

Neuroinflammation, Fatigue and Pain Lab Stop
We were having a case of déjà vu as we drove around the surprisingly large campus. Getting into the NIH to see Avindra Nath had been a nightmare. It turned out that the NIH would only allow the big van through one access point and we’d ended up mortifyingly late to our appointment. Now here we were in another big campus with what my partner felt were inadequate directions.
I thought Jarred and I had it going, though. He said he would meet us at the parking lot and let us through the gate, but there were lots of parking lots. Plus, because we couldn’t stop, we had to keep driving around the campus and hope we met up with him at the right parking lot at the right time. It did seem a little dicey but I was confident I’d figured it out.
My partner, though, wasn’t having it with the sketchy directions or the reliance on male directional genes. She could see it happening – we were going to be late again.
“Men,” she said, “how do you ever get anything done?”
As it turned out, Jarred and I were in sync: we both showed up at the gate at about the same time and he led us into his surprisingly large facility. Once again, we forgot to take pictures, but our timing couldn’t have been better; Younger had just wrapped up one of the most exciting studies in memory.
But first a little history…
Neuroinflammation – The Japanese Way
Researchers have thought for decades that neuroinflammation is probably present in chronic fatigue syndrome (ME/CFS), but it’s only recently that the technology has been able to pick up the lower levels of neuroinflammation believed present in diseases like ME/CFS and fibromyalgia. The Japanese were the first to take a crack at it.

Neuroinflammation has long been thought to play a role in ME/CFS but only recently have the tools to study it become available.
They have long believed that inflammation produces central fatigue (fatigue emanating from the brain), which plays a major role in ME/CFS. In 2013, Watanabe proposed that inflammation in the brain was whacking the “facilitation system” which pops up when we are fatigued to boost signals from the motor cortex to keep our muscles moving. He also hypothesized that an inhibition system was turning up the fatigue in ME/CFS.
A 2016 study rounded the circle when it found evidence of reduced dopaminergic activity from a part of the brain (the basal ganglia) which activates the motor cortex. That fit in just fine with Miller’s results, which suggested that problems with the basal ganglia could be producing both the fatigue and the motor activity problems in ME/CFS.
The big breakthrough came in 2014 when the Japanese startled just about everyone with a PET scan study which found widespread neuroinflammation in the brains of ME/CFS patients. The study was small (n=19) but the findings appeared strong.
The neuroinflammation was widespread but was highest in the areas of the brain (thalamus, amygdala, midbrain, hippocampus) that had shown up in ME/CFS before. Plus, the Japanese were able to link specific regions of inflammation to specific symptoms. Inflammation in the thalamus was associated with cognitive impairment, fatigue and pain; inflammation in the amygdala was associated with cognitive issues; and inflammation of the hippocampus was associated with depression.
Anthony Komaroff called the findings the most exciting in decades. The Japanese began a much larger (n=120) neuroinflammation study. This year they published a large number of papers on ME/CFS in the Japanese Journal, “Shinkei Kenkyu No Shinpo” (Brain and Nerve). One of the papers was specifically on neuroinflammation but the findings have not yet been published in English journals.
Neuroinflammation – The Younger Way
Jarred Younger – who runs the Neuroinflammation, Pain and Fatigue Lab at the University of Alabama at Birmingham has also long believed that neuroinflammation plays a major role in chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM).
In 2015, he noted what a hot subject neuroinflammation had become. Seven years ago, he said, there was almost nothing on the microglia at the pain conferences. Now they’re loaded with presentations on microglia.
These immune cells are sensitive to so many factors and can be triggered in so many ways that virtually any stressor, from an infection to toxins to psychological stress, can potentially trigger a state of microglial sensitization in the right individual. With their ability to produce dozens of different inflammatory mediators, Younger believes that the difference between ME/CFS and FM could simply come down to small differences in how the microglia are tweaked.
Both diseases could be triggered by high rates of immune activation which, over time, sensitizes the microglia to such an extent that they start pumping out inflammatory factors at the first sign of a stressor.
The Neuroinflammation Man: Jarred Younger on Inflammation, Fibromyalgia and Chronic Fatigue Syndrome
New Non-Invasive Technique
Younger had just finished up his ME/CFS brain thermometry study. He used a new, less invasive way of assessing the brain called magnetic resonance spectroscopic thermometry (MRSt). The technique, which aims to create a thermometer for the brain, uses a magnetic resonance imaging (MRI) scanner. While Younger was assessing the temperature of the brain, he was also examining its chemical makeup.
My partner asked him how he glommed onto the heat mapping idea? It turned out that Younger had been trying for quite some time to find a non-invasive way to assess neuroinflammation. He needed a technique he could safely use again and again in his longitudinal (Good Day/Bad Day) studies.
None of the present techniques, however, fit the bill; they were all heavily invasive. The PET scan approach uses radiation to image the brain. Another approach using magnetized nano particles is supposed to be safe but it still requires putting little bits of metal into peoples’ brains…
After hitting several dead ends, he hypothesized that because inflammation produces temperature increases, he could try and create a heat map of the brain. Looking through the literature, he realized that thermometry was already being used in the brain to assess stroke and cancer patients. It turns out that the brain’s attempts to repair the damage from stroke and cancer results in huge temperature increases. The stroke and cancer researchers, though, were just focused on small areas of the brain.
Because Younger didn’t know exactly where in the brain to search in ME/CFS, that technique wouldn’t work for him. He had to develop a method that would produce a heat map and a chemical signature of the entire brain, and found a Florida researcher who developed a way to do that.
With this technique, it takes just 20 minutes in the machine to get an entire 3-D heat and chemistry map of an ME/CFS patient’s brain. After The Solve ME/CFS Initiative (SMCI) provided funding, he got to work and ultimately scanned the brains of 15 ME/CFS women and 15 age and sex matched healthy controls.
Widespread Neuroinflammation Found in ME/CFS Patients’ Brains
“The markers were truly elevated” Jarred Younger
It turned out that Younger’s brain-wide search technique was right on. Looking at single areas of the brain in ME/CFS patients would have produced misleading data. It turned out there was no single area or even a group of areas in the brain that were abnormal in ME/CFS: most of the brain was.
Younger found lactate – a product of anaerobic metabolism – widely distributed across the brains of people with ME/CFS. He opened a chart showing an amazing array of lactate-engorged brain regions. He picked out a few: the insula, hippocampus, thalamus, and putamen, which had particularly high levels. They were virtually the same regions the Japanese had found in their 2015 study. The fact that the temperature increases overlapped with the lactate increases provided further confidence that Younger had identified some key areas.
