed? This is the most promising idea I have heard in years. I react badly to most broad spectrum drugs side effects so a drug trial targeted on one thing is an exciting prospect. It would be heaven to stop being on red alert! Looking forward to their progress & your next update. 🙂
Audrey I am so sorry to hear how severe your stress responses are. You have aptly named it ‘red alert’. I feel my nervous system has also been on red alert. The smallest things stress me out. I can also relate to chronic stress over many years which I had to keep suppressed and ‘in control’, as fitting the Cortene scenario. When I got the me VIRUS-because I believe that is what it was, it must have been ‘the last straw’ for my nervous system. Let’s hope my prayers for a cure have been answered.
As I understand it the way the HPA axis is configured in ME/CFS impairs the ability to fight off intracellular pathogens.
I feel simnilar – like my body is on red alert as well…well said!
Me too. It’s a horrible feeling.
Audrey/Christina. I totally identify with red alert. After years of major stressors (mom w/ALS, husband/terminal bladder cancer & major conflicts with families in both instances while havng to earn income), I was entirely revved up, on overdrive. Inability to sleep for years – really thought I was heading for disability. Like a car on overdrive that can’t be kicked down but running on empty. Doctors were no help. Eventually through a FM support group, and the help of meds, things are better and stable. This Cortine sounds very promising, a reset. The heart rate monitoring connects with me too. I have no fuel to exercise, the muscles burn. I would love to get into the trial. Thanks Cort and how nice they named the new drug after you 😉 Well deserved!
God bless Cortene for taking this on. I hope Big Pharma doesn’t profit from this drug in the future.
My son has Narcolepsy so when this work is done perhaps Cortene would like to tackle that debilitating illness which in many ways is similar to ME/CFS?
Hi Cort,
thank you for giving us hope! Although there have been many promisses made in research an many promissses broken. over decades I speak.
I wanted to ask you something that is not clear to me.
Would it also help with sleep because I can take wathever I want and I hardly sleep and when I sleep, it is mostly during the day because after 2 years of illness I suddenly had a day-night time shift with my sleep.
since ’94 I did not sleep well (hardly)and when oly this drug could give a good night sleep, I would be excited.
thank you!
Because the drug will hopefully turn down the stress response (sympathetic nervous system) which Vollmer-Conna’s study suggests plays a big role in the poor sleep in ME/CFS if the drug works – a big if for any drug – it’s very possible it will.
Those time-shifts are rough!
Konijn – I like you couldn’t get any sleep. Exhausted and with a headache everyday. I just accepted having to function as best I could. I look back now and don’t know how I functioned at all. I’d forget automatic things like putting the car into park. Bed was a warzone. Instead of being a comfort, a place of restoration, I dreaded going to bed because it was futile. I was up to all hours every night before lying down and not sleeping – it got to the point where it felt as though electrical connects were happening in my head – a awful sensation. I’d been taking gabapentin and nortripilene which helped with pain but not sleep. Other drugs like lorazapan, trazodone didn’t help. I could understand how Michael Jackson wanted to be hooked up to a drip-anything to get sleep. What really helped me was LDN. LDN helped calm my system. If you haven’t ever tried it, it might help you. It was a wonderful drug for me. Bed is no longer a warzone.
Hi Cort, Thank you for this thorough write-up and all the explanations making it comprehensible for a layperson. Perhaps this will be the breakthrough we need. I’m certainly not a scientist, and therefore don’t understand all the implications of hyper-seratonin state. I want to feel optimistic about this trial, but in my gut I’m not quite there. What are the implications for factors such as: hypersensitivity to light/audio/movement stimuli; food intolerances; MCS; hypersensitivity to molds & the side effects from that? These are just a few anomalies that seem to affect PWME more specifically than healthy people. In the section about stress and that another stressor could set off ME again is also a bit disconcerting. Certainly in my own disease development there were physically stressful events leading to full blown severe ME: vaccines, pesticide poisoning; and concussion. I can’t say for certain if I had a viral event or genetic predisposition. “Stress” is so arbitrary, and varies from person to person, life experience to life experience, psycho-social situation, etc.
