Could the microvascular system – the small capillaries and the red blood cells that run through them – hold the key to energy problems in chronic fatigue syndrome (ME/CFS) and perhaps fibromyalgia? The idea that the blood delivery system – not some metabolic derangement per se – is causing the problems with energy production is not new.
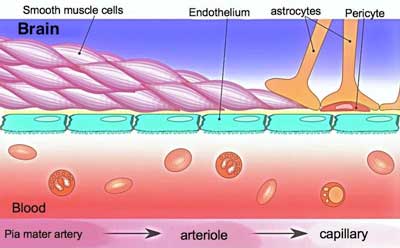
At first Shungu thought ME/CFS was a mitochondrial disorder but his studies convinced him it was probably more a disease in which poor microcirculation impaired the ability of the mitochondria to produce energy.
Over a decade ago, brain scans from Dr. Cheney’s ME/CFS patients caught Dikoma Shungu’s eye. The only other time he’d seen lactate levels like that before was in people with primary mitochondrial disorders.
Three vital seed grants from the Solve ME/CFS Initiative allowed Shungu’s work to proceed – work that is still going on today and that’s mushroomed into millions of dollars in funding from the NIH. That work has produced one of the most consistent findings in all of ME/CFS research: Shungu’s five studies have all found increased lactate levels in ME/CFS patients’ brains.
Over time Shungu developed a startling hypothesis. While ME/CFS brains may look like they have a mitochondrial disease, they don’t. That doesn’t mean the mitochondria are working well – they’re not – but they’re not underperforming because of a metabolic problem. Instead, Shungu believes they’re getting pummeled by oxidative stress, resulting, at least in part, from a dramatic decline in the antioxidant system that’s designed to keep oxidative stress in check.
Shungu believes that an immunological change or pathogen triggers the production of pro-inflammatory cytokines and the potent free radical peroxynitrite. When the muffled antioxidant system fails to mop up peroxynitrite, the free radical smashes into and rips up the lipid membranes of cells, and in doing so forms a major menace in ME/CFS – isoprostanes.
Potent vasoconstrictors, the isoprostanes then compress the blood vessels, reducing blood flow and producing a hypoxic or low oxygen environment. That low oxygen environment forces the cells to rely on anaerobic energy production.
In Shungu’s view, then, ME/CFS is not a mitochondrial or metabolic disease; its much simpler than that – it’s an oxidative stress induced micro-circulatory disease. The oxygen the muscles and other energy intensive tissues such as the brain need to produce abundant amounts of energy? It’s there, but it’s just not getting through.
Shungu is not alone in his belief that circulatory problems, not the mitochondria, play a key role in the metabolic problems in chronic diseases. A 2018 review of skeletal muscle performance in metabolic diseases proposed the same idea.
… it may be hypothesized that the primary site of dysfunction with earlier stages of metabolic disease may lie at the level of the vasculature, rather than at the level of the mitochondria. Frisbee et. al.
He also lamented the lack of attention given to the role that problems in the microcirculation may play.
While there has been extensive effort dedicated to determining the major factors that contribute to the compromised performance of skeletal muscle with chronic metabolic disease, the extent to which this poor outcome reflects a dysfunctional state of the microcirculation, where the delivery and distribution of metabolic substrates can be impaired, versus derangements to normal metabolic processes and mitochondrial function, versus a combination of the two, represents an area of considerable unknown. Frisbee et. al.
An Oxidative Stress Disease?
Nor is Shungu alone in suggesting that increased levels of oxidative stress may be playing a key role in ME/CFS. Over a decade ago Martin Pall’s NO ONOO hypothesis – which Shungu’s work is partly based on – proposed that runaway oxidative stress is causing ME/CFS.
The oxidative stress data in ME/CFS is abundant and remarkably consistent. So many studies have found increased levels of one or more free radicals in ME/CFS that Michael Maes, the foremost proponent of the oxidative/nitrosative stress hypothesis in ME/CFS, has proposed redefining ME/CFS as”Neuro-Inflammatory and Oxidative Fatigue (NIOF)”.
Many of Maes’s studies suggest that oxidative stress plays a major role in ME/CFS, including a 2011 study in which Maes differentiated people with ME/CFS from healthy controls using levels of plasma peroxides. Other researchers have had similar results.
Recently, measures of oxidative stress were able to differentiate people with ME/CFS from healthy controls. A 2010 study found significantly higher levels of the free radical malondialdehyde in ME/CFS patients. Five years ago, Meeus proposed high levels of reactive oxygen species (ROS; which cause oxidative stress) were behind the pain in FM and ME/CFS. Jason suggested that low antioxidant levels could be affecting HPA axis functioning. The list goes on and on with at least 8 more studies from 2005 to 2010 found elevations of reactive oxygen species after exercise or at baseline in the muscles and blood of ME/CFS patients.
San Jose University/Stanford Study
Now comes an Open Medicine Foundation funded San Jose State University/Stanford study overseen by Ron Davis which manages a trifecta by bringing oxidative stress, red blood cell problems and the microcirculation together. It’s not the first study to suggest that something is wrong with the red blood cells in ME/CFS. Way, way back in 1989, New Zealand researcher Les Simpson came to this conclusion:
“Samples from subjects with myalgic encephalomyelitis had the lowest percentages of normal red cells and the highest incidence of cup forms. The results provide evidence that myalgic encephalomyelitis has an organic cause.”
and even suggested the altered red blood cell shapes could constitute a diagnostic biomarker. A 2003 study that brought news of increased oxidative stress in ME/CFS patients RBC’s, was followed up by a 2004 De Meirleir and and a 2007 study which suggested the same. Things seemed to be hopping with regard to free radical damaged RBC’s in ME/CFS, but except for a small 2010 study from the Griffith’s group in Australia which found no evidence of red blood cell deformability in ME/CFS, that was it for the next 11 years.
Erythrocyte Deformability As a Potential Biomarker for Chronic Fatigue Syndrome. Amit K Saha, Brendan Schmidt…Anand K Ramasubramanian and Ronald W Davis. Blood 2018 132:4874.
“Altered microvascular perfusion can be a possible cause of ME/CFS symptoms.” Saha et. al. 2018
It was a small but comprehensive study – the type that Ron Davis seems to prefer. Rather than throwing a few measures at a lot of patients, Davis seems, once he thinks he’s onto something, to prefer throwing the kitchen sink at smaller numbers of patients in order to dig as deeply as possible into the problem.
Red blood cells carry oxygen from our lungs to our cells. They’re also quite susceptible to free radical damage.
Because red blood cells (RBCs) actually scavenge free radicals, they’re particularly susceptible to damage by them – including changes to their shape. Because RBCs carry the oxygen mitochondria need to power our cellular engines, it’s essential that they quickly and efficiently get from our arteries to our capillaries where oxygen transfer occurs. In order to do that, they need to be smooth, rounded, and above all, elastic.
Once they deliver oxygen to our cells, our RBCs serve as cellular cleaners by removing CO2 – a waste product – and passing it to our lungs where we exhale it out.
The Study
Mimicking how our red blood cells operate in our capillaries, the San Jose State University group, lead by Anand Ramasubramanian, PhD, recorded how long it took RBCs to enter two differently sized tubes and pass through them, and how elastic they were (how much squishing they tolerated as they squeezed through the tubes). The “squishing” the RBCs go through increases their surface to volume ratio and allows them to pass more oxygen to the cells.
ME/CFS patients’ RBCs struggled more to get into the tubes (~12%, p<0.0001), took longer to get through them (~17%, p<0.0001) and deformed less (lower elongation index, ~14%, p<0.0001). The highly significant data (p<0.0001) indicated that the deformability problems were very consistently found in the ME/CFS group. The findings suggested that in ME/CFS patients, the red blood cells were likely having trouble delivering as much oxygen to the cells as was delivered in the healthy controls.
The Gist
Shungu’s brain findings suggest high levels of oxidative stress and low levels of antioxidants are constricting the blood vessels.
The blood vessel constriction is producing a low oxygen environment which forces the cells in the brain to rely on anaerobic energy production.
High levels of oxidative stress are amongst the most consistent findings in chronic fatigue syndrome (ME/CFS).
Our red blood cells provide the oxygen our cells need to do their work. In order to flow properly through the capillaries to our cells they must be round and elastic.
The SJSU/Stanford study overseeen by Ron Davis suggests that ME/CFS patient’s red blood cells are often no longer round and take longer than usual to enter the capillaries and flow through them. Their membranes are stiffer than usual as well and they contain high levels of free radicals.
The red blood cells still contain normal levels of hemoglobin but their distorted shapes and inelastic and damaged membranes may be keeping them from delivering normal amounts of oxygen to the cells.
The red blood cell issues and Shungu’s findings suggesting that narrowed blood vessels are creating a hypoxic or low oxygen environment in the brain provide another possible way to explain for the low energy problems in ME/CFS.
Three more grants by the Open Medicine Foundation and Ron Davis will attempt to further characterize the red blood issues in ME/CFS with an eye to producing a cost-effective biomarker.
Then they found that ME/CFS patients’ red blood cell membranes were 30% (30%!) less fluid (30% stiffer, less elastic) and were consistently (p<0.008) packed with 30% more reactive oxygen species (free radicals).
Digging even deeper, the researchers used scanning electron microscopy to find all manner of strange RBC shapes and other issues (biconcave disc, leptocyte, acanthocyte and burr cells; area and aspect ratio; levels of RBC aggregation) in ME/CFS patients’ RBCs. The fact that recovered patients’ RBCs were normal suggested that the disease was indeed associated with RBC abnormalities.
Finally, as was probably suspected, hemoglobin levels and arterial saturation were normal – the RBCs were carrying their full load of oxygen.
The question then became whether the oxygen was getting through. The problems with deformability suggested perhaps not. The authors proposed that free radicals were damaging the membranes of the RBCs, stiffening them up, and impairing their ability to flow through our capillaries and deliver their vital loads of oxygen to our muscle, brain and other cells. No wonder everyone is so tired!
The results of this study seem to jive nicely with Shungu’s hypothesis that free radicals are constricting the blood vessels to create a hypoxic (low oxygen) environment which fosters anaerobic energy production in ME/CFS.
Combine narrowed blood vessels with damaged red blood cells and you seem to have another very possible explanation for the mysterious energy problems in ME/CFS.
Treatment Implications?
The treatment implications of the study are unclear but they seem to be familiar: find a way to stop the inflammation and oxidative stress while boosting the performance of the antioxidant system. At the last IACFS/ME conference, Shungu reported he used NAC to good effect in ME/CFS and both Dr. Klimas and Theresa Dowell RN are reporting good results with intranasal glutathione to boost brain antioxidant levels.