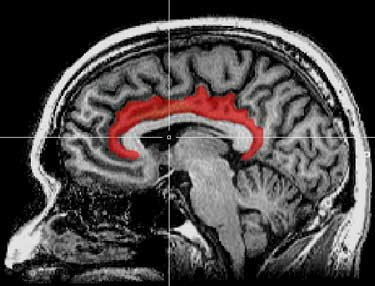
The interior cingulate cortex, in particular, which Younger called “the seat of suffering” in the brain, showed up in spades. It’s associated with a lot of nasty symptoms (malaise, fatigue and pain) and it’s shown up in both ME/CFS and fibromyalgia studies in the past. The high choline signal in that region of the brain suggested that inflammation there was producing a pattern of destruction and replacement; i.e. quite a bit of damage – even possibly neuronal damage – was happening there.
Overall, the lactate levels weren’t as high as in other diseases – they were just consistently present. Younger didn’t expect to see really high levels; really high lactate levels would have meant irretrievably damaged neurons – the kind of neuronal damage seen in M.S., Parkinson’s and Alzheimer’s – the kind of neuronal damage that is really hard to reverse. The fact that Younger saw inflammation in ME/CFS but not neuron-destroying inflammation is good news indeed for people with ME/CFS.
It’s possible that some damage such as neuronal reprogramming and synaptic pruning could be occurring, but determining that would take an autopsy. (Some groups are collecting ME/CFS brains at a couple of autopsies that have been done.)
Remarkably, the healthy controls didn’t show evidence of a single analyte such as lactate being elevated or a single area of the brain being heated up. It’s highly unusual to find zero evidence of an abnormality in the healthy controls. Usually the results of studies apply to groups, not individuals; some healthy controls typically will have findings that are similar to the MEC/CFS patients and vice-versa, but not here – the two groups were absolutely distinct. Even though this was a small study, such black/white results strongly suggest that neuroinflammation of the brain is a key element of ME/CFS.
Lactate
Magnetic spectroscopy studies have found increased lactate in the ventricles of the brain in ME/CFS before but not in the brain itself. Shungu’s spectroscopy studies have, in fact, produced some of the most consistent results in all of ME/CFS research. Three times he’s probed the ventricles and three times he’s found increased lactate. Shungu, however, is examining an area just outside of the brain. His findings may indicate inflammation is present in the brain or it could be confined to the cerebral spinal fluid.
Younger’s new approach looked at the entire brain and found signs of inflammation almost everywhere. When asked what could cause that, Younger said that any neurodegenerative/ neuroinflammatory disorder like MS or a severe brain injury that tweaks the microglia (immune cells in the brain) enough to produce a sustained period of inflammation, burns up the oxygen in the system. Once that happens, the cells resort to anaerobic metabolism and lactate builds up just as it does in the muscles during exercise.
My partner asked another intriguing question. (Thank god her brain was functioning.) What about intervention studies? What about whacking ME/CFS patients with exercise and seeing what happens to their brains? Younger, it turned out, had already laid the groundwork for that study.
Therapeutic Implications
Documenting that neuroinflammation is present and is affecting functioning in ME/CFS could have dramatic treatment implications. It could lead the scientific and medical communities to focus less on drugs that target the nervous system and more on ways to reduce inflammation. For example, attempts could be made to modify current anti-inflammatories so that they pass through the blood brain barrier (most do not). Health Rising will focus on some way that might happen in a future blog.
Fast Mover
Throughout this process, Younger has moved extremely quickly. He completed the thermometry study as quickly as possible, and then as the dramatic results began to come in, rapidly applied for a nice, fat ROI grant from the NIH. The results were so convincing, in fact, that he didn’t wait for them all to come in and applied for the grant using half the data from the study.
It’s hard to imagine that that grant application won’t get funded. When it is, Younger will have plenty of money to pursue the neuroinflammation angle further, including challenging ME/CFS patients with exercise – something he’s never done before – and seeing what that does to the inflammation in their brain. It’ll be fascinating to see if it rises, how long the inflammation lasts, how it tracks with post-exertional symptoms, and where it’s most evident.
Cause?
Younger speculated that people with ME/CFS have an immune-triggered metabolic disorder. The widespread neuroinflammation provides a clue, he thinks, to what’s going on. That pattern suggests that immune cells are breaching the blood-brain barrier in multiple areas; like a flood overwhelming a dike they’re essentially pouring through gaps across the brain. Why that may be happening he’s not sure, but his next step in ME/CFS is to demonstrate that that’s happening. How he proposes to do that is the subject of the next blog.
Stay tuned also for Jarred Younger at the Open Medicine Foundation’s Stanford Symposium. He’ll be participating in the Working Group and presenting at the Symposium.
The Solve ME/CFS Initiative’s Ramsay Award Program Scores
The other big winner in this exciting saga is the Solve ME/CFS Initiative’s (SMCI) Ramsay Award program. The SMCI knew a good thing when they saw it and funded Younger’s new approach to neuroinflammation with a Ramsay Award. Without that funding Younger wouldn’t have gotten to where he is, we wouldn’t have validation that neuroinflammation is occurring in ME/CFS, and the NIH wouldn’t have a nice grant application sitting in their lap.
The Ramsay Award program is designed to fund pilot studies that produce the data needed for major grant applications. It’s done that in spades; three of the four recent grantees are applying for major grants. That’s a big boost for a field that direly needs more grant applications.
Your Support Is Needed
Health Rising’s East coast trip provided a wealth of information inspiring the article you just read and the ones below. Several more articles will come from the Younger stop plus Dr. Klimas’s exercise study, a possible new treatment for fibromyalgia, an Avindra Nath interview, and – on the return home – the folks at the Bateman-Horne Center. Next up on the travel agenda is the Stanford Symposium.
Travel provides many opportunities but travel to the East Coast, in particular, is expensive for a small organization like Health Rising which is still attempting to recoup its trip costs. If you find conference reports and other travel related blogs helpful and want to see these in the future, please support Health Rising.