I don’t comment on your blogs very often, but while I’m here I guess what I’m also thinking about are the other researchers/projects that have not been completed…and what we are to make of that? The Microbe Discovery Project, for instance, and anything Dr. Ron Davis has been working on? Will anyone still be advocating for Ampligen again? So, I know this last paragraph is completely tangential, but I felt I needed to mention it. Mostly, my concern is that different institutions are approaching MECFS in different ways. My concern is that there is lack of continuity and cooperation.
Thanks Cort, great blog post!
Great questions!
My guess is that this serotonin hypersensitivity to stress state could probably sensitize whatever pathways are amped up in MCS.
Microbe Discovery project – not sure but if I remember correctly at least one of the NIH research center is digging into the gut and Lipkin and Hornig have a paper under review on it now I believe.
Ron Davis is working on a bunch of stuff – his nanoneedle and measuring energy in ME/CFS cells; the severely ill project will be wrapping up hopefully by summer, a big family study is look at all sorts of things, he’s working the Mark Davis at Stanford to examine T-cell clonal expansion and he’s working with SJSU to look at problems with our blood vessels.
There is absolutely a lack of continuity. The NIH research centers will help a little bit with their collaborative study. Davis wanted/wants to get a group of researchers and experts together and plot out an organized path but that hasn’t happened. One good note – a gold standard for ME/CFS studies has been made in conjunction with the NIH.
Hi Nick. The Amygdala is central to the limbic system and is your brain’s stress alarm, however it is an ancient organ and barely distinguishes between threats to the body. Which explains the different triggers many of us now have, regardless of whether we were initially triggered by stress or pathogen. Prolonged exposure simply makes the Amygdala sooper sensitive, and as it is always on the lookout for trouble & threats from anywhere anyway this then becomes sensitivity to noise, light, temperature etc… The role of the Amygdala is very well understood neurologically and from my experiences this maladaption seems to be very much what is happening with my condition… which is why I am now waiting with baited breath to here about the results of this study as it all stacks up.:)
A CFS hypothesis ought to explain PEM. This CRF/Serotonin theory may explain the fatigue and weakness, but I don’t see how it can account for the delayed sickness after an exertion. Any idea?
I don’t know but I am struck by Dr. Klimas’s ability to watch what’s happening when PEM occurs after exercise. She’s found that inflammation produced by the exercise causes a cascade of effects including dysregulating the autonomic nervous system. Since Cortene’s hypothesis involves a major system in the body which regulates the immune system I think something similar might happen (?)
While I am still not “feeling” the serotonine angle completely yet, I could see a potential angle here: serotonine can mess with vasodilation and constriction. If very high doses would suddenly be present in the blood that could disrupt blood flow.
I still fail to see the bigger picture of how this cascade would work if it involved serotonine, but I believe disrupted blood flow is a big part in the PEM cascade. Just not sure how to fit in serotonine and making it fit with the rest.
=> In https://en.wikipedia.org/wiki/Serotonin I find:
“In high concentrations, serotonin acts as a vasoconstrictor by contracting endothelial smooth muscle directly or by potentiating the effects of other vasoconstrictors (e.g. angiotensin II, norepinephrine). The vasoconstrictive property is mostly seen in pathologic states affecting the endothelium such as atherosclerosis or chronic hypertension. In physiologic states, vasodilation occurs through the serotonin mediated release of nitric oxide from endothelial cells.”
=> The combo norepinephrine (and ephinephrine aka adrenalin maybe too) and serotonine may be quite potent vasoconstrictors here.
I praise all of you, Cort, who put so much effort into finding a way. I just get so
downhearted, as if this new “drug” could not accommodate new stresses, as I think you said, then all is lost, as life keeps throwing up major stress that would make toast of a normally healthy person (for some of us). Yes the mystery illness. Anyone tried
scenar? (energetic healing – helps me for pain) Anyone tried Methylation cycle compounded vits? This treatment includes a “promotor” full of amino acids. Don’t
even know why I am writing this. However, am glad there are people like you, Cort,
and your team. Blessings be upon you.