Engineers Enter the Fray
Who would have ever thought that engineers might hold an answer to ME/CFS? In another demonstration of Davis’s drawing power, a team of engineers has entered the fray. Four engineers participated in the three-day Working Group session that occurred prior to the Stanford Symposium. Bringing an engineering precision to the discussion, they pushed the group with tough questions at times and participated heavily. It was gratifying and quite frankly surprising to see how fully engaged the group was.
Giving the study’s findings, it’s no surprise that the Open Medicine Foundation (OMF) is funding more red blood cell work. The OMF is funding three engineers to write more red blood cell grants. The end goal is familiar to those who know Davis’s work – create a low-cost diagnostic device for ME/CFS.
Creating devices like that is Davis’s forte. Rahim Esfandyarpour, an engineer in Davis’s lab, used microfluidics, electronics and inkjet technology develop a “lab on a chip“. The remarkably low-cost chip can perform complex, minimally invasive analyses of single cells without specialized equipment and personnel. Now Davis is attempting to do the same for the red blood cells in ME/CFS.
The first Open Medicine Foundation grant is to a chemical engineering team lead by Eric Shaqfeh, PhD that will create a 3D model of blood channels which will be able to better characterize what’s going on with the red blood cells in ME/CFS.
That group’s results will be used to assist Anand Ramasubramanian, PhD, another chemical engineer, in developing a “microfluidics” chip that will be used to distinguish the ME/CFS RBCs from those of controls.
Finally, Juan G. Santiago, PhD, a mechanical engineer who participated heavily in the Working Group sessions, will develop an imaging device which will be able to automatically identify and track the position and shape of thousands of RBCs under relaxed and stressed conditions.
The end goal of all this – a diagnostic chip for ME/CFS. If ME/CFS needs anything, it’s a diagnostic biomarker. Let’s wish them luck.
Serendipity strikes?
The red blood cell findings were wholly unlooked for in more ways than one. Ramasubramanian’s group at San Jose State University (SJSU) came out of nowhere. SJSU is down the peninsula from Stanford but they weren’t on Davis’s radar at all. Instead, Davis was on their radar. Davis has turned out to be an ace at recruiting researchers, but he didn’t recruit them – they recruited him.
To their credit, Ron Davis and the Open Medicine Foundation recognized a good thing when they saw it. Davis is a molecular biologist – not a hematologist – yet he apparently immediately recognized the potential significance of this group’s findings and he and the Open Medicine Foundation supported them.
Conclusion

Red blood cell and blood vessel problems provide another possible way to explain the energy production problems in ME/CFS.
The exercise and other studies indicate that people with ME/CFS have an oxygen problem. Either their cells are not getting enough of it or they’re not able to use it.
Shungu has shown how oxidative stress may be constricting the blood vessels and creating a low oxygen environment in which anaerobic energy production dominates. Now this finding of deformed blood cells, damaged membrane, and increased oxidative stress, points to another way energy production may have gotten whacked in this disease.
However it turns out, it feels like this field is slowly starting to come together – cohering around issues of inflammation/oxidative stress, energy production and cardiovascular issues.
What we obviously need is more and more funding and more and more research. The grants by the Open Medicine Foundation stand out because for once an organization actually had the money to quickly follow up on an exciting finding. That’s rarity in this field.
The lightning speed with which the OMF provided these grants indicates how important nimble private research foundations like the OMF are to this disease. No year-long waits are needed to see if an NIH grant application got funded. Private organizations like the OMF, the SMCI, MERUK, the Simmaron Research Foundation and others have the ability, if they’re given the funds, to quickly follow up on promising findings. The SMCI, for instance, kept Shungu’s research alive with three successive small grants which allowed him eventually to get two large NIH grants.







I definitely go with the idea that the constricted vessels and lack of oxygen delivery in combination with huge body wide oxidative stress are at the core of our disease.
I’ve been writing lengthy comments/rants about it on Corts blog for some time. Now I’m aiming at a more exact route as to how this rampant body wide oxidative stress impacts our biochemistry and blocks our energy production and making good progress.
I definitely am glad fellow engineers took on the battle, and healthy ones with full capacity none the less! In a systemic disease where the larger system and physics/mechanics/material strength comes into play engineers can certainly add quit a bit to fill in some of the missing pieces.
I do believe that about all of the described things in the blog hold true except for one major thing: it is but one major component of this devastating systemic disease. But understanding it and modeling it is vital! It takes great doctors like Ron and organizations with vision like the OMF to take such unconventional route. Many Many thanks for that!!!
In case anybody that could put it to use does read this, I’ll add what I believe are a few under investigated related points.
“the RBCs were carrying their full load of oxygen”
In situations with rampant oxidative stress and depleted anti-oxidant defenses, as described in the blog, this statement is very unlikely.
Hydrogen peroxide has quite a strong affinity to RBC. One molecule of hydrogen peroxide that is bound to a RBC blocks 3 other positions for a total of 4 positions that could hold molecules of oxygen. That is: 1 molecule of hydrogen peroxide is firmly attached to one position and it makes 3 molecules of oxygen very firmly attached to the RBC.
As the 1 molecule of peroxide makes 4 positions unavailable for frequent O2/CO2 uptake and release each 1% of peroxide bound to RBC decreases oxygen blood carrying capacity by 4%. That’s crippling during anaerobic exercise!
As 3 out of 4 of this positions show up as carrying oxygen it does require tests to take into account that those 3 are not “oxygen” but “dead weight oxygen” that do not serve us at all. It’s a bit like a person sitting all day on the same seat on the subway to keep warm in winter: in effect it’s a lost seat for transportation.
Peroxide is well known to bind to RBC in case the anti-oxidant defenses are depleted, a state described in the blog. So investigating it may be worth it.
As peroxide acts a bit as “the small brother of carbon monoxide”, overbreathing or “flushing the peroxide out” by “washing them (in the lungs) with large amounts of air saturated by oxygen” is a reasonable strategy of the body to get rid of it. That likely is part of the reason why we “hyperventilate”.
In situations with rampant oxidative stress and depleted anti-oxidant defenses, as described in the blog the RBC will be under heavy attack by the high oxidative stress. It is considered as one of the reasons why our RBC are less flexible. That in turn reduces blood flow and forms a vicious circle.
But it gets worse. Heme, the iron complex binding the oxygen in hemoglobin (and myoglobin in the muscles) is very vulnerable to oxidative stress. To be more correct, heme and what it is embedded in…
Heme is essential to good oxygen delivery. But damaged free heme generates copious amounts of oxidative stress everywhere it is. So, we have got another vicious circle: oxidative stress damages heme creating free heme creating more oxidative stress.
But it still gets worse: under situations of heavy oxidative stress the body tries to reduce the amount of heme in the body. As such, less can be damaged and (future) oxidative stress can be reduced a bit. As RBC hold much hemoglobin and that holds much heme one way to reduce heme in the body is to reduce the amount of RBC. In our bodies, many of us have normal RBC per volume of blood but strongly decreased blood volume levels (around 70% of normal). I don’t say this is the reason why, but coincidentally it results in 30% less RBC bound heme in the body.
But it still gets worse. One step in the Krebbs cycle vitally depends on another sort of heme. If this same heme gets damaged (by oxidative stress) then oxidative stress production gets rampant !in the mitochondria!. That gets us very close to the CDR effect seen by Naviaux.
On top of the strongly increased peroxide generation in the mitochondria due to this mechanism, it is logical to assume the body tries to reduce the amount of heme in the mitochondria too in order to get this source of rampant oxidative stress under control.
Result: more oxidative stress and less NADH/ATP production in the mitochondria (due to less heme and due to more half-damaged heme functioning less good). That’s enough to sustain another sort anti-oxidant depletion/rampant oxidative stress vicious circle.
Catalase and peroxidase, two prime anti-oxidants able to convert/remove massive amounts of ROS per molecule of catalase or peroxidase, also contain heme.
If the body would, due to body wide oxidative stress, also reduce heme for these two key enzymes then we have another nasty vicious circle.
One can argue that the body would be stupid to take away heme from those two key enzymes as it obviously would make oxidative stress worse. But made at that stage we are already at a point where, in near all of human history, people that sick would have lived very shortly.
In a farm based society, they would have failed to survive winter. In a hunter gatherer based society they would have been left behind as they could not walk from camp to camp each day and carrying them for weeks would be too costly.
Harshly said, maybe people close to ME patients died too easily in order to guide evolution so human kind didn’t evolve a mechanism to survive long term a situation that anyhow would cause short term dead.
Can you comment on how this would impact iron levels and might cause iron deficiency anemia? I’ve had ME/CFS for 14 years. The only two labs I’ve ever had that were off, were low iron and high Carbon Dioxide at the onset, and low iron and RBC labs that indicate iron deficiency anemia in the last few years. Iron levels and RBC were ok in the years in between.
Would taking iron supplements be a helpful or harmful thing based on this new theory. If I understand correctly, I think it might be both.
Hi Rae,
I didn’t respond to your mail as I didn’t knew yet how to feel on iron deficiencies. Cort gave me a clue in his latest blog.
My GUESS is it might indeed be both. See my comment to Cort in http://www.healthrising.org/blog/2018/12/14/healing-chronic-illness-with-nutritional-balancing-dardens-me-cfs-and-fm-story/#comment-843478.
See also
“Iron eaten in foods has no effect on the levels of iron that accumulate in the brain. It is the haemoglobin [sic] levels, rather than iron that need to be tackled,” Dr Bangham said. “Iron is vital for the body, and should not be reduced in the diet.”
in Cort’s link http://www.specialtypharmacytimes.com/news/the-impact-of-hemoglobin-levels-on-multiple-sclerosis
This doctor suggests that under normal and maybe even under MS conditions eating iron rich products should not be avoided. I too believe it is the damaged heme that is problematic.
Can it make things worse then comes down to: does increased iron supply lead to increased heme production? My guess is: yes, to some extend.
Is that a bad thing? My guess is: it could be both. It will depend on the question: will the body increase the stock of heme in the body or will it reduce the “life time” of heme in the body while keeping heme levels at a constant low level?
In the latter case more heme would be recycled in I think the spleen, no need to look it up now. By recycling it earlier the percentage of damaged heme would be reduced.
In reality I think it will be a bit of both: a bit more blood volume and a bit higher heme recycling speed. Both MIGHT balance roughly out.