Articles From the East Coast Trip
The Jarred Younger Series
- Widespread Neuroinflammation Found in Chronic Fatigue Syndrome (ME/CFS)
The IVIG Series
- An IVIG Chronic Fatigue Syndrome (ME/CFS) / POTS Treatment Success Story: IVIG#1
- Are Chronic Fatigue Syndrome, POTS and Fibromyalgia Autoimmune Dysautonomias? IVIG #2
- The Case for IVIG Treatment in Chronic Fatigue Syndrome (ME/CFS), Fibromyalgia, Small Fiber Neuropathy, and POTS: IVIG#3
- Winning the Lottery: “Novel” Treatments Return Severely Ill POTS Patient to Near Health: IVIG #4
Advocacy
From the Dysautonomia Conference
- 2018 Dysautonomia International Conference I: Small Fiber Neuropathy, POTS, MCAS and Vagus Nerve Stimulation
- The 2018 Dysautonomia Conference Pt. II: Could You Have a Spinal Fluid Leak? An ME/CFS, POTS, FM Perspective
- Dysautonomia International Conference Pt III: The Autoimmunity Revolution in POTS
- “Sticky Blood” – Antiphospholipid Syndrome, POTS, Chronic Fatigue Syndrome and Fibromyalgia – The Dysautonomia Conference #4
- Stagnant Hypoxia – Where Chronic Fatigue Syndrome and Hyperadrenergic POTS Meet?
- Promise Fulfilled – A New Chronic Fatigue Syndrome / Fibromyalgia Practitioner Steps Forth









Cort, thank you for always doing work that gives us hope to carry on.
Have we got too many hopes to carry on?
Thank you Cort this is the most hopeful information I’ve seen yet. Those of us that suffer with this disease know very well that it’s brain brain brain! I’ve always tried to tell my doc that my brain is hurting! I hope Mr Junger gets all the funding he needs to pursue this. Fingers crossed!!!!
A couple of comments.
1. We already know that there is inflammation in our brains, all they had to do was ask us! That’s why we ice our heads. Literally, ice packs on my neck are a regular “treatment”, in a world without treatments. Another patient reports wrapping his entire head in ice for relief from symptoms all over the body. So I guess it’s good to have “proof.” Yay!
2. Proof of serious physiological disease in ME/CFS has not typically been good enough for NIH to fund it. After all, it mostly affects women, so who cares? With six times more spending designated for male pattern baldness, the NIH is guilty of a long term pattern of blatant discrimination, negligence, and imcompetence. I hope for the best from NIH, but I certainly don’t expect it.
3. It would be awesome if Jarred could include in his follow up study a third group – the physical exercise group, the control group, and a MENTAL effort group (playing chess, reading and trying to retain difficult material, debating, writing an essay, achievement tests, etc). I’m beginning to suspect that when I read, talk, study, think – I am as likely to crash as when I walk, run (hah!), etc. It would be really interesting to see if that bears out in a controlled setting. It might give us new insights and flexibility to help with “staying in our energy envelope.”
Thanks for all you do, Cort.
The NIH has been very niggardly with it’s ME/cFS (and FM) funding. I don’t think it’s just women, though. I think it’s women with invisible diseases like ME/CFS, fibromyalgia, IBS, interstitial cystitis, migraine – they are all horribly underfunded.
Honestly, though, I cannot imagine that the NIH will not fund Younger’s next study. This grant application reminds me of Ron Davis grant application involving Mark Davis. These grant applications are a kind of a test: they are so good that if the NIH can’t fund applications like that what will they fund?
I’m confident they will fund it – Younger’s grant – which is already in – fills all the NIH’s dots – exciting new technology and great results – and Younger will be able to do the stress tests – the exercise tests and/or mental effort tests – and see what happens to what happens to ME/CFS patients brains.
Stay tuned for the next blog for more exciting stuff from Younger. He’s a very creative researcher.
Honestly the reason they may fund male pattern baldness more may be that one study (multiple?) that linked it to heart problems, and because they don’t acknowledge that anyone with ME has died from the disease and people do die from heart disease maybe that’s how they justify it.
Or maybe they do consider men losing their hair to be more painful than losing your life, livelihood, family and housing to a debilitating disease? That would be awful. (I do understand there’s pain for anyone losing their hair but I’m sure we can all agree that it’s an unfair comparison to ME/CFS).
((Cort you may want to reconsider using that one word in your first sentance in the comment above due to its painful connotations for many people, even though I know you didn’t mean it that way there are many people who find the base of that word distasteful including those who are ill or allies and it would be a shame to alienate them over a word choice issue.))
I absolutely agree the NIH has done us wrong, of course and I feel that it’s plain as day from their funding, I am not even sure how they justify it. Also the history of takinf money from us for other illnesses, they also ignore the CCC and ICC exist in a bizarre way and I’m not sure if its an American-centric thing or what.
It’s clear women and other minorities work still is not considered equal in the US and other countries, and so when illness effects us it is considered to be less dire to fund the devlopement of tests to prove what many of us have been saying all along. Part of me wonders if the issue is that a lot of people with other illnesses seen to have Cfs as a secondary diagnosis and we tend to have multiple syndromes. This overlap means that they may see it as many of us will never be able to work so it’s easier to pretend at times that we don’t exist, though its not true obviously since people with cfs and multiple syndromes/illnesses work every day. I’m following the progress on endometriosis because previously the tests to prove and treat have been invasive and it does effect primarily women and they’re trying to make treatment, biomarker, and tests for it less invasive. Would be nice if there was a simple scan to register pain one day, though I’m not sure that will happen.
Absolutely no reason to remove the word “niggardly”
Why certain individuals take offence at certain words (yet accept a continuous barrage of four letter words in movies and on TV) defies logic. It is way overdue that certain members of society removed their “chips on their shoulders” niggardly
ˈnɪɡədli/Submit
adjective
1.
ungenerous with money, time, etc.; mean.
“he accused the Government of being unbelievably niggardly”
synonyms: mean, miserly, parsimonious, close-fisted, penny-pinching, cheese-paring, penurious, grasping, greedy, avaricious, Scrooge-like, ungenerous, illiberal, close; More
adverbarchaic
1.
in a mean or meagre manner.
I agree with Shy re the use of a certain word. The very similar-sounding word staring with an “n” has an extremely negative connotation, & the meaning of the word used in this thread can appear to imply that African Americans (& black people with other ethnic backgrounds) have the various characteristics listed in the actual definition of said word. Words are derived from other root words because they are related, so this is not a stretch, by any means. (True in this case, one word is not actually the root of the other, but not many people would know that, as they sound so similar.)
And, no I do not have a “chip on my shoulder” at all. I am also not black, not that that should matter re this subject. I do, however, despite prejudice in any shape or form, as well as the appearance of such. And, even if I didn’t, I agree that there is no point in alienating people when there are so many synonyms that convey the meaning as well, if not better as the chosen word.