Thanks Billie. Cortene hopes the drug will reset the system enough so that it would take a major stressor to turn it back. Let’s hope!
For those who are suffering from a ‘red alert’ stress response, may I suggest Atenolol? Early on, it seemed that my body had no way of buffering the effects of adrenaline: it felt like acid coursing through me making simple things like standing while having a conversation with someone literally sickening. Atenolol, a beta blocker, helped quite a bit. I take 50mg in the morning and 50mg in the evening. Not anything new but hopefully helpful.
Thanks for contributing that Neal.
Who is the Stanford researcher who will be overseeing this?
Suzanne Vernon at the Bateman Horne Center is overseeing the study with Cindy Batemen.
One promising aspect of this hypothesis is that it seems consistent with Jarred Younger’s preliminary data from his ‘Good Day/Bad Day’ study (https://www.youtube.com/watch?v=QHIvcw9SNFo). If I remember correctly, Younger found that the symptoms of one group of ME/CFS patients were correlated with relatively small changes in signs of infection (as in Montoya’s recent publication); the symptoms of another group correlated with relatively small changes in a marker of autoimmunity; a third group didn’t fit these patterns. Thus, different, relatively minor “stresses” seemingly resulted in the big problems of ME/CFS, as if something in us is over-reactive.
Just a guess: The delay before PEM (about two days for me, usually) might result from the time it takes for viruses to reactivate or autoimmune cascades to develop, etc., and then for our overly sensitive systems to respond.
Even if this trial doesn’t work, I hope that researchers continue to consider similar hypotheses.
Could this approach theoretically be compared to ‘DNRS’ ? ( as in Annie Hopper/Gupta)https://retrainingthebrain.com/how-the-program-works/
Both in different ways ‘reset’ the limbic system.
Ever grateful for these updates Cort.
As well as suffering with ME/CFS I have severe migraines and the only drug that stands a chance of getting rid of the pain is Sumatriptan which I believes blocks serotonin and constricts the blood vessels.
I also have SNPs that stop my body from getting rid of excess serotonin so it will be interesting to see if this research pans out.
My Migraine Consultant thinks there is a huge serotonin connection in Migraine.
Thank you Cort for your great write-ups of new research which can definitely be pretty complicated.
Yes there is with migraine – but its too little serotonin in that case, as I suspect is also the case in CFS.
Dave,
you will find a lot information on Tianeptine on the website
selfhacked.com: look under nootropics.
You did not see any reaction from european patients because
it is very rarely prescribed for FM patients.
I searched in patient blogs in France (the drug was patented by a french society) and found ample ractions from patients with depression and anxiety. In general the drug has few side effects (less than other antidepressants
Some patients were negative in their comments, but patients who did bad on other SSRIs- like you- were very enthusiastic.Those were all patients with depression only:
No reports of effect on FM pain.
I also looked at blogs from German FM patients: I found one female patient who was very enthusiastic; fantastic mood and huge reduction in pain; she did not understand why de drug was almost never prescribed for FM patients.
There is a recent article on the ” Effects of tianepine on symptoms of fibromyalgia via BDNF signaling in a fibromyalgia animal model ” by Hwayoung Lee et al in
Korean J Physiol2017 jul; 21(4);361-370.
Levels of BDNF in blood serum were lower than normal.
That is in rats… but it is a start!
If you try it let me know!
Cort, you’ve been in the m.e world reporting for lone than me. It seems that for years, there have been numerous studies asserting Knowledge and hopeful treatments/cure to no avail. Is the tide turning now? What is your view? Can I dream of a day with no m.e? I hate my lack of belief – alone in the UK it’s so hard at times to see a way out…
I honestly don’t know. I think there are more possibilities on the treatment end now with Cortene and Nancy Klimas’s and Bob Naviaux’s upcoming drug trials.