I think trying and reducing oxidative stress is the prime thing to do here, more so then changing iron intake. Even in order to increase blood iron levels in the long run. If you’d decide to increase iron intake it might be wise to take it in a more natural food form as it will be complemented by some vitamins and enzymes to optimize its use (and disposal?) in the body. Also, as it would likely increase blood volume to some extend it would allow you to do a bit more. Don’t!! as that would generate more oxidative stress risking to backfire.
If you Pace for a long time at pre iron supplement levels you might decrease oxidative stress due to the combination of slightly better heme quality and somewhat increased blood volumes lowering oxidative stress and hence counteracting potential increased oxidative damage of heme by ROS due to higher availability of heme due to supplementing iron.
As with many things that improve ME health I’d advise: only “consume” 20% of health improvement and use the other 80% to start finally creating the so much needed buffer. In reality I’m happy if I can get myself to stock over half of the progress in a buffer, but 80% sounds ideal to me. Using 100% of progress to do more is bound to backfire big time some day.
I have consistent below range Mean cell haemoglobin concentration. Could this be a result of body reducing heme levels due to high oxidative stress?
Also a lot of my main symptoms seem to be circulation based
“I have consistent below range Mean cell haemoglobin concentration. Could this be a result of body reducing heme levels due to high oxidative stress?”
That is the hypotheses, yes.
I didn’t know there was some sort of test for cell hemoglobin concentration. I guess you don’t talk about hematocrit value, which is volume % of RBC per volume of blood (or that it is a real test and not an estimate the lab did based on hematcrit values). Is that correct?
Never heard anyone talking about it. Would you have more info on that test? That could be important with more of us.
Thx,
dejurgen
I see another related thing needing investigation: the relation constricted (returning) capillaries in the brain and Cerebral Spinal Fluid Pressure.
Capillaries are very thin tubes made out of a substance a lot less strong then steel or copper. They carry blood that is under pressure. It’s obvious we don’t want them to rupture massively in the brain, so they must be able to withstand the pressure for a long time.
Just imagine a cylindrical tube made of very very fine cut ham, so thin it’s totally transparent. Would you make a garden hose with such flimsy and weak material? Could it ever withstand something close to the pressure of your water supply? Of coarse not! It could only support a tiny fraction of it.
If the ideas mentioned in the blog hold true, then the high amounts of oxidative stress do damage these very fine tubes and the lack of sufficient blood flow reduces the speed at which they can be repaired. This makes the assumption that the capillaries in ME patients brain are a lot more fragile then those in healthy patients very reasonable.
Now engineers can determine the material strength of our blood vessels, take into account the dimensions, add the variations on top of it to find the weakest spots of the capillary and take into account the damaged state of our ME brain capillaries and model it to determine the maximum allowable pressure. Add some margin for mechanical acceleration such as falling or bumping the head.
I haven’t got the resource to do it myself but I have a very strong hunch the allowable pressure will be tiny. It would be unworkable tiny. But the allowable absolute pressure can be increased by having the capillaries surrounded by tissue at a pressure only very slightly less then the pressure of the blood in these vessels. Give or take a bit and that pressure is the Cerebral Spinal Fluid Pressure.
Now, unlike in the conventional medical models for brain blood flow, the pressure in the brain cannot be zero. The blood must flow back. But if the blood must flow back trough these tiny constricted vessels and later all blood coming from the brain must flow true a bigger constricted vein in the neck then it’s easy to see that just gravity alone wont do it. Don’t forget our inflexible RBC make things worse in the capillaries! It needs more pressure! In order to allow for this and not let the brain capillaries rupture, the Cerebral Spinal Fluid Pressure must be increased a lot above what is seen in healthy people.
So IMO it’s simple: ME (and to an extent FM) patients have inflexible RBC plus constricted capillaries and veins in the brain so they have a lot higher Cerebral Spinal Fluid Pressure. In order to keep the Cerebral Spinal Fluid Pressure within safe limits the brain must make a compromise between too high (damaging) pressure on the brain and too low blood flow. Both will get hurt.
Now see what happens at night: at night the brain could do with some less blood flow. But we go sleeping so gravity isn’t helping with letting return blood from the brain to the hart.
In healthy people, a bit less blood flow to the brain and some blood vessel dilations at some places and contractions at other places will do.
But our blood vessels lack the chemicals to easily dilate (due to the long lasting high oxidative stress). So blood flow away from the brain comes to a trickle.
Result: bad nightly hypoxia, “spent” blood piling up in the brain getting toxic, oxidative stress at night from this bad combination, even worse oxidative stress in the morning to clean up the ischemic damage all of this did during the night in the brain…
Is it a wonder we get up more exhausted then we went to bed? Is it a wonder that we are that tired during the day: we need to “repair/regenerate” during the day and work at the same time when healthy people regenerate at night and work during the day. Is it a wonder we have more energy in the evening, at the end of our regeneration phase? Is it a wonder we are wired and tired at night? Adrenaline prioritizes blood flow to the brain and we need this option most at night.
I’d like also to see a well modeled engineers view on POTS. The classical view is that when a person with POTS stands up, the hart can’t adapt quickly enough to increase the blood pressure in order to provide sufficient blood to the brain.
One key element is omitted though: as described above the Cerebral Spinal Fluid Pressure must be in close relation to the pressure inside the capillaries in order them not to rupture massively. Standing up from laying down and the reverse cause quick changes on the local blood pressure in the brain when not counteracted. That’s just the plain effect of gravity.
But not only the pressure in these blood vessels drops when for example standing up. The Cerebral Spinal Fluid is located in a large bag near the brain plus some long tail running down our spine. Both are deformable to some degree. Having plenty of body wide ROS and potentially some leaks will make this bag more deformable compared to healthy people. So standing up will shift the location of the Cerebral Spinal Fluid more towards the lower parts. This will cause a significant drop in Cerebral Spinal Fluid Pressure at the brain.
If now the hart “functioned normal/reacted sufficiently quick” then there would be a strong imbalance between the pressure in the capillaries and the Cerebral Spinal Fluid. In combinations with our flimsy weakened capillaries that is a recipe for massive brain hemorrhage.
So it could well be that the slow and sluggish reaction of POTS patients when standing up is not faulty but rather life saving (allowing more time for Cerebral Spinal Fluid Pressure to adapt) and that taking meds to “help” the hart to increase pressure more quickly are damaging to the brain capillaries and cause plenty combined brain capillary plus micro brain hemorrhage that over times makes brain inflammation and POTS worse.
Brain overactive, can’t get sleep…
I just envisioned a constricted vessel having a thicker and hence stronger wall. Let’s make a mechanical model:
Let me take a “dilated” cylindrical vessel of 100mm length, a diameter of 20mm and a wall thickness of 1mm. This has pi*diameter*wall_thickness*length = 3.14*20*1*100 = 6280mm^3 of “wall material”.
Now let this same cylindrical vessel constrict to a diameter of 10mm. As solid “wall material” can’t disappear then the wall becomes thicker. The new thickness is 2mm as 3.14*10*2*100 = 6280mm^3 too.
=> A) A constricted vessel has a significantly thicker and hence stronger vessel wall.
Now I took an old basic strength calculations handbook. For a cylindrical vessel with a thin wall the needed wall diameter is: (pressure*diameter)/(2*maximum_material_strength)
That means that for the same pressure, a double as big diameter needs a double as thick wall.
Now one can say that a double as big diameter quadruples the area of the tube and thus allows pressure to drop by four so that is good. But static forces caused by gravity must be met to and are dependent on position (standing, sitting, lying down…) rather then vessel dilation.
So the blood vessels must withstand the worst case combination of both static pressure and “flow related pressure”.
=> B) Optimal diameter to withstand worst case pressure will likely tend to be more constricted. The lower the blood flow, as is the case in ME, the more constricted this optimum is. More accurate models need to be made.
This also happens to describe an experience of mine well. I took a drug to dilate the blood vessels. It did reduce mind fog a lot and made my mind clearly stronger. Unfortunately I had to stop it as it increased leg pain a lot and decreased my ability to walk by 3 to 4 times measured in distance.
That makes sense with this model: the legs are far worse affected by gravity then the brain.
Having more dilated vessels in the brain improved blood flow, blood flow related pressure and gravity pushes few on the brains capillaries.
In the legs however gravity is a main force. Weakening the vessels by dilating them with drugs could well cause large scale damage to the blood vessel and surrounding tissue.
Note that my arms were clearly less affected. As they are positioned higher that makes sense.
This could be a fairly useful hint as to what affects FM (strongly increased pain due to this vessel dilating drug: micro damage?) and ME (in the leg muscles, strongly reduced ability to exercise mainly in legs: micro damage?).
If both ME and FM were very strong body wide oxidative stress related diseases, an important parameter determining how much ME and how much FM you get in the mix could be the amount of vessel dilation or constriction. That could be a useful hint to at understanding both diseases and their relation.
One can say the body better just regulated dilation of brain and leg vessel dilation separately. It actually does to some extend, but how ROS interacts with things like NO and other constrictors/dilators is bound by chemistry. As long as both blood vessels in brain and legs are made of the same stuff it’s hard to let both behave very different.
Forgot to mention: using drugs to just dilate blood vessels in this model could make things worse in the long run.
I already was puzzled a long time as to why the body tends to constrict blood vessels when it experiences a combination of hypoxia and oxidative stress. It did make few sense. Now it turns out it acts a lot like a defense mechanism.
Last one before sleep.
Long time strong body wide oxidative stress disrupts the reduced glutathione (GSH) to oxidized glutathione (GSSG) balance.
Reduced glutathione binds to countless (far over hundred) chemicals in our body. It protects/buffers them from oxidative stress and alters their function.
Plenty of chemical processes or key enzymes are influenced/activated/inhibited by either GSH or GSSG. Among those are processes in the main energy generation pathways (Krebbs cycle, glycolysis and the PPP).
Dejurgen; you seem to have a lot of insight and I recognize a lot of what you describe.
Could you; in a short post, tell what supplement, drugs, and doses you benefit from and belive in?
Thanks!
Hi Tiril,
I benefit from very few drugs and have zero clear improvements from supplements. My approach is mainly complementary to it.
I do take Isoprinosine and Cymbalta for a long time. They clearly benefit me. But they could not prevent me to be in a nasty downward spiral after insurance forced CBT-GET. That was the 5% of functionality and lowering level I fell through.
Isoprinosine gives me clearly more energy.
Cymbalta is good for my neuropathic pain and a bit for my energy. Note however many call Cymbalta diabolic due to side effects and withdrawal effects that plague me few.
Now I gradually reduced both to very minimal quantities. I probably could have higher functionality with more of them but as long as I keep slowly improving I prefer not to risk long term side effects or suddenly loosing there backup when its really needed due to overuse.