Even if one thinks that “It is way overdue that certain members of society removed their ‘chips on their shoulders'” this thread is not the place to fight that battle. We already have a huge battle trying to get people to understand & research this illness. There’s no point in adding a red herring to that. Plus, the people who tend to take offense at a certain n-word have more than sufficient reason to do so & anyone with a heart (esp anyone with an illness that is also greatly stigmatized) should understand that, & should also find that word offensive. (And, again, yes, I know they are two distinct words, but there is clearly reason why many people would not know that).
I think in the future I will find a different word :).
I looked up the etymology – who would have ever thought an ME/CFS blog would lead to an etymological search?
Although the two words sound similar they have completely different etymologies. Niggardly comes “can be traced back at least to the Middle English word nigon, which has the same meaning, and is related to the Old Norse verb nigla, which means “to fuss about small matters”.[1]
The other word was taken Spanish/Portugese and French words meaning black and can be traced all the way back to the Latin term which means black as well.
That should read “despise prejudice” not “despite”.
And while we are at it, let’s make sure we never refer to the country Niger ever again. Also, no more nagging each other. Don’t want to be seen as a bunch of naggers.
Honestly, this weak victim culture is getting ridiculous. The word niggardly has nothing to do with that detestable N word. If someone takes offense to it, it is their fault for being ignorant toward its true meaning.
Cort,
What a wonderful article! I found this evidence found in research to be so validating for the absolutely bizarre cognitive issues I find happening to me that a person couldn’t possibly predict would happen! Who forgets how to read when they are an Avid Reader? Who forgets how to spell their name let alone how to form a letter that they have in their head as what they need to write? and I mean a letter of the alphabet. who in the world forgets how to talk, or become so fatigued from the slight amount of mental effort involved in a short conversation that they can’t stay awake 4 days afterward in any predictable fashion? I’m still suffering from an appointment last Thursday when my doctor’s office wanted me to fill out a six-page form on my pain. I have to do this every 3 months now because of all of the current hyper concern over ill people that happened to have opioids as part of a pain management program. The appointments exhaust me and I know that I can’t write without experiencing severe pem. I keep telling them I can’t write and they don’t seem to understand and they will not help me. that means I get about one page done before I’m no longer able to write in a legible fashion or even put marks on an anatomical drawing of where my pain is located. instead it looks like a one year old got a hold of an ink pen. I have physical symptoms 36 hours later this time I took pictures of my grotesquely swollen hand and sent them to my doctor. but my doctor isn’t here to see me fall asleep while dunking a cookie in a cup of coffee I was holding. I woke up really fast when the coffee spilled all over me and my bed though! And now I can’t move my right arm unless I put my shoulder and my ear angles to where they come right up to touching each other. for some reason when I use a muscle group and it becomes injured from overuse it seems to have some sort of a chain reaction type of phenomenon as the days go by. Overusing my hand went from my hand being swollen all the way up to my arm aching and my shoulder and my armpit the side of my neck and along the right-hand side of my scalp and the top of the shoulder area from the edge of the neck. The pain is absolutely excruciating and I just hope the Chain Reaction has gone as far as it’s going to go and now I can just work on healing. But these are for the most part caused by mental effort as well as the physical effort of actually using my hand. Inflammation in the brain as well as lactate I think that’s what you called it, as well as it being on the area that causes pain it certainly seems to fit with what I go through! I get so ill from just visiting with my family for half an hour from my bed! And I’m so happy when they come by and I get to see my grandchildren and my daughter and her husband! I laugh and have so much fun with the kids and just grin from ear-to-ear on seeing those big smiles they have for me! And they even give me hugs of their own accord! I don’t even ask for one because I don’t like to do that to kids. So when your four-year-old grandson grins from ear-to-ear and runs to you with his arms open and just throws himself onto your bed and hugs you with all its strength that should make us feel healthier because of the joy we feel inside! and isn’t smiling supposed to cause the release of certain chemicals in the brain? Why then am I so ill after the visit for probably 2 weeks? I can barely speak and can’t even watch a TV show without falling asleep. I gave up driving years ago because I kept falling asleep at the wheel! and I was only in my early forties when I gave up driving. I know now that it was caused by neuro cognitive fatigue. Finding inflammation in the brain with relatively simple to administer test we’re just be fabulous if it turns out to be as rock solid and dependable as it’s beginning to look! Such a thrilling discovery!
I am very glad that you can see that grammar is something that we should be more realistic about. So many people have become snowflakes and I think it’s a terrible mindset to be looking for insult in the most innocent of things. and to take something that is proper grammar and try to vilify it and the person who’s using it is ridiculous and impossible to avoid! Things are just really getting to the point of being afraid to say anything. I made the mistake of referring to something Asian as Oriental. Everybody about had a cow that was under the age of 20. Oriental had been a word that I known for my whole life that was often used in referring to something having to do with the Orient! when I was a kid every Friday we went out to an Oriental restaurant. I never had one negative thought when I heard the word oriental used as it meant we were going to get to go get some really good food and that lovely pot of tea in tiny cups they bring to the table!
Nobody sent a memo out telling me that that was a politically incorrect word now. I meant no harm. I don’t believe you meant any harm at all and it seems a bit hyper sensitive did anybody even took notice in the direction it was taken. Thank you again! you bring such interesting news and I love when I’m able to read your columns! I’m probably going to suffer for this one, as I’m already having some PEM hanging on., but it was so worth it!
I wonder if this is why taking Klonopin at night to sleep helps me feel so much better, it calms down the brain, not due to anxiety for me but due to this brain issue you mentioned. I think it should be part of the treatment for us all I even go through deep sleep with it at a low dose of.05 mg, without it I don’t ever go into deep sleep
Hi Katherine,
I read the whole piece and as always feel so grateful for all the information on this wonderful site.
Reading your comments I have to say that my experience shows there is definitely, for me at least, a massive link between any kind of ‘brain work’ and increasing pain and fatigue. I know too that any physical activity greatly reduces my ability to function cognitively and vice versa. In order to pace my limited energy I have to consider everything I do, both physical and mental. For example, a five min phone call can be as devastating as a five min walk etc.
I still haven’t mastered ‘pacing’ as my body continually moves the goalposts, but at least I feel I’m ‘trying’!
I have the same experience. Mental work can produce amazing symptoms. I read somewhere that the brain – the most energy intensive organ – uses up about 20% of our energy or something like that. If there is a metabolic problem in ME/CFS – and it sure seems there is – it makes sense that using the brain could have it’s effects.