With regards to research this field still has not cohered around a central or a couple of hypotheses. Plus there’s the problem of subsets – there’s no organized approach to delineating subsets – different subsets pop up all over the place!
I really wish we could start a “War on ME/CFS” and take an organized approach to this disease – lay out a strategy – knock off possibilities one by one – do large-scale studies – like what Ron Davis proposed. That takes money and a collaborative approach. We really don’t have either at this point but we shall see – we have good researchers exploring exciting topics. I’m particularly interested in what Nancy Klimas is doing. I think the community is going to be surprised. I’m certainly more hopeful now than ever before.
Thanks for your views – i value them a lot. A war on m.e! Now that sounds exactly what we need. Soon I hope!
Thanks Cort. I hope science finds a way out soon.
Can you refer me to what Nancy Klimas is currently doing with research? My fog brain can only find one recent video (homeostatic reboot?). I would love to read more…
BUT … decreasing Seratonin levels is just what I thought I didn’t need. Currently on long term Zoloft to increase Seratoniin and it has helped with my life long skid downward to clinical depression.
Or does this group think other hormones like Dopamine will take over for lowered Seratonin?
Hi Cort, your work is so exciting! Does this mean that taking neurotransmitter supplements that increase serotonin such as 5htp or tyrosin will worsen the condition?
Because Cortene’s drug is focused on just one part of the brain I really couldn’t say. I know that some people certainly benefit from 5-HTP.
Thanks so much Cort, for keeping us informed. I see no downside here. If it works even for some people resulting in even minor improvements, it’s a major WIN. Then they might be able to tweak it or further develop the hypothesis. If it doesn’t work, nothing is lost. That you are optimistic speaks volumes as you’ve been following the research for so many years. The more research the better! Thank you Cortene for investing in ME/us.
Cort – Thanks for the detailed reporting. All – the discussion is informative and appreciated.
If available in the public domain – I would like to be informed about what subset of patients will be targeted for the Cortene trial. Also, an overview of any risk analysis conducted by Cortene – especially real and hypothesized concerns about what could go wrong – specifically the potential downside risks of taking this drug.
I agree that a defined big picture view starting with standardized subtypes and identifying research activity might be helpful for patients. I wonder if the patient community could engage with hope by contributing answers to subtype profiling questions? I for one would like to know more precisely where I fit – this could also be of benefit to the emerging precision medicine approach.
As for the “psychological aspect” and “exercise will make you better” – there is a saying in the southern U.S. – oh she’s mental translated to she’s emotional/crazy – ugh that would get my ire up! … And then a nurse friend told me she viewed it as just a brain or cellular process. Don’t know if that helps anyone but it sure helped me! Besides who isn’t mental? Just take a look around.
Hi Janet,
The criteria for participation in the trial will be announced after FDA and IRB approval – probably in a month or two. The human data – which did not include ME/CFS patients – suggests that the risks of significant side effects are small but this is a different population….
How is the drug administered? Pill or injection?
Also do you think programs like Gupta and ANS REWIRE are good options to try as they seem to address the brain dysfunction side of the illness? Thank you
i hope this is a major breakthrough i have cfs severe and fibro for almost 6 years started after severe stress i think they are on to something big i can only hope and pray this will help so many of us suffering and not many people get it they can not see what we are going through anyone ever tried stem cell therapy for cfs me or fibro and got some help i hope this is the big answer for so many of us will know soon hope we all can be in the trial traveling and being broke is no fun but looking forward to this thanks cort and everyone else JR
Interesting theory, I always noted hat I get utterly exhausted and foggy on serotonine enhancing supplements. And on the other hand serotonine enhancing drugs help me to calm down when my hpa axis is in overdrive (my hpa axis pendulates between over and underactive).