The closest thing to a supplement that works is Symphosan, a root extract helping somewhat with muscle stiffness and pain when applied externally. It’s like Saint Johns Wort oil but better for me.
So what broke that nasty downward spiral? Please see my comment to Peter lower in this blog as to why I am low on details.
* Relearn basic mental and physical skills by breaking them up into the tiny component tasks they are and train them step by step. It’s like men that only can play darts well when drunk because they learned it in the pub. All skills we learned are for a healthy body and mind we no longer have. When falling rapidly in health I could no longer get out of a chair, walk around a corner or get true a door while bumping into it. I had no clue how to do it. I did not forget. My experience was just worthless with my current mind and body. Then I realized I had to relearn things that I still could do somewhat too, as well trained skills are a lot more efficient and friendly to the body. Decompose and practice very basic sub-skills, just doing something a lot isn’t enough or can further deplete the brain.
* I learned to breath more efficiently. I learned the hard way that that is totally different from breathing more. Breathing equally bad with less effort is a big gain. Having some reserve “on the breathing apparatus” because of spending less average effort is very valuable when you get into real trouble. Real trouble like PEM+ and “who removed oxygen out of the air” is VERY expensive so having some spare then is very valuable. I only started learning to breath well when I met a very good physical therapist. When you learn to automate breathing more efficient after a looong time, you get both more breath and spend less energy 24/7.
* I learned to recognize signs of oncoming exhaustion way earlier leaving time to act and avoid PEM. PEM is very costly, avoiding it a big gain.
* Circulation exercises were a big thing too for me (see physical therapist too). It reduces muscle strain and with it pain, energy cost for over-straining muscles (limited effect) and biggest one: decreases (additional) constriction of blood vessels by force of muscles. That is for me A LOT better then increasing blood flow with drugs as the latter still does not allow the body to chose and adapt blood flow.
* I try and improve my diet to be less inflammatory and more anti-oxidative. Works better then supplements for me as they often have low bio-availability and have high effect per gram but are taken in far smaller weights then food. Still in experimental phase but avoiding overeating and keeping blood sugar between upper and lower limit for non keto diet seems important.
* I learned to decrease the “cost of night” a lot; more information soon on the forum.
* I learned to think in a way that cost a lot less focus/energy.
All the above got me over 20% functionality.
* I learned this summer my gut is in far less good shape then hoped and I have plenty of food intolerances; working on that now.
dejurgen, how much isoprinosine do you take? How much Cymbalta/duloxetine do you take? I take Cymbalta and it moderately helps my Fibro, but it doesn’t help in a huge way; I can’t figure out if it helps any amount with my fatigue. On the topic of something you mentioned months ago, have you posted any description of your protocol for intermittent sleep and breathing, circulation exercises?
Hi ElizaBee,
Only just saw your comment. It’s not always easy to see what posts are new.
I take now 2 times half a 500mg tablet Isoprinosine (also known as Immunovir) for a total of 500 mg a day. That is down from double the dose originally. That is my best drug out of the 2 as it has clear benefits with moderate side effects for me. Benefits were clear after a week, kept growing during a month; initially it was hard on the stomach but that feeling disappeared. Still, the effect on the stomach is probably still there and that is why I reduce dosage as long as I have other things that keep me improving.
Cymbalta I take 1 pill of 30 mg every other day. That is down from 1 pill of 60 mg each day so a reduction by 4. It works best against my strong pain shoots but also reduces other pains somewhat.
After doing circulation exercises it gave me extremely huge and frighteningly dangerous levels of adrenaline as a bonus. It seems that it’s effects are strongly increased by improved blood circulation. That is a major side effect. For me, I am one of the *very* rare ME patients that can slowly improve on high doses of adrenaline and keep the improvement. Note that no other ME patient ever reported this, but plenty reported the devastating effect that long turn adrenaline rushes had on their health. I *very strongly* advise not to go that dangerous route. Hart going out of control and a combined serotonin syndrome (dangerous for suicidal behavior) combined with huge waves of adrenaline (calling for action, very dangerous in combination with a suicide risk increasing serotonin syndrome) needs to be managed long term in a very calm way.
I’ll intend to post more on my sleep and breathing protocol probably this month. Working on describing it as clear and safe as possible. Not easy ;-).
Hi dejurgen,
Replying to a 4-year old post here, but wondering if you ever published the tips on improved breathing & sleep protocol? I can’t find it but would really be interested to read more. Thank you for all the time you put into these posts, they are extremely insightful.
I agree almost. This is the core problem. But don’t forget low bloodvolume 🙂 and an oxygen problem could explain everything in my view. It is importent for all systems! Also the abnormal stress respons. This respons is necessary for compensation.
So breathing problems like hyperventilation are explained too 🙂 Not in the mind at all!
Hi Gijs,
I agree with the low blood volume. That’s in part an indirect effect of hyperROS IMO. See the RBC reduction equals blood volume reduction due to reducing heme under high oxidative stress.
See also the damage to the blood vessels due to oxidative stress. I experience more blood noses since I improved from 5 to 25% of normal health. Those stopped when I was at worst. I believe this was due to too few blood to “bleed well”. Also wounds did bleed less so bleeding due to leaky blood vessels can be reduced by having less blood volume IMO.
The blood in the stool thing returned too when getting better and eating something I am intolerant too. So bleeding gut seems to be reduced by less blood volume too.
Peroxide acts like a somewhat smaller brother of carbon monoxide as described in another comment. So oxygen uptake problems are normal. Also impaired GSH and GSSG levels have a major impact on glycolysis, Krebbs cycle and the PPP. They inhibit or activate key processes. In effect, this has a direct strong impact on oxygen uptake.
And high adrenaline levels would be a very good help/medicine for ME patients if they hadn’t that much strong side effects IMO. Better breathing, prioritizing blood flow to the brain and liver, boosting NADHP/glutathione production,…
There is one extra component that is needed in many cases IMO: an “enduring” source of high oxidative stress. While there are many feedback loops that can maintain and worsen the dysfunctional body wide hyperROS state I believe for most patients without an additional source of oxidative stress the disease would slowly fade away with pacing.
In my case I believe multiple food intolerances, leaky gut and a strong immune reaction to ?undigested? protein is a major source of ROS feeding the nasty vicious circle.
I have had CFS/ME for 45 years. And now, I have pulmonary hypertension, which is a lethal disease (five year survival is 50% WITH the meds that cost $115,000 a year for just one – most take several) and it is caused by HYPOXIA. CLEARLY CFS/ME has restricted my oxygen and on top of that, POTS has also caused me to have 20% less red blood cells that normal.
WARNING – once you have CFs/ME for 40 years, you will likely end up like me with a hyxpoic disease that is fatal.
and worst of alll, it’s all in my head, right!
Sorry to hear that Carol.
By chance, would you happen to:
* have very fast fibrillation like hart beat late at night / early morning while sleeping
* breathe deeper at night then during the day
* have very unrestful sleep waking up many many times a night especially as the night progresses?
I wish you all the luck you can use.
Love the rants/comments Dejurgen. I agree that it is probably one part of a vast multisystemic disease which involves multiple breakdowns.
Rings true to me. I have ESR of 2. Related muscke and circulation issues. I took NAC twice. Both times I experienced a “thawing out” of tight shoulders and neck. It was great, everything loosened up and the crunchiness disappeared. Then, 3 hours later there was a bad rebound effect and everything was worse. After 2 attempts with this, i got spooked and threw it away
This theory also sounds quite like sickle cell disease which is genetic. The blood cells are deformed also, resulting in poor oxygenation. Reading about it. It is treated with hydroxyurea, for symptom management
H2 tablets definitely help me with PEM. It doesn’t get rid of it entirely, but minimizes…H2 is a potent anti-oxidant, and easily crosses the BBB. I am still experimenting with front loading, in addition to drinking it after a workout.
I have also been using compounded intranasal glutathione, but have not made the connection that it may be helping.
what is H2 tablets?
do you have a order-link?
Thanks!
6 papers have their manuscripts accepted now in Plos One soon for Publication from McMaster University pointing to Radiation in CFS they are now posted on Gail Kansky’s website the National CFIDS Foundation
there is also a recent Video dealing with mitochondria ATP findings as well…Is radiation the Cause of CFS? Time will tell if this passes or not but I still do not believe it is any Cause especially at low doses
& it was not around back in time when Ehlers Danlos Syndrome was mentioned way back in the 12th Century which is another link to CFS. The links are on their Homepage now scroll down once opened they are
all there now including the recent Youtube channel link from McMaster University in Hamilton, Ontario, Canada…
I was tested for Red Blood cell deformation about 20 years ago by a doctor in New Zealand who was retiring. He modestly called himself a simple country doctor who was about to retire. He tested my blood for free. I just paid for shipping from USA. My results were quite funky. Huge deformed red blood cells. Still have the photo. He thought it was a possible biomarker. In my case – yes!
Wow great information to have on your health record Scott! I was wondering about a possible test for RBC deformity is it an expensive test?
Scott the name of that Doctor is Les Simpson! He also tested my blood about 25 years ago. These new researchers should track him and his publications down!
Thanks Scot. I just found Simpsons 1989 paper and included it in the blog. He was truly a pioneer.
Ramsay’s Disease, Myalgic Encephalomyelitis (ME) and the Unfortunate Creation of Chronic Fatigue Syndrome (CFS) , LO Simpton and N Blake contains a full account of Les Simpsons research…based on hematology, which is not taught in medical schools, he observed and described non-deformable erythrocytes and their appearance and effects in ME. He also wrote a book ‘Blood Flow – the Missing Link in Modern Medicine. His work is in a chapter in Byron Hyde’s book about ME/CFS. Les explained that the not-deformable shapes could be observed if the blood samples were ‘immediately fixed’…otherwise the usual process of leaving the sample in saline for 30 min allowed the red blood cells to revert to the biconcave discocyt form. The paper, by Sonya Marshall, which dismissed his work did not use his laboratory protocol..they didn’t immediately fix their samples. If this paper has been responsible for the neglect of Les’ work, they have a lot to answer for. Les wasn’t sure if it could be a definite biomarker, as thee changes also appear in MS, Huntingdons and other conditions.
The fact that the non-deformable cells cannot traverse the microcirculation, therefore causing oxygen deprivation and a build up of lactates in areas most dependent on these functions – muscle cells, cognitive areas of the brain, the endocrine system – certainly seems to offer a simple explanation for many of the features of ME/CFS.
Les recommended taking 6g of fish oil, or 4g of genuine EPO, pentyoxyfillines, or vitaminb b12 as hydoocobalamin…each of these could improve the wel-being of some patients, so he recommended trying each to see what you might respond to.