It is odd that if I dance I do not feel fatigued at the time, and if I party and “banter” with friends I don’t get it. But if it is work, physical or mental, it is always worse. Could be there is some real healing in laughter and joy?
(by the way, I certainly do not dance or banter often or long!!)
For sure….Stress ignites pro-inflammatory immune factors. If Younger is right and the microglia in your brain are hypersensitized then the stress of working might be able to tweak them enough to start releasing those factors producing fatigue, pain, etc. On the other hand, laughter, joy, calmness calm down the microglia and give your parasympathetic nervous system – the relax and digest system – a boost which then calms down the sympathetic nervous system – the fight or flight system.
@Weeroo: it may be in part the effect of dopamine, the reward hormone. After reading “found evidence of reduced dopaminergic activity from a part of the brain (the basal ganglia) which activates the motor cortex.”
I decide to search for a link between dopamine and NADPH (oxidative stress related, NADPH regenerates glutathione). First hit https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4061571/: “D5 dopamine receptor decreases NADPH oxidase, reactive oxygen species and blood pressure via heme oxygenase-1”
So dopamine can reduce ROS and with it potentially inflammation. Enjoying a rewarding activity should increase dopamine. It’s however also the addiction hormone.
Combine it with the effect adrenaline has on many of us: more energy for a while and potentially more NADPH generation so less oxidative stress. Both could have a positive effect on ME patients, when used in moderation. But adrenaline makes us blind for exaggeration. And dopamine can turn something into an addiction. Are we chemical driven towards being adrenaline junkies? Is that why we have the oh so predictable “push through and crash cycles” which make us feel so silly afterwards?
Maybe you found the way in between too few and too much ;-)?
Katherine I complete agree about that cognitive group I really hope he gets a grant and decides to include them. Science and research are sometimes so frustrating how they prove something in small groups, especially with this illness which is so diverse it’s hard for me to take large studies that disprove earlier findings from small studies terribly seriously because of that.
It’s a situation of bizarrely the small studies seem more accurate to me personally sometimes. I strongly feel subtypes and definitions used can alter results, and that many studies done with weak definitions will need repeated for the more specific groups down the line.
I hope that a larger study verifies the earlier findings in this case. Also I think what’s novel was the study shows inflammation in the whole brain which wasn’t necessarily my first guess but it does help explain the long list of dysfunctions and symptoms this involves. It feels tragic that despite the name of the illness most docs will never believe without nice expensive big studies.
I’ve been icing my head to help my brain lately too. I haven’t heard this talked about too much until recently (but then I don’t wander the full depths of most forums, I’m a surface dweller).
Hi Kathrine,
Thank you for sharing the above, I fully agree with your observations in the comments. I find that “mental” stimulation triggers me to crash more and it takes much longer to recover compared to low/moderate physical and other “mindless” activities. Reading, writing and especially focusing, problem solving and as well as a “busy mind” are my most vulnerable forms of “activities”.
Thanks again for sharing.
When you think of how much energy the brain uses then a “busy mind” one of the weird byproducts of ME/CFS – must eating up a lot of ATP…
1. Asking people is not a scientific method and does not constitute proof. This level of inflammation is not treatable by the application of external ice packs any more than cancer can be treated by frozen lemons.
Thanks for your support Tamesin. This is a really exciting finding. I imagine we’ll going to be hearing from the big Japanese PET study soon as well. Younger’s is particularly noteworthy because of the non-invasive technique he and his team have developed.
Thank you Cort as always, sounds like an amazingly productive trip. I am greatful that they’re finding verification of the brain inflammation that’s been suspect for a long time in ways that are not invasive. I do wonder what the IOM criteria’s response to these studies will be since they claim the Japanese studies and none on brain inflammation exist in their summaries. I sincerely hope they issue a correction as many patients seem to use that criteria to educate their doctors, imperfect though it may be.
I wonder if the blood brain barrier also contributes to why many of us don’t seem to flag for inflammation on the tests they run in rhuematology? (So instead of absorption, not allowing the markers outside the brain somehow?) Not a Dr or scientist so this is all speculation on my part as I would like an answer to the question “Why don’t we show up generally as flagging for CRP?”
I would imagine its because that particular test doesn’t actually fit our inflammatory pathways which may be different from those of a traditional autoimmune disorder, hence we will hopefully have scans or a bloodmarker, perhaps from cytokine patterns or a future biomarker. Do we have a form of seronegative inflammation? My assumption is it’s a watch and wait situation. Does anyone know the answer who may be reading? I might just be using the wrong keywords when I try to search.
I think you’re right. Dr. Montoya has said that other types of inflammation are being uncovered and he suspects that we will have new tests for them.
In regards to the studies, are inflammation markers not being found on blood tests? I have a history of Kleine-Levin Syndrome, now at 29 years old I suffer from EXTREME fatigue daily. I had blood tests to check for inflammation and everything came back normal within range.
My GP found an elevated ESR, (erythrocytes sedimentation rate) and sent me to the rheumatologist who thought that the ESR was not a meaningful marker. She also gave me a prescription for a psychiatrist. … anybody else with an elevated ESR ?
“the rheumatologist who thought that the ESR was not a meaningful marker”
Maybe the rheumatologist is not aware that most people with ME (and FM?) have reduced blood flow, sticky blood and constricted veins. Under such conditions, small debris can reduce blood flow far much more in the capillaries then when one has normal blood flow, blood viscosity and vessel constriction.
Compare it with trying to flush a thick soup with pieces of beans through a small pipe of the sink compared to poring pure water through a wide clean pipe connecting the toilet.
Cort, thanks again for a great article and for keeping us up-to-date on the active research. I wonder if Younger and Dr. Systrom have ever spoken? I also can’t help but wonder how much faster we would find treatments that help if all of the information patients have gathered over the years of their illnesses as well as the patients’ hypotheses were considered?
That’s a great question. Note that Systrom is finding metabolic problems in the body, Younger and Shungu in the central nervous system, Ron Davis, Armstrong, McGregor, Hanson and others in the blood….At some point all this – if it works out – has to come together. It seems like the metabolic problems are body-wide.
Cort- couldn’t agree more….all of it should come together!
Much is tied together by Sickness Behavior, which has fatigue as a major component, but we all know the difference… When fatigued, it feels pretty good to lie down and rest. When sick, we lie down and wait to feel better. That’s the test & the way to spell it out to our doctors.