What I currently don’t understand: there are drugs like Trazodone recommended for insomnia in CFS. As I understood Trazodone works on blocking a certain kind of serotonine receptor (the 5ht2-receptor), thats why it is called serotonine receptor antagonist. But as it is said to increase serotonine in the brain, how can it be an antagonist at the same time? As I understood an antagonist actually blocks the effect of a certain substance (serotonine in this case). So when it’s blocked, how can it lead to increased serotonine? I really didnt get that. Or is it that when an receptor is blocked the body compensates for that by increasing the amount of serotonine? Maybe sb could explain. My doctor couldnt, haha.
My doctor always highlighted the importance of having enough serotonine in ME/CFS. But actually it seems to be the other way around!? I am completely confused now :-).
According to my own research there is also desensizitation at the level of the pituitary (so it becomes insensitive to CRH, doesnt produce enough ACTH and not enough cortisol). At the same type a hyperresponsiveness to cortisol at the level of hypothalamus/pituitary leads to an increased feedback mechanism, also called “super-supression” (can be meassured in the dexamethasone supression test, CFS folks tend to shut down the hpa axis on significant lower levels of cortisol than healthy controls).
It’s so complex! Any input appreciated 🙂
Hey Cort thanks for the info! Can you describe in detail your connection to Cortene Inc. (I.e. why a link to this page appears on the home page of their website) and also why they might already purport to have developed a curative treatment for this condition before it has ever been administered to any pwc’s? Thanks
My connection is that Cortene requested that I produce blogs introducing their trial. After learning more about their hypothesis and the people involved in it including ME/CFS researchers I decided to do that. I am not affiliated in any other way – I haven’t received money from them, I am not on their board etc.
The link to this blog makes sense since the blogs explain the hypothesis in detail.
Cortene has never said this drug will work – they said it might work and they have explained why. Throughout the blogs I’ve been careful to emphasize that this is a highly experimental drug.
Hi Cort, Thank you for all of the information and wonderful explanations in your blogs about Cortene, their theory and CT38.
Is there any new news regarding FDA approval and start date for the trial? Thanks
I haven’t heard anything lately but I think we’re still about a month or so away. It always seems to take a bit longer than expected….
Cort, Have you done any reasearch or come across any literature on TMS therapy for a limbic system reboot?
I know some TMS studies are underway in FM trying to reboot whatever is going on….
Any idea when trial info will be avail
Here’s an update from June 13: https://cortene.wordpress.com/2018/06/
Cort,
Has the trial started, and is it at Dr Bateman’s? Are her patients the ones in the trial.
Grateful for any update.
I have had fibromyalgia for 24 plus years. This article is most encouraging. I have done a ton of research over the years and this makes sense to me. Hope and prayers that this research tells us something new and moves the needle toward a cure/treatment for those suffering for so long.
i am a 59 year old male in north dakota with severe cfs and fibro after 2 surgeries tried antiviarls no help many many things now broke still can not work a job hard to function from day to day but was wondering if anyone tried stem cell therapy for cfs the testimonials look hopeful for relief from symptoms i did one stem cell therapy and am going to do another one in my spinal fluid this saturday for headaches anyone else try this am waiting for cortene trial like all of you but want some relief the thing that helps the most is steroid shots in my neck for about a month they are kennolog shot seroid anyone tried either one or the other let me know if you did thanks hope cortene is a breathrough for all of us i hope and pray soon JR
If excess serotonin is the problem, shouldnt SSRIs exacerbate the symptoms? I was put on zoloft once and while it didnt help and made me a little more tired, it didnt effect my myriad of other symptoms like the pain and brain fog.
I have never responded favorably to any of the anti-depressants like SSRI’s that have been pushed at me since I first got sick almost 30 years ago. Even tiny portions of a pill/or even tried liquid, make me wish I could jump out of my skin! I hope and pray this Cortene trial is successful!
I have the same response, Lane. I can’t tolerate psych meds of any kind.