He sent me an article showing that treatment with pentoxyfilline for type 2 diabetes seemed to prevent the circulation problems which lead to amputation.
It is interesting that it is engineers who are taking this up, as hemorheollogy, Les’s field, is about the physical, not the chemical properties of blood. But this is not taught in medical school, it was difficult to get articles published, and the Journal of Hemorheology closed down.
Les died a few years ago…he would have been delighted that finally, after years of neglect, his information is finally being taken seriously and further research is now being done.
We both used to write cross letters to journals…starting perhaps with the Pace Trial, no, earlier. Eventually we started writing to each other, and when he told me he had a manuscript that needed publishing I blithely offered to help self-publish it…he graciously invited me to include my story, and eventually to come is as co-author. So Ramsay’s Disease is a kind of alternating double act, as I proofread but did not alter his sections.
There is so much research out there that seems to point to the red blood cell problems, but until now, has not acknowledged them. So pleased about this development!
He was a real pioneer wasn’t he? And he sounds like a compassionate and delightful man. It would have been so nice if he could have seen what Stanford and the SJSU and modern technology are now dong with the red blood cells.
I was tested for RBC deformation over 20 years ago by a Dr in the UK who had previously worked with Dr Cheyney. Video microscopy showed my cells to be very deformed a ‘text book case’ he said. I had glutothione injections for 6 months along with calcium injections but, sadly, to no avail. I am still bedbound
I think its pretty clear that while boosting the antioxidant system is a good idea it’s not going to be a panacea. Whatever is causing the inflammation/oxidative stress in ME/CFS has to be addressed as well. Drugs that get at the small blood vessels may be needed too…We may have to find a way to get more oxygen to the cells.
The OMF must have rich donors I notice they raise an enormous amount of money each year. Cort, it is remarkable how much you do in getting so much information out to us. Health Rising’s fundraising drive will surely be a big success!
Let’s hope! We’re about 35% of the way there. I maintain that the better off Health Rising is the better off everyone will be. One of HR’s main goals, after all, is to highlight the work of good researchers so that others can support them. The more we can spread the word the more people who will be able to support their work.
Yes, let’s make sure we all donate something to health rising! How lost would we all be without his updates?!
This rings so true for me. I often say that I feel like ‘my cells are suffocating’.
Bingo! In my heart of heart or my body of bodies I think, feel, intuit…whatever that the microcirculation is key. We shall see 🙂 The muscle research done by Ron Tompkins at Harvard is going to be fascinating.
Leslie O Simpson wrote a book about his theory on red blood cell deformability and capillary restriction in ME, together with Nancy Blake. It’s called “Ramsay’s disease. Myalgic Encephalomyelitis (ME) and the unfortunate creation of CFS” (2013).
His theory goes back as early as 1986. Simpson speculated “that ME is a hemorrheological disorder in which symptoms are manifestations of the consequences of impaired capillary blood flow.” How fascinating that more than 30 years later, scientists are looking at the same issue in patients with ME/CFS (It’s also a bit sad and reflective of the lack of research into this disease).
Simpson saw blood cell deformability to be a key element of the pathophysiology of ME, but he didn’t think it was unique to this disease. “The changes in red cell shape populations which occur in ME also occur in other chronic disorders”, he wrote, “so red cell shape analysis alone is not diagnostic for ME. The observed changes are probably of importance in the pathogenesis of tiredness.”
Here are some relevant papers/abstracts:
https://www.ncbi.nlm.nih.gov/pubmed/3093959
https://www.ncbi.nlm.nih.gov/pubmed/2886780
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(89)90399-1/fulltext?code=lancet-site
https://www.ncbi.nlm.nih.gov/pubmed/2927808
http://orthomolecular.org/library/jom/1997/pdf/1997-v12n04-p221.pdf
http://orthomolecular.org/library/jom/1997/pdf/1997-v12n02-p069.pdf
https://pdfs.semanticscholar.org/fb5a/d891d78ee0e74849fe348503263230770fef.pdf
https://www.ncbi.nlm.nih.gov/pubmed/3588027
Hi Michiel,
Thanks for the links. A pity of some paywalls…
It’s a very early idea but reading orthomolecular.org/library/jom/1997/pdf/1997-v12n04-p221.pdf, what struck me was the amount of cup formed RBC. There shouldn’t be many of that shape at all IMO. It doesn’t make much mechanical sense.
In en.wikipedia.org/wiki/Red_blood_cell I believe I saw in the movie the RBC shortly take on a cup form shape, probably to better enter small capillaries. But it reverted back to normal shape as it should. Why a RBC would stay cup form shape still puzzled me.
Then on the same page on the topic of flippases, floppases and scramblases I *believe* that these 3 are mechanisms to change shape. I believe that 2 out of 3 of these mechanisms use ATP to reverse. Something we lack a lot.
Once the RBC takes cup form shape shortly, one of sides will face the direction the RBC is going in. The other side wont. Flow will “fix” what side is on front, just like shape is determining how an umbrella is carried away by the wind.
In an environment with few ATP to begin with *and* low ability of the RBC to produce ATP with glycolysis (as they don’t have mitochondria), availability of ATP could become asymmetric IMO.
That could well reinforce the asymmetrical shape over time and require to add a pretty big amount of ATP on the ATP poor side to counteract the other side’s pull to become normal shaped again.
Maybe this is one of the reasons why the cells throw out their hardly won ATP out of them (as Davis/Naviaux observed), to give to the needy RBC?
And while a cup shaped RBC has slightly smaller diameter, it has a much larger stiffness due to its shape, impacting deformability.
Maybe someone with more knowledge could tell?
I’ve been using a glutathione oral spray for a couple of months and immediately felt better. This was after trying ala and glutathione ivs which made me feel worse. I read that a doctor recommended the nasal spray because you can dose it better, more often, more easily and cheaper than iv. I couldn’t find the nasal spray online so I went with the oral spray and luckily it helps me. But I’m curious where people can find the nasal spray.
One other thing I feel is worth mentioning is that I started taking montelukast for some difficulty breathing and that also seemed to help me a lot with my energy. So that makes me think they’re on the right track with the oxygen angle. I’m curious if there are others out there with asthma or allergies that have tried meds to help with breathing that made a difference in their cfs symptoms?
Improving my breathing and using a glutathione spray have been the best two things I’ve found yet in my three years with me/cfs.
Good news. Thanks for passing that on. A blog is coming up on intranasal glutathione. Dr. Klimas is testing it out in GWS.
is the intranasal formulation not available yet?
This type of oxidative stress also occurs in the hypometabolic state of hibernation in mammals:
Effect of Hibernation on Oxidative and Antioxidant Events under
Laboratory Conditions in Anatolian Ground Squirrel, Spermophilus
xanthoprymnus (Bennett, 1835) (Mammalia: Sciuridae) from Central
Anatolia.
Link to PDF file: zsp.com.pk/pdf46/177-183%20(23)%20PJZ-1543-13%2029-1-14%20proof%20reading.pdf
Abstract:
“The oxidative stress is an integral part of the metabolic depression machinery associated with
hibernation, estimations and tolerance to freezing, dehydration, hypoxia and anoxia In the present study, we aim to
investigate the role of hibernation on lipid peroxidation, nitric oxide production and glutathione levels in various
tissues of Anatolian Ground Squirrel Spermophilus xanthoprymnus. A total of 9 female ground squirrels were
collected from different localities in Nigde, Bolkar Mountains, Turkey. All squirrels were in three different conditions:
at hibernation, aroused and non-hibernation stage. They were sacrificed under anesthesia. Glutathione (GSH), reactive
nitrogen oxide species (NOx) and malondialdehyde (MDA) levels were measured spectrophotometrically. GSH levels
of almost all tissues were lower in the hibernating group compared with the aroused group. NOx levels were found to
decrease in all tissues except the brain tissue examined on hibernating group compared to non-hibernating group.
MDA levels were found to increase in brain, lungs and heart tissues examined on hibernating group compared to nonhibernating
group. In conclusion, our data show that an impaired balance exists between oxidative stress and
antioxidant systems in most organs and tissues during hibernation.”
Blood vessels problems could also be part of a hypometabolic state:
https://www.britannica.com/science/dormancy/Dormancy-hibernation-and-estivation-in-warm-blooded-vertebrates
“Changes in the circulatory system involving constriction (narrowing) of posterior vessels and the favouring of anterior circulation allow the brain temperature of hibernators to remain a few degrees warmer than the environmental level. This enables the temperature of the brain to remain constant despite fluctuations in the temperature of the skin.”
I have just started using nasal Glutathione so too early to tell if it’s going to make a difference. Here’s the link to it https://glutagenic.com/product/glutaquick-nasal-spray/. Not cheap and not available here in the UK so I had somebody send it over.
Last week following a full round of blood tests, my GP informed me my RBCs are ‘enlarged’. But as is often the case dismisses it and I’m told the usual, let’s do another blood test in a few months. A couple of years ago I had my blood looked at under “blood microscopy” and was told in all his years of doing this he’d never seen such poor looking blood cells. Pointing to peculiar shaped cells, strange round large dark looking cells with spikes. It was fascinating and also horryfying, I still have the pictures. So for me I feel certain they are on the right track and there’s a serious problem with the blood cells.
Dejurgen’s words sure hit the mark “Is it a wonder we get up more exhausted then we went to bed? Is it a wonder that we are that tired during the day: we need to “repair/regenerate” during the day and work at the same time when healthy people regenerate at night and work during the day. Is it a wonder we have more energy in the evening, at the end of our regeneration phase? Is it a wonder we are wired and tired at night?” I wake each morning feeling like I’ve been poisoned and remain in this stupefied state for ages until it wears off a little and I can function minimally. Best time of day is nearly always later afternoon and evening when I’m starting to feel clearer headed. Then of course I can’t sleep when it’s time for bed and half the night is spent tossing and turning or in light fitful sleep until morning and the cycle starts again.
How fascinating (and a bit horrifying (lol)) it must have been to see those weird red blood cell shapes show up.
Here are some of the ones that study found:
leptocyte – Leptocytes are thought to be erythrocytes in which the cellular envelope or membrane is unusually large in proportion to its contents
acanthocyte – refers to a form of red blood cell that has a spiked cell membrane, due to abnormal thorny projections. (thorny projections!)
burr- have serrated edges over the entire surface of the cell and often appear crenated in a blood smear
Now how in the heck is a red blood cell with a thorny project or with serrated edges going to easily slide down a capillary?