Turns out that we just need a combination of 3 main cytokines signalling to our brain to induce the sickness immobilization: TNF-a, Interleukin-1 & Interleukin-6. There are plenty of causes of inflammation signalling which cause those crazy-complicated cascades which typical have sickness behavior as one of their common endpoints, which is why viruses, bacteria, injury, etc tend to all make us feel the same. This article spells it all out: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2740752/
The catch is that there’s 2 signalling routes: blood circulation (slow route) & nerve stimulation (fast route). The nerves are activated in the periphery by paracrine/autocrine action & the cytokines don’t need to enter circulation at all. Our vagus nerve is a major route for this. Signals shoot right into our brains, which is gonna be hard to detect, right?
Post Exertion Malaise, at least the exercise kind, seems like it’s connected with Interleukin-6, which is both a cytokine & a myokine (muscle cell signaler). It’s released with muscle contractions, especially aerobic exercise & strength training. But this is more like the PEM we all know and love:
“As a matter of fact, eccentric exercise may result in a delayed peak and a much slower decrease of plasma IL-6 during recovery.” (From https://en.m.wikipedia.org/wiki/Interleukin_6)
IL-6 sounds like it’s normally a myokine, so its release into circulation wouldn’t be unusual, except when TNF-a (major inflammatory cytokine) is in effect. Then IL-6 acts as a cytokine if TNF-a is being released. IIRC, it enhances IL-1’s effect in causing sickness symptoms.
The thing is, most of this is just normal immune function. It just needs to be triggered continuously enough to keep sickness signaling going. And that can happen via too many ways to count. I’ve only covered the tip of the iceberg here & am forgetting to add plenty, but I just need to get started before I poop out… The links seem to bring together much more of what’s going on.
Oh, also since aging is associated with immune activation, it wouldn’t surprise me if a larger study showed some older people in the control group as having some inflammation too.
I’ll try to get back on this soon. Hopefully someone’s interested in this too… I’m seeing way too many arrows pointing to this to ignore.
Thanks Stephen!
Yes, the vagus nerve connection to the brain. We’ll see in the next blog that Younger has developed a new way to figure out where the inflammation in the brain came from. He too believes that there are probably dozens of ways to get those microglia sensitized.
I’m seeing Dr Robert Dantzer as a common author on several of these reports. Is he known to us? More links:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2919277/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1850954/
https://en.m.wikipedia.org/wiki/Interleukin-1_family
https://en.m.wikipedia.org/wiki/Tumor_necrosis_factor_alpha
Also, see if the IL-6 wiki page’s entry for Enterovirus 71 is interesting.
Very interesting and finally gives hope.
Thank you Cort for taking the energy and trouble keeping us updated.
I wonder if this brain inflammation shows up on an MRI?
I’m not all up on brain scans but I’m positive it will not show up on a standard MRI. You either need a PET scan with a tracer, or Younger’s method or the metal bit method. There may be some others – but not a standard MRI.
I can thoroughly concur with the brain-on-fire condition. Whenever I am pressured to think/act while I’m struggling, my brain actually feels as though it has caught on fire. This has been so extraordinarily nasty that I now refuse point-blank to put myself in any stressful situation or job. I will not do that to myself.
Cort, thank you for this exciting news, and for making the enormous effort and spending your time and energy on our behalf! You are a hero in my eyes! … Now, if only the funding comes through!
(Shy: Google the word… etymologically the two similar words are not related.)
Your excellent article mentioned groups who were collecting ME/CFS brains for autopsy. I have spoken briefly to Stanford, who expressed interest, but I’m hoping that perhaps you might be able to refer me to someone or the someone’s who might be most interested a “great” ME/CFS brain when I kick it. I have a rather severe case and would like to have inflammatory mess of a brain go to someone who could use it to further their work.
Younger mentioned that he thought that Stanford had some brains. There was brain autopsy done in San Diego I think it – for this case report – https://jim.bmj.com/content/65/6/974.full. I don’t know about others but I suspect some others are gathering them or in the process of being able to do that.
Yes, I wanted to donate my brain as well. However, when I looked into what my family would have to do to donate my body and/or just my brain to the Solve ME/CFS Initiative BioBank, I decided it was just too complicated. This was back in Feb. 2016, so maybe they have now made it easier. The contact I had back then was: VManohar@solvecfs.org. I hope this helps someone. I’d still like to donate my brain if they have been able to make things easier on the surviving family. (And, honestly, now I can’t remember what put me off–otherwise I’d tell you.)
Excellent. I’ve felt for a long time that brain inflammation is a key part of this illness, but there seemed to be a stalling in momentum. The Japanese have been doing some great stuff.
Cort you mention treatment potential. Is that on Jarred’s horizon? Hopefully it is.
Younger’s been looking at treatments that can calm down the microglia for some time. He just completed a study I believe which looks at one of them. He’s the guy that convinced the FM and ME/CFS world to give LDN a try so that kind of stuff is definitely on his radar.
There’s also this from Jarred…
https://www.youtube.com/watch?v=E14Ust5BN3c
Hi Cort. Thanks as always for your great articles 🙂 Do you know if Dr Younger still strongly recommends LDN as a treatment? Or has he moved on to other things? – I feel like I haven’t heard anyone pushing LDN for a while! – Is it still favoured?
LDN doesn’t work for everyone but it really helps some people and the studies have backed that up. LDN is here to stay. That’s not to say that something better won’t come along and he is looking at a variety of substances.
When diagnosed in early 1990’s had good insurance that covered tests to determine my level of disability. Neurological involvement was one of my ‘top 3 symptoms; as Cheney Clinic patient, then, I was referred to Dr. Myra Preston, PhD, who was doing Brain Mapping on ME/Cfids pts. My abnormalities were pronounced and part of my medical records that helped (after two denials) the court to approve my SSI Disability. Decades later, I am at a plateau w/relapsing brought about when a trigger of physical or mental stress occurs. Thanks for sharing this important research, Cort. Your blog is very helpful.
This is interesting…if I understood correctly…that chronic neuroinflammation burns up oxygen in the brain faster than if it wasn’t inflamed. I wonder if this could be another reason why so many of us have symptoms consistent with too little oxygen in the brain (both direct symptoms of low oxygen, like faintness, as well as symptoms that could be attributable to the body trying to increase blood flow, like fast heart rate)?
This also implys that anybody who is suffering from both neuroinflammation and poor oxygen delivery to the brain for any other reason might be in double trouble.
For anyone who’s interested in related ideas from the perspective of functional medicine, Datis Kharrazian’s book, “Why isn’t my Brain Working?” has a ton of information about various links between many inter-related subject matter like neuroinflammation, neurological autoimmunity, neurotransmitter imbalances, and low brain oxygen levels, and the factors that are currently thought to often underlie those circumstances, such as GI inflammation, leaky gut, neurotoxicity, vagus nerve dysfunction, and dis-glycemia, amongst others.