Although I have long thought that CFS/ME is not one disease with a single cause, I believe that a subset has a toxic onset. In this case, this new theory would not apply. Toxic substances like heavy metals, pesticides, drug use, and some prescription drugs can cause permanent damage to the nerve cells that make serotonin and other neurotransmitters. Approximately 250,000 of the 697,000 U.S. veterans who served in the 1991 Gulf War are afflicted with enduring chronic multi-symptom illness, a condition with serious consequences that is in most respects identical to CFS. Gulf War Illness has been attributed to exposures to pesticides, nerve gas agents and/or pyridostigmine (anti-nerve gas agent). A study of 100 symptomatic Gulf Veterans published in Environmental Health Perspectives found increases in antibodies against Epstein-Barr virus, cytomegalovirus, herpes simplex virus type 1 (HSV-1), HSV-2, human herpes Type 6 (HHV-6), and Varicella zoster virus (VZV). The infections and viruses found in CFS and GW patients are opportunistic not new active infections. Why aren’t we investigating toxic exposures that may trigger CFS? Many people are exposed to highly toxic substances in their homes and workplaces and don’t have a clue about what these exposures can do. I was surprised to learn that the Tahoe outbreak of CFC followed a toluene spill. This is a highly toxic solvent that is stored in body fat. Chemicals can be released from body fat because of sudden weight loss; heat and sun exposure; aerobic exercise; stress; in sum, the same things that trigger symptoms of CFS. I am co-chair of the National Institute of Environmental Health Sciences’ Public Interest Partners, a group that represents health organizations where environmental toxins may have caused or triggered the disease represented by the member organization. National groups represent breast cancer; learning disabilities; autoimmune disease; autism; birth defects;lung disease and others.
Betty,
I believe that toxic drugs are the cause of ME /CFS FM in some cases. I found it interesting that Cort mentioned the drug Lupron (developed for prostrate cancer but it is given to women to treat fibroids and endometriosis) in this article. This drug has actually been the cause of these illnesses for many many women!
I wonder if how Lupron turns off the estrogen receptor and inhibits a stress response in the body therefore causing these illnesses. Would cortene’s drug be helpful in this situation?
I believe there is a lot to this new trial. My CFS was caused by an ssri. I’ve been ill now for 12 years. I’ve always believed there is a connection to serotonin receptors densensitzation and a subset of cfs.
I wonder if this would work for people who have been damaged by SSRIs. I have permanent sexual dysfunction (PSSD), emotional numbing, genital numbness, severe insomnia, no libido, no taste or smell, physical issues, etc. It’s like I’ve been lobotomized and castrated. It is theorized that a permanently desensitized 5HT1A is the root cause. Does anyone think this drug could help? Cause suicide is the only other option.
Hey Cort, this is a very interesting research!
I came across this looking for research done on dexamethasone. I have breastcancer and get chemo, and one of the things I get is dexamethasone against nausea. And while most people suffer from increased fatigue from chemo, I am perking up like nobody’s business. I have been able, for the first time in TEN YEARS, to have two showers in one week, and no rest needed after that. The bath I had a week before that had me in bed for two hours. So that really has been an enormous change.
I’ve come across this article (also with Suzanne Vernon) where they also talk about dexamethasone. But I have no real scientific background and my brainfog is enormous, so I don’t exactly understand what they’re saying about it. Could you help figure that out? The bit about dexamethasone is about 55% of the complete text.
https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1000273
Hi #Cort—any news from Cortene?
Any update on this trial, Cort? So fascinating! I believe this is what happen when I was given Lupron (for fibroids). The stress response got stuck after estrogen was taken from my body so quickly. I find it interesting that Lupron is mentioned in your article.
Im a doubter on this one. Nearly all models of neuroinflammation and CNS states that result on elevated extra-cellular glutamate result in reduced serotonin not excess. Most prominent cytokines suppress tyrosine hydroxylase (dopamine and norepinephrine) but also interfere with the cycle that results in serotonin synthesis.
My take – elevated cytokines promote extracellular glutamate, suppress astrocyte activation and suppress central norepinephrine and dopamine release. Reduced central norepinephrine itself promotes further cytokine elaboration and stops central alpha 2 buffering of peripheral sympathetic activation