Has anyone here had Ischemic Optic Neuropathy (ION, NAAION, etc.)? It’s a problem of blood not circulating in the capillaries that supply the optic nerve. I’m bringing this up because of the description here of abnormal blood cells attempting to squeeze into capillaries. A few different pathologies could cause ION. It seems to reason that if we have blood flow issues, as a ME/CFS population, then ION might be a known issue among us. I’m glad that it isn’t common. (Or is it?) I’ve had at least 2 ION episodes on my left optic nerve where it meets up with the eye. Which means a loss of some vision of that eye, with each episode. I haven’t had a blood cell morphology test done but hope to have one in the next couple of months. My neuro-ophthalmologist and I mostly figure my ION could have been triggered by not drinking enough fluids, which lowers one’s blood pressure,or possibly too many sweets (sticky blood cells). I’m thinking that if anyone reading this has experienced ION they would really remember it. You go to look at something with the one bad eye, like I did one day when putting on eye makeup, and I could only a fraction of my face. I went to my regular eye doctor, who looked in and saw 2 hemorrhages at the back of the eye by the optic nerve attachment. The capillaries back there are of course really small, therefore a circulation problem, lack of oxygen, and a type of stroke. Anybody else have anything similar? If not (which is a good thing!) — *why* not?
Yes! Same here. I feel like I’ve been poisoned every morning when I wake up. Or hit by a truck. Horrible feeling. And I’ve been waking up to this for 34 years. I actually feel better when I sleep poorly.
“I actually feel better when I sleep poorly.”
Good observation. It makes total sense to me.
Using similar observation led me to one of my bigger (and stranger) “tools” for improvement. Probably it’ll be only useful for a small subgroup of patients. I’ll probably describe it this month in Healthrisings forum. It’ll depend on how well I can retrace all steps in order to describe it well.
I have my own version of the “Or hit by a truck” thing. Or at least I had. I used to feel every morning like a truck ran over my chest.
I did wake up every morning with a deep feeling of nausea too. Wouldn’t call feeling like poisoned but who knows how that feels? No need to know that. Maybe it resembled the feeling of food poisoning more.
I could use your input here to get a better overview on the idea/technique: do you by chance wake up a *lot* at night and more as the night progresses? Do you have frequent and very strong nightmares late night/early morning? Do you happen to wake up breathing like a horse from time to time? Do you get irregular or very fast hart beat late night/early morning?
If not, or if I miss some important nightly symptoms, please feel free to share observations/ideas. It could be of real help for those in a similar situation.
Thx,
dejurgen
Cort,
Thank you for an excellent job presenting the pieces of this puzzle. Dejurgen, your comments are interesting, too.
My naturopathic doctors have been working with me on the problems you’ve described, with good results. I don’t have time to wait around waiting fir the researchers to perfect this theory, so I’ve been willing to try using Maes and Pall’s theories to help and pay for treatment my insurance won’t cover, as this is all “experimental.” But it has led to vast improvement, especially if I take liposomal glutathione before snd after exertion.
Unfortunately, one must get to the root cause of the oxidative stress (infections? toxicity? more activity than the body can handle?) and reduce the causes for treatment to work.
And, though NAC, ALA, and intramural glutathione can definitely help, one must treat the entire system to improve.
I believe the spiky cells are due to cell membrane damage. Garth Nicolson’s work with lipid replenishment with NT Factor along with mito cofactors like carnitine helpful, as well as IV phosphatidyl choline.
High MCV, the size of the cells, can be due to depleted folate or B12 or both. Folate (the 5-MTHF firm, not folic acid) and B12 are needed to make glutathione. And the cofactors needed to make it have other jobs to do, like B6 is needed for heme and making glutathione, and B2 is needed in mitochondria complex II.
One also needs adequate amino acids _found depleted in ME/CFS patients) to make glutathione. NAC is useful, but many of us are depleted in glycine (or glutamine) so without this trilogy, glutathione can’t be made.
ALA is extremely useful, but worth some caution. It can recycle both water soluble and fat soluble antioxidants. It is also helpful in removing toxins from the mitochondria, so taking it without adequate support for fully removing the mobilized toxins from the body can make people feel very sick. This pints to a comprehensive strategy.
The transsulfuration pathway, which requires B1 and molybdenum is needed to move the body’s netabolic trash into the intestines for excretion. People complaining if sulfur smell need more support here.
Antioxidants work as a coordinated network. Studies investigating only one antioxidant have concluded that they don’t work (because they didnt provide adequate support with the other antioxidants). We need the entire system balanced. A great bssic read on tn this system is “The Antioxidant Miracle” by Lester Packer who ran the world’s foremost antuoxidant lab at UC Berkeley for many years.
To put together a program, my ND runs a Genova Diagnostics NutrEval test every 9-12 months. Over time, these tests have shown depleted glutathione, ALA, A, C, E, selenium, B1, B2, B3, B6, B12, magnesium, and molybdenum, all of which are used in the process of reducung oxidative stress. It also has showed high lipid peroxides and high 8OHdG, a marker of ongoing DNA damage. And problems with precursors to phosphatidyl choline.
Doing an HDRI Nitrotyrosine test showed that I indeed am producing peroxynitrites.A MitoSwab test showed low complex I function (which csn be caused by peroxynitrites) and huge oxidative stress from complex II (where riboflavin, manganese, and succinic acid are needed) and complex III and IV.
So, my doctor put me on an oral supplement program, which may work for some. But as my gut isn’t in tbe best shape, due to being celiac and having had antibiotics for chlamydia pneumoniae, I’ve also been doing a weekly IV protocol, too, which has helped – I’ve gained more energy over time, and am in the process of retesting to see how far I’ve come.
Another component has been addressing my lousy ATP production, by using NAD+ (nicotinamide riboside doesnt work). We need B3 to make it, but dint want too much B3, as that can reverse what the methylation methylating nutrients above do. So small amounts. The kynurenine pathway that Phair has been taking about lesds to NAD production, so if that’s compromised, making NAD voukd be a problem. NADH works, too, but NAD+ works better.
The last piece is oxygen therapy. I do hyperbaric oxygen 70 min 3x a week, ehich my doctor says helps pysh the nutrients deep into my tissues.
The down side of this is that it costs money. I feel fortunate to be an n=1 experiment here, but I wish theyd hurry up and finish the research so our healthcare systems would support this treatment.
One last note, the FDA is slowly rolling along evaluating snd banning many of the bulk drug substsnces that make this protocol a reality. They aren’t even looking at ME/CFS and the problems you desctibed in this blog The ME/CFS community needs to be aware that this is going on and mobilize to make our voices heard that we need these tools before they become unavailable.
“A MitoSwab test showed low complex I function (which csn be caused by peroxynitrites) and huge oxidative stress from complex II (where riboflavin, manganese, and succinic acid are needed) and complex III and IV.”
That is pretty much in line with the
“heme is used in the Krebbs cycle; under high oxidative stress the amount of heme in the body is reduced as oxidative stress damages heme and damaged heme causes massive oxidative stress; As such reducing heme slows the Krebbs cycle and thus reduces energy production while the damaged heme produces plenty of ROS; both combined resemble the CDR response to hydrogen peroxide a lot”
comment I made.
complex II, III and IV are heme based and is in line with the idea of massive oxidative stress by heme in the mitochondria.
* https://en.wikipedia.org/wiki/Succinate_dehydrogenase
* en.wikipedia.org/wiki/Cytochrome_c_oxidase
* en.wikipedia.org/wiki/Coenzyme_Q_%E2%80%93_cytochrome_c_reductase
For complex I, I can find no evidence of it being heme based yet.
However if oxidative stress from complex II, III and IV is significantly larger then from complex I as your test results seem to indicate then we are in some big trouble:
https://en.wikipedia.org/wiki/Respiratory_complex_I
“During reverse electron transfer, complex I might be the most important site of superoxide production within mitochondria, with around 3-4% of electrons being diverted to superoxide formation.”
=> complex I is suspected to be the biggest source of potential superoxide production at 3-4% of electrons
=> A lose interpretation of your data seems to suggest that complex II, III and IV each (easily) top this 3-4%.
OUCH!!! if that were true!!! That would get very close to the CDR proposed by Naviaux where entire mitochondria turn from producing mainly ATP to producing mainly ROS. If electrons are directed to ROS production then they are not used for ATP production.
=> Would the “signalling function of peroxide to turn on CDR as stated by Naviaux” be mainly the depletion of anti-oxidants outside the mitochondria combined with the reduction in heme in the mitochondria and the damaging of said heme in the mitochondria?
You seem to be on a pretty good roll :-).
I have to retry vitamin B supplements. They seem to be pretty important in some of the key paths to better health. I tried once and as with any other supplement I didn’t notice any improvements. I suspect I have to get my gut in better shape first or find ways for better uptake.
Many of the thing you do make quite a lot of sense. Unfortunately getting access to many of them isn’t easy or cheap. And some things like ALA or NAC I avoid since I had a few experiments firing back big way.
Still I believe there is plenty of useful information to be extracted out of the simple models I make. It ain’t easy and I often have to revise ideas so far.
But I managed to turn around being at 5% and on a steep decline into improving steadily and being at 25% yet. The path from getting away from that 5% was hellish. I’m now slowly trying to make it more reliable for myself and hopefully the next step is to make it bit by bit more useful for other patients. My path will never be easy however and require plenty of tailoring and good senses.
Success with your ongoing improvement!
Do you feel comfortable sharing whom your ND is???
High level of lactate appears not only in CFS brains, but also aging brains and injured brains. Glial cells have been implicated in producing/shuttling brain lactate as well as inflammation. So it is possible that lactate is a correlation/symptom, or a signaling molecule playing a role in the whole mess, rather than the actual cause of fatigue.
As for the SJSU study, I’d wait for the replication study. If it was that easy (p<0.0001), someone should be able to replicate and turn it into a biomarker.
Finally, my pet peeve. How does RBC abnormality explain PEM lasting several days after a minor exertion? I wish researchers focus more on PEM and less on energy production problem.
Happy holidays!
Nancy Klimas has found the problems during exercise start with inflammation and proceed to increased oxidative stress, autonomic nervous system. Maybe the system kind of pushes the blood along during exertion- shoves in it there – and then it gets stuck in a hypoxic environment and that is where the PEM really comes in….???
Once again we see that science is amazing. However, in the meantime what can we observe if we step aside from our story?
1. We are only observing the body’s stress response system in action. Why is our stress response so active?
2. How does a person end up with CFS in the first place? What are the commonalities that every sufferer has?
3. Why is it that so many people recover all using the same basic method?
4. Where are the recovered people, what did they do and how much effort have I placed on connecting with them?
Many thanks for another great summary.