I am super happy whenever I see ME/CFS researchers validating such important aspects of this condition. But it also feels important for those of us in this community (which can often feel somewhat isolated/marginalized, comparatively) to be aware that many of the specific findings that this field’s experts end up zeroing in on (including neuroinflammation) are turning out to be the very same factors that practitioners in functional/holistic/naturopathic medicine have been pointing to (for years, and often decades) as the key underlying conditions in the cause and perpetuation of most chronic illness. Gratefully, this also means they have accrued a lot of ideas about how to address/reverse these factors right now (and not only after more research is done).
(This is not to imply that those practitioners will ultimately prove to be correct in all of their theories and deductions, just to say that anyone who is looking for interventions to explore right now will find more material for experimentation within those “alternative” conversations.)
I view studies like these as solid hope for the diagnostic clarity and future targeted treatments that we so desperately need. But also as further encouragement to listen even more carefully to the other branches of the medical field, which generally get so much less respect, and therefore much less press.
I always find myself wondering…are smart, open people like Jarred Younger even aware of the work being done by people like Datis Kharrazian…and vice versa? And how much faster would we progress if there was solid and steady cross pollination…?!
Thanks, Cort, for another great report!
Thanks. One thing I really like about this finding is that it fits so well with other findings. Yes, there’s inflammation but the presence of lactate also indicates that the brain is relying on anaerobic metabolism to produce energy. That’s the same thing that’s happening in the muscles! My understanding is that that means either the brain is not getting enough oxygen, it’s ability to use oxygen is limited or something (inflammation) is using up the oxygen.
Thanks for the book tip 🙂 Your comment on functional medicine makes me think of Bredesen who is apparently doing pretty well using that approach with Alzheimer’s (!)
Yes, Dr. Bresden seems to have had better success having his ideas take hold…maybe because he’s addressing an illness that is causing so much undeniable suffering? Or maybe because he is a traditionally trained physician (MD)?
But it seems like they’re all circling around the same set of basic ideas…
Just today (while researching SIBO-related issues) I ran across the work of Dr. Nemechek, an Osteopath out of Arizona, who places microglia activation/sensitization at the root of autonomic nervous system dysfunction. He has designed a treatment protocol to encourage neurological regulation and healing by addressing GI inflammation (most specifically SIBO) and microglial activation, and using vagus nerve stimulation to help re-set the system.
https://www.nemechekconsultativemedicine.com/screencasts/
(Ignoring the self-promotional flavor to his writings/videos) I find his perspective very interesting, especially as it is so rare for anyone to directly addresses the potential of neurological damage (specifically sensitization of the microglia and damage to the ANS) from so many varied sources (viruses, head injury, trauma, vaccines, etc.)…many of which are very familiar to this group.
Awesome Cort – thanks for breaking it down for us!
🙂
Is there any research being done to determine what homeopathic remedies could be used to bring the brain inflammation down? I had not made the connection with the left side of my head bothering me and inflammation when I am under extreme stress or fatigue. This makes complete sense to me now and I will start researching the brain and neuro-inflammation. For me personally, mental stress, fatigue and gut distress go hand in hand with my CF and Fibromyalgia and is always preceded by tinnitus and head pain. Cort, keep up the good work. We need all the help we can get.
I think a lot of people immediately discard homeopathy but I met one person who had been ill for over a decade who tried everything she could and it was a specific homeopathic remedy that worked for her. Similarly I heard of a person with M.E. married to a top researcher who similarly tried everything and homeopathy returned her to health.
I wonder if Dr. Younger and Dr. Klimas should be working on this together? I know Dr. Klimas was (or still is) studying Gulf War patients, where she was going to reduce neuroinflammation, then block the HPA axis long enough to force it to do an internal reboot. I don’t know what drug she was going to use to reduce neuroinflammation, but she was going to reboot the HPA axis with Mefipristone.
Yep….first she hits the inflammation with Etanercept – after that drug is given to do its job then she hits the HPA axis with Mifeprestone. That’s what her models say should work.
Cort, do Dr. Klimas’ models say that method should work for ME/CFS or are you referring to GWI? I’m hoping both!
I don’t think etanercept crosses the blood brain barrier
Keto diet suppresses brain inflammation – thoughts?
https://www.google.co.nz/amp/s/articles.mercola.com/sites/articles/archive/2017/10/09/amp/ketogenic-diet-anti-inflammatory-effects.aspx
So does IV NAD+ and glutathione, oxaloacetate and lactate. I felt much calmer when doing my 3 month keto experiment. I crashed much less. It’s just for the first 6 weeks while your body is adjusting to using fat for energy I had very little energy. Felt improvement after that though.
Fascinating! A couple years ago my PA prescribed Meloxicam when we thought maybe I had RA and it was going to take months to see a rheumatologist. I don’t have RA and the inflammatory markers they tested were all negative. I’ve always wondered why the Meloxicam helps my fatigue so much if I supposedly don’t have inflammation. Well, now that makes sense.
Something another commenter said about icing her head caught my attention. Summer heat absolutely debilitates me. I’ve tried wearing ice cubes in a bandana around my neck, but tying an ice pack on top of my head is far more effective (even though it is counter to the common advice to cool your neck to cool the blood going to the brain). I wonder if my issues with heat are related to the brain inflammation or if it is something else. Curious!
Thank you Cort, this is such hopeful news! I can’t believe how much the research field has changed in the last couple of years. It’s so encouraging.
Agreed! With Ron Davis, Ian Lipkin, Unutmaz, Armstrong, Hanson, Scheibenbogen and others in the field things seem to be breaking in the right direction…
yay for Jarred! (and us ;))
think next up should be finding newbie patients to show they inflame right away and not after years of being a couch potato (just thinking of what the Wessely’s of the world’s response will be)
and my guess for the anearobeness would be EBV and other creatures that seem to thrive on low oxygen, but am prolly repeating myself…
Good point…That would be a great study. I too think viruses are still in the mix…..
I think the teqnique and method from Younger has not been accepted by neurologists at this moment. That is a real problem, also the brain problems are most likely secondary due to bloodflow issues. I don’t understand why the pet scan isn’t confirmed yet.
Good point. Thermal mapping of the brain is accepted and used; its the technique to thermal map the whole brain that is new but Younger already has some validation in the form of the lactate signals which are overlapping with the temperature increases. So while its true that the technique is new, hopefully acceptance won’t take too long.
It has taken the Japanese a long time to get that PET scan done that’s for sure. If it really was 120 people it must have been horrendously expensive.
Thank you for this article. This is my main problem anymore, the inflammation in my head is so bad and I don’t explain it well. They come back with ‘headache’ even though I keep saying it’s not a headache. We left that station a long time ago. It would have been nice to know there was research going on in Alabama when I was living close-by. I’d like to be informed of the progress if there is a list to join.
I’m a veteran. I’ve been suffering with many symptoms – chronic fatigue and fibromyalgia. I have had numerous MRIs and my white matter lesions have increased from “several” to “30-40” in 6 years. Also have abnormal lumbar puncture results – increased IGg synthesis rate and increased protein but no oligiclonal bands. I’m very interested in any treatment possibilities because I usually feel like I did an extreme workout, and my muscles feel acidic, and my head is burning up (mostly at night). Thanks for the info!
I don;t know about treatment – except have you tried LDN? Any other microglial inhibtors? Anti-inflammatory treatments?
You are similar to many I think – they find something but it doesn’t fit normal disease patterns…
bless you for all this information. It gives me hope that my 48 year old daughter with Chronic Fatigue for the last 5 years may someday get her life back. Meanwhile we can try icing the head for maybe some relief. So thankful her husband and 12 year old son are supportive
I am 73 and have had Fibromyalgia for about 20 years. Could I have passed a bad gene to her in the womb?
Not many studies have been done but it appears that genetics plays at least something of a role in both FM and ME/CFS. It takes more than genetics – I have an identical twin who is healthy – but the genes are in there somewhere.
My mother had a severe case of Sjogren’s Syndrome and someone on her side of the family had severe chemical sensitivities.
We’ve had two cases in England where autopsies of ME patients have revealed inflammation of the basal ganglia in the brain. This may be linked.
Just one thought; wouldn’t elevated lactate levels in the brain show up in cerebrospinal fluid analyses?
I would think but I don’t know. I don’t know if anyone has looked. Both Shungu and Younger used spectroscopy to look chemicals.
Your reference to spectroscopy brings this to mind i.e. use of Raman spectroscopy to diagnose ME/CFS using blood cells.
1) abstract: https://pubs.rsc.org/en/content/articlelanding/2018/an/c8an01437j/unauth#!divAbstract;
2) full paper: https://sci-hub.se/10.1039/C8AN01437J.
I’m surprised that this hasn’t been picked up more widely i.e. since it’s a potential blood based diagnostic test. It’s based on Chris Armstrong’s, and Fluge’s and Mella’s, finding that there is a metabolic shift to using amino acids for fuel. Ron Davis has tried to find the blood compound/s which change the metabolism; that could help to understand mechanism/treatment.
I recall that you did an article on Shungu’s work. He used MRI/MRS to measure lactate [high] and glutathione [low] in the brain. MRI/MRS is a non-invasive technique (no tracer); however, the range of compounds which can be detected is low, and the sensitivity isn’t great, hence PET is also used.
I’m not a scientist by the way; I have a family member who’s affected.
Wow, what a comprehensive article, Cort. Here are some of my thought.
1. Lactate is a brain fuel. I did some googling and found one article that says that the lactate uptake of brain increases during strenuous exercise. Maybe the brain is so sensitized to somatic exertion/inflammation that it increases lactate uptake even during a minor exertion, resulting in brain inflammation and CFS symptoms.
2. If immune cells can breach BBB, so can nano-viruses. Is there any evidence of elevated beta amyloid in CFS patients? If not, maybe it is just the brain’s lactate uptake that’s causing inflammation, not the BBB breach.
3. The brain inflammation model still needs to explain PEM that is delayed by 24-48 hours and lasts 3-5 days. If the brain inflammation is triggered by somatic inflammation via lactate uptake, that could explain PEM perfectly. Somatic inflammation comes in waves after an exercise, with some cytokine spiking up after 24-48 hours.
Interesting that post-migraine symptoms resemble ME/CFS – period of high fatigue; could hypoxic events be triggering problems that take days to resolve?
Years ago Baraniuk thought he found evidence of amyloid proteins in cerebral spinal fluid study…
Good point, maybe migraine is causing the inflammation in certain parts of the brain as the Japanese researchers described to cause the postdrome symptoms. PEM though is “worsening of symptoms after exertion”, so we need a mediator between exercise and the worsening of the brain inflammation after 24-48 hours. But I guess Dr. Younger will have to establish first that the brain inflammation gets worse 24-48 hours after an exertion, and then figure out what the link is between exertion and the worsening. I’m glad your partner brought that up!
This is a good reminder that I need to spray my head with pain spray and put ketamine and lidocaine lotion on my neck and upper back when they’re aching – which is most days. Also, I need to relax and laugh as much as possible and not work for long or too hard.
Thanks for your hard work, Cort. It is much appreciated.
I did neurofeedback for a year. One of the principles is that neurofeedback calms the brain ( very elementary explanation for sure ). It made a significant, transformative difference. I have practically no anxiety – and the most obvious of my cognitive symptoms have been cut by 2/3rds. Curious to understand if there is a relation to the inflammation
Congratulations Noelle! I suspect there is. There’s probably some sort of feedback loop going on – the inflammation tweaks the neurons in the central nervous system – causing anxiety – which causes more anxiety – and more inflammation. I imagine there could also be an autonomic nervous system connection – again produced by inflammation working on the brain.
Has it helped with fatigue and the ability to exerise? What kind of neurofeedback did you do?
My daughter is 38, hasn’t worked in 2 years, I’m going totally broke supporting her and trying to get her health care by a Doctor to feel better. I know she won’t feel better and this is just her life now, but hopefully they will find a cure or something to make it better.
We live only hours from Alabama and would like to know if she could be in any study, or recommend any solutions for us here.
Hello, while you wait to find a doctor that might know something at least check out Dr. David Hanscom, MD in Seattle…
I think after 20 years of daily misery and pain I might have stumbled across an answer
I have debilitating CFS yet if I take aspirin, within 15-20 mins, I am up out of bed and fatigue is gone, and I have good energy.
Sounds like inflammation is a big deal for you. How long does it last?
The fatigue has lasted now for 4 months, although I’ve had it on and off for shorter periods over the years. I’m feeling a bit scared because my memory has become poor and I now have intermittent pain in my head, which is unusual. Will a strict anti-inflammatory diet help?
Thank you so much for replying.