I’ve just started taking Arginine. Arginine changes into Nitric Oxide (NO) which helps blood vessels relax and also improves circulation. Some immediate clearing of cog fog but too early to tell if lasting or just placebo effect.
Also looking into Nimodopine (which I think you have a link to somewhere, Cort) for if the Arginine doesn’t work out. Goldstein had some success treating ME with it as have a few other knowledgeable ME docs. Surprised it hasn’t been mentioned again with all the talk of restricted blood flow in/to the brain.
Would like to get hold of a nasal or oral liposomal glutathione spray in the UK that doesn’t break the bank. Suggestions welcome. Sue Jackson wrote about taking Glutathione in her blog earlier this year: http://livewithcfs.blogspot.com/2017/03/increasing-glutathione-in-mecfs-related.html
Interesting about arginine.
Remy has written a blog about Nimodopine which is coming up. She’s finding it quite helpful. I think it’s a fascinating drug 🙂
Thanks for the tip about Sue’s blog as well. I hadn’t seen that.
Good luck with the treatments.
On http://simmaronresearch.com/2018/11/immune-study-evidence-energy-production-chronic-fatigue-syndrome/ Wayne is very positive on Citrulline (in the form of Malate).
Looking deeper into it, the idea seems to be that Citrulline has better uptake and would convert to more Arginine then taking Arginine itself. Haven’t tested either myself.
Looking up the relation between RBC shape and ATP, I did found something else.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3131338/
“RBCs are known to release ATP, which acts as a signaling molecule to cause dilation of blood vessels.”
“ATP is a well-established vasodilator”
“Furthermore, reduced deformation of RBCs has been correlated with myocardial infarction and coronary heart disease. Because ATP release has been linked to cell deformation”
=> So we produce few ATP, have constricted blood vessels and ATP does dilate blood vessels. Maybe that is why our cells throw out hard won ATP? To dilate blood vessels?
=> As the RBC are supposed to produce ATP themselves and have some spare to release for blood vessel dilation, having largely inhibited glycolysis won’t be a good thing for our blood flow.
In http://www.physiology.org/doi/full/10.1152/ajpheart.2000.278.4.H1294
“During this time, the RBCs sense the hypoxic environment and release ATP. This ATP then diffuses to the vascular endothelium and binds to its receptors, resulting in activation of a vasodilator mechanism operative in these vessels, possibly NO”
“Although an 8% increase is small, it is important to remember that, on the basis of Poiseuille’s law, vascular resistance and flow are determined by the fourth power of the radius. Therefore, even a small change in vessel diameter will have a dramatic effect on both parameters.”
But also
“human RBCs release ATP when exposed to severe hypoxia in the presence of hypercapnia”
=> We have combined hypoxia and are closer to hypo-capnia due to our “hyperventilation”, possibly removing this anti hypoxia mechanism.
Thanks, dejugen, for all of your insightful comments. Very intriguing. Perhaps I’ve missed it in previous posts but a summary of the different approaches you have used to lift yourself from 5% to 25% energy would be of interest to many of us.
One thing I have trouble getting my head around is how something as basic as deformed red blood cells would not have repeatedly jumped out as a bio marker for CFS – and begs the question: Shouldn’t all of us ask our GP to order a test evaluating RBC morphology? Theoretically all of us should have abnormal RBC, if this theory holds water. It would be great if 25 or so people would report back here their blood work. Would tell us a lot.
Many doctors seem to shrug their head when they see something that is so called impossible in my experience.
Maybe because so many of them have busy lives, are overworked and have ever 5 more patients waiting in the queue?
Hi Peter,
All of us have tried many many things and were disappointed so many times or experienced getting worse a lot without easy way to reverse it.
I do not wish people try some of my ideas and pay with their health for it.
I neither wish that people try out ideas I put out too early and many are just disappointed. Those early poor experiences would let patients put these ideas with the trash even once they are better developed, tested and described how and when they are expected to work.
I’ve actually picked up a really discredited one that does not work as explained but by looking at it in a different way and using these new ideas I could it make work for me.
Another thing is I believe I am close to putting together a sort of theoretical framework/model I use myself already. The idea is to make it more coherent and iron out the possible problems. IF, big IF, successful it could provide good insight in some important aspects of the disease and point at potential small treatment options such as the several I do use and provide some ideas to research in a more professional way.
I know the chance is small, but the potential of having a model that can describe a disease resembling a lot ME and being in line with current medical science is too great to hurry. I know it’s very ambitious and chances to fail are huge.
It will have very few chances to come back if the initial version fails due to rushing. At the very least a flawed initial version will give researchers all the munition they need to break it down. Even good and sound ideas like the RBC deformation studies seem to be snowed under for 30 years…
But waiting forever ain’t the best either. So this is the current plan.
I’m currently confident enough in some ideas to be in talks with Cort to put together basic elements of this theoretical framework and put it somewhere on the forum for all to critisise, find gaps, errors, problems and see if it can survive this phase. Then there will be another phase ;-).
As to what helped, I will from time to time create a topic in the forum discussing what I did and why I think it helped me. That is asking for people to share their ideas and experiences and see if they can add to it rather then me giving advise.
My papaya topic is one of it, but nobody tried this simple thing or did not report it did not help them. Probably it’s for a small subgroup then.
Next will probably be something on my sleep routine. But first I have to be able to repeat my results. I stopped the routine for some time in order to try and solve my newly discovered food intolerances. If I can’t get the previous results of my sleeping routine back myself then it’s not really useful to anybody else I think. With luck I can start it this month.
Hi Deurgen! Lots of really relevant info and ideas. Thanks for putting so much effort into writing. I don’t have that much stamina for writing right now as it is late and I am procrastinating on the nightly ordeal. I too wake a lot especially during much of night and then get into deeper sleep when it is almost morning. But I wanted to tell you that reading Les Simpson’s book Ramsay’s Disease 10 months ago gave me insights that led to a protocol that helped me a lot but like you I am hesitant to recommend something that could harm someone. Also I could get you your copies of research papers through a university library and send PDFs to you by email. Ask Cort for my email address. He is doing such a great job on blogs. This one is amazing in the ways it ties current research to past, and I will try to respond meaningfully as soon as I can. But now I need to at least try to sleep. Cheers. Ruth Ross
P.s. Where can I find your “papaya topic”?
Hi Ruth,
“like you I am hesitant to recommend something that could harm someone”
I still am hesitant, but I decided I’ll write it from a perspective of what I experienced and what I think it is, with clear warning that it’s not advice but rather a starting point to exchange idea’s and experiences.
Also, many of us had to find out do’s and more importantly dont’s by costly experience. Sharing my experience including what went wrong and telling were I took much of a risk might be less harmful “on average” then writing nothing and let many others make the same mistakes over and over again.
Especially fighting a symptom that in fact tries to improve your health is a double loss. Sharing a “complete” experience including success, doubts and points of failures plus inviting others to join the discussing and pointing out other possibilities and caveats may be better then silence.
Still, I try to have very repetitive results for myself and even then I do need to see a sort of logic/sense in them before I put them on the forum.
Thanks for the generous offer of providing improved access to papers!! I’ll think I’ll start first with writing a basic “open literature based” text to only later go to checking by “closed literature”. Wright now I read many sources diagonally in search for something I can use, and for ease of doing so these need to be in the open.
The papaya topic is here https://www.healthrising.org/forums/threads/eating-papaya-with-protein-rich-meals-gave-me-a-significant-improvement-in-health.5980/
The first and last post are the short version: what I did and why I do think it does work for me. In between is a lengthy discussion that may lead to something new ;-).
All I can share here is my absolute willingness to be a test subject to find a cure or at least relief from this horrible existence. All of my independent research has led me directly to ME/CFS. MY GP’s have been zero help and tell me it’s all in my head. If I can be of help in finding a solution to this please contact me. I will go anywhere, anytime. Poke me, prod me, stick me, cut me, measure me, study me, ANYTHING! I’m right here waiting. I’ve been looking for a clinical trial and have been unsuccessful. Please help me help you help me and everyone stricken by this terrible disease! You’ll be heralded as geniuses and hero’s!
Thanks, dejurgen. I look forward to anything protocol-wise you feel comfortable sharing in the future. Like any number of conditions, ME is a clearly a multi-factored disease, yet there is bound to be a kind of Venn diagram ‘sweet spot’ where we all share something in common about the illness, and where treatment will ultimately overlap, at least to some extent. Anything you have to offer is of interest to me because there is a chance it’s inside the overlap. Who knows? You have thought as deeply on the subject as anyone has. Keep it up. It’s appreciated.
Thank you everyone for the most intelligent conversation I have read about CFS/ME. This information is invaluable to those of us trying to find a way out of this almost “hell on earth” existence. It is a complex disease but it sounds as if some fundamental revelations are emerging. It cannot be expressed enough how desperate people are to get some sort of relief from the mind bending fatigue and pain that this disease brings about. Thank you again for such good info.
We have come a long way from the Yuppie Flu days. Thank God there is now interest and hope. Many have faded but some like Dr. Nancy are still with us, thank you for the struggle, it could not have been fun without money or support.
Now all we need is time and more hope and maybe a prayer.
For many of us our money is gone, down rabbit holes.
I do think more is involved but I don’t understand why the delivery of more oxygen would eventually result in negative consequences if the delivery part is somewhat fixed. If the blood vessels are being constricted I also wonder if hbot would be enough. I wonder if anybody’s tried a blood vessel dilator and antioxidant booster in combination with hbot.
Thanks for a lot of useful info from yall. I’m scandinavian. I’ve had ME since -92 and now my son has it too:-( . I have tried all sorts of treatments and protocols during the years. Some has helped and I am no longer bedbound, but not well…..i take/ have taken B12 iv in the form Hydroxycobalamin to avoid liver issues. B12 is a great detoxifier and was used for gold miners that was toxic from QuickSilver used in mining. Magnesium iv helped a lot to relax muscles and increase bloodflow. But can’t get it anymore:( i take a lot of vitamins. Liposomal C and gsh gluta5hion from Altrient. I take all b vit. Have started taking Biocidin which I belive has great properties getting rid of biofilms,parasites and virusets! and now i have started citrulline to help get rid of ammonia build up which my former ME dr told me was a great problem. I think this is a good Idea..? Too much ammonia causes a kind of encephalitis and constricted bloodvessels. If you cantolerate lactose, Lactulose can help the body reduce ammonia. To help my liver I take glycine, and Thorne liver Cleanse. I don’t use alcohol, but still has type of fatty liver..? Toxins, ammonia? are to blame I think! ? To keep herpes reactivation away I take l-lysine and avoid Arginine, which made me really sick as it activates herpes. I get shingels. I had very very high levels of hydrogensulfide when DeMeirleir tested and i was near death. Detox is really important. Takusumi bamboo coal can help and chlorella etc.. As mentioned above I also have always very low blood sedimentation rate! So I never get help when I have severe infection which is a great trauma for me! Some real difficult suffering because of it. I really hope this hellish disease is solved soon! So thankful to all researchers that work with it!!! Hope real info get out to dr ’s that we patients meet soon. Its awful to be un treated this way! Good luck everyone!
Marie
I’ve been investigating whether I have a circulation or micro-circulation problem that underlies my symptoms. My primary symptoms are fatigue, muscle/connective tissue pain and stiffness, polyneuropathy in feet and hands, RLS and a variety of neuro-psychiatric symptoms that are worst at night and morning and severe while sleeping.
The clue to circulation and tissue hypoxia are pain in the tissue on which I lay while sleeping such as the shoulder, hip and arm on which I lie. I have an Oxygen generator and using O2 while sleeping greatly lessons this pain and awakening symptoms. My O2 saturation drops to the low 90% range without O2 and stays in the 97-99% range with O2. So I suspect poor circulation is reducing O2 delivery to my body and is worsened by the pressure of laying on muscles.
I’ve also had some unusual drug hypersensitivities that seem to cause similar symptoms 4-12 hours after ingestion. This includes all opiates whether oral, by patch or sublingual.
So what might be going on? I came upon the type III or IV hypersensitivity as one model that could be the connection between both problems. I’m exploring the type III.
It is caused by an excessive of immune complexes in the blood circulation due to the “antigen” binding to IgG antibodies resulting in circulating immune complexes. These complexes activate the complement system which binds to the complex and attracts neutrophils. If the neutrophils are unable to phagocytize (gobble up)the complex, it degranulates damaging tissue causing inflammation and pain. Mast cells are also attracted and will also degranulate causing inflammation and pain.
This mechanism is a common cause of inflammation and pain which can also cause damage to the blood vessels and decrease the delivery of Oxygen to the tissues. So what would trigger this cascade besides a drug hypersensitivity and its large amount or IgG antibodies?
One reasonable possibility is a gut permeability problem which allows excessive food and gut organism byproducts and liposacharides into the blood. If I eat any sugar and to a lesser extent carbs, the symptoms flare. So is my gut permeability problem leading to excessive circulating immune complexes which in turn is simulating a type III hypersensitivity which is also known as Serum Sickness. Serum Sickness is the triggering of this cascade due an influx of excessive antigens. Does gut hyper-permeability lead to something similar to Serum Sickness?
So how can one test for it? Possibly test the compliment system. When these immune complexes are detected by the complement system, C3 and C5 break into C3a, C3b and C5a. If C3a is high, it suggests this compliment cascade is active. C3 might also be low by using excessive C3. There is also a panel for detecting on group of complexes seen in Lupus, C1q Binding Assay and Raji Cell Immune Complex Assay. There are others but these might be helpful in detecting excessive Immune Complexes and Complement activity.
So how might one try and reduce the complexes and see if it helps? Proteolytic enzymes break proteins down. Immune Complexes are proteins. One possibility is taking bromelain, papain, pancreatin, trypsin, chymotrypsin, and rutin enzymes in an effort to assist or own proteases in reducing these circulating complexes.
So that is where I stand in my investigation as to whether Circulating Immune Complexes are triggering my Complement system and causing the symptom cascade I described. My doctor ordered the Complement tests and I’ve just begun taking the Proteolytic enzymes. I’ll report the results. Thought this line of thinking might be useful for others and I’m curious of others opinions and experience?
So nice to have such a clear possible explanation Tom.
Certainly complement issues have been found in ME/CFS at times, particularly, interestingly enough after exercise – which could be inducing leaky gut syndrome
https://www.ncbi.nlm.nih.gov/pubmed/30325737
https://www.ncbi.nlm.nih.gov/pubmed/26116897
https://www.ncbi.nlm.nih.gov/pubmed/19015737
Hi Tom,
The first 2 paragraphs are very familiar to me. I just haven’t measured O2 saturation levels and don’t use oxygen supplementation but I am very glad you mention measured values before and after. Thanks!
That confirms my suspicion and reduces the time I’ll need to get everything in order to write about my nightly routine in the forum. I’ll expect it to be this month and would greatly appreciate your feedback as comments to it as you seem to have a plenty similarities with my case. I just “miss” the drug sensitivities..
I strongly suspect blood flow to drop at night below bare minimum in many ME patients (due to no leg movements, head lying flat, hormones,…) resulting in poor blood flow including lung blood flow, acidification and toxicity of blood, upflaring oxidative stress…
Having frequent aphtous ulcers in the mouth? If so you might want to take a look to the papaya topic I made on the forum. In the middle a lot of lose ideas, but the first handful of posts and the last one are more to the point.
As to drug or in my case food hypersensitivities I have written elsewhere about the following idea:
Poor blood flow = less blood per minute passes along signal receptors = less signal molecules per second are activating the receptor = faint signal for plenty of chemical processes
=> the body adjusts and grows more receptors in places with (frequent) poor blood flow (mainly the capillaries)
but with our general low blood volumes and low blood flow + resistance of blood flow goes up when blood flows slow (worse then water, blood starts to stick together)
=> blood flow in capillaries in many ME patients shows a far larger fluctuation or ratio between max flow and min flow then in healthy people.
=> result:
very poor flow in capillaries * (strongly) increased amount of receptors is still underwhelming signal strength of signal molecules
“high” flow for us in capillaries (due to exercise, thinking, adrenaline…) * (strongly) increased amount of receptors is too big / overwhelming signal strength of signal molecules
=> result: frequent swinging between under and overreaction to many many substances; often causing symptoms and wasting energy at the same time due to unappropriated reaction strength.
Hi dejurgen
All my symptoms seem to be triggered by food after eating with sugars = horrific and most carbs = bad while proteins have little reaction. I have a known mild egg, milk, casein, gluten, and beef IgE allergy. I also have an IgG4 Casein allergy. Casein is broken down to Casomorphin which is an opiate receptor agonist – suspicious with my opiate sensitivity.
My total IgE ranges from 300-1100 depending on my avoidance of IgE allergens. This seems excessive unless I have an intestinal permeability problem.
I also have a high CK which is actually CK isoenzyme type 1 which is a CK bound to an IgG antibody = an immune complex. This is associated with myositis.
Sporadic high EOS and low RBC and hemoglobin and high MCV. Suggests a mild hemolytic problem which damages red blood cells. Careful diet improves this.
Everything suggests excessive antigens entering my blood stream from my gut. I was treated for C Diff one year ago and just received a G.Map Stool test with high C Diff Toxins A & B.
These toxins are known to damage intestinal lining. C Diff Toxins may be damaging my intestinal lining allowing excessive antigens into my system along with Toxin B .
These excess toxins/antigens including food, LPS and toxins may triggering IgE allergic symptoms and causing excess immune complexes that are leading to tissue damage due to “frustrated” Neutrophils and Mast Cell degranulation plus direct C Diff Toxin B damage.
This in turn is causing both pain neurological, lung and other symptoms after eating which can last a few hours to a day. One of the problems it seems to cause is blood vessel damage which may be inhibition O2 delivery to cells which is exacerbated during the low blood flow rate and low (90-95%) saturation during sleep. That might explain why O2 supplementation helps.
A Hypothesis not yet fully tested…
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4885049/
“Clostridium difficile is mainly mediated by two exotoxins: toxin A (TcdA) and toxin B (TcdB). These toxins primarily disrupt the cytoskeletal structure and the tight junctions of target cells causing cell rounding and ultimately cell death. Detectable C. difficile toxemia is strongly associated with fulminant disease. However, besides the well-known intestinal damage, recent animal and in vitro studies have suggested a more far-reaching role for these toxins activity including cardiac, renal, and neurologic impairment. The creation of C. difficile strains with mutations in the genes encoding toxin A and B indicate that toxin B plays a major role in overall CDI pathogenesis.”
Hi Tom,
The browser crashed just before sending a lengthy comment. So here is the short version:
One of my strengths in fighting ME back is my ability to see patterns in things.
If I combine yours, Not Dead Yet!’s and my observations with some gut related patterns I come close to a common gut related pathway to ME disease for a subgroup. The special thing about it is that it would include quite a lot of specific details and traceable processes IF wright.
From gut cell infection on (as opposed to infection growing on the inner tube surface of the gut) I’m working together with Cort on a model that can explain plenty off this disease.
Both parts combined might (plenty and plenty of chance to go wrong but my intuition says too valuable to ignore) form a usable full enough description off the bulk of ME for this subgroup. IF that could work out that would be nothing but a breakthrough.
Please take a look at Not Dead Yet!’s topic https://www.healthrising.org/forums/threads/rheumatic-fever.6092/#post-34577
were I will continue writing stuff related to this idea.
I started developing this idea in my papaya topic in the forum, but more in a brainstorm fashion.
Please make sure Cort can contact you if desired. I’ve got this overexcited Eureca moment very strong now :-).
p.s.:
Got often pain in the bowel that is point/sphere like with from time to time additional pain shoots originating from it?
About sugar: did you try to differentiate between slow carbs that convert to dominantly glucose and sources that are or convert to high loads of dominantly fructose?
What if you have orthostatic intolerance as part of your M.E and are on vasoconstricting meds like midodrine? Can this make your M.E worse?
How does a layperson like myself determine if my wife (who has ME/CFS) has the shape distortions in her red blood cells that could be causing her cognitive fog? Is there a blood test one can order from a local medical lab? …or a place to send a sample?
Live Blood Cell Analysis – Most Naturopathic Docs probably offer it – I had mine done once and the Naturopath said my blood cells were some of the most damaged he’s ever seen. I’m only 27 and my fatigue is severe most days and as a result I’m unable to work much.
I haven’t heard this being super common with MECFS patients but when I first got ill, and ever since then (12 years) I have had hypochromic microcytic anaemia – this is not iron deficiency anaemia or thalassaemia – so that’s been a mystery for me for the last 12 years – low MCH and low MCV so the normal hemoglobin doesn’t apply to me. Absolutely feels like constantly out of oxygen.
What’s hypochromic microcytic anemia?
Wow, this is alarming. Our focus should be more on preventing diabetes, because once you get it, there’s no cure for it. One way of doing this is improving our microcirculation, as this improves our blood flow in the tiniest of vessels, reaching all parts of our vital organs. There are a number of microcirculation therapy out there. You guys might want to look it up.
Great article! Does anyone know of a vasodilator or blood thinner that might be used to treat? Thank you!