

Disturbances in circulation and provision of oxygen to tissues could underlie many symptoms of ME/CFS. The authors
Metabolomics has quickly become one of the hottest research trends in chronic fatigue syndrome (ME/CFS). The metabolomic work in ME/CFS started with the Australians, picked up speed when Ron Davis and Bob Naviaux took it up, and is now being done all over the place. Hanson, Unutmaz and Lipkin make up the short list of new researchers involved.
With Ian Likpin and the Simmaron Research Foundation producing the first metabolomics study of cerebral spinal fluid (CSF), we should soon learn if the metabolomics profiles found thus far – which Hanson’s study reported have demonstrated an unusual consistency in ME/CFS – extend to the CSF and therefore the brain as well.
The Study
Prospective Biomarkers from Plasma Metabolomics of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Implicate Redox Imbalance in Disease Symptomatology. Arnaud Germain, David Ruppert, Susan M. Levine and Maureen R. Hanson. Metabolites 2018, 8(4), 90; https://doi.org/10.3390/metabo8040090
This study, partially funded by the Solve ME/CFS Initiative (SMCI), demonstrated how hot a topic metabolomics has become. The SMCI did a clever thing when, seeing that ME/CFS samples from prior NIH awards to Dr. Hanson were available, paid Metabolon to analyze them for her.
This study was, with its 832 metabolites surveyed, easily the deepest single look into the metabolomics of ME/CFS ever. Metabolon, the company doing the analysis, broke down the metabolites found into eight super pathways and 83 (!) sub pathways, making its analysis a very fine-tuned one.
No magic bullet was found – no single metabolite is yet able to explain ME/CFS – but the study did point an arrow in a promising direction.
Energy production
After an extensive analysis, nine metabolites belonging to four super-pathways (cofactors and vitamins, energy, nucleotides and peptides) were highlighted in the chronic fatigue syndrome group.
With several studies suggesting that the energy production processes in ME/CFS have taken a hit, having a key factor in the Krebs energy cycle – alpha ketoglutarate – pop up was rather stunning. Oddly, though, the levels of alpha ketoglutarate were higher, not lower, than in the controls- something the authors did not attempt to explain.
Oxidative Stress – The Major Stressor in ME/CFS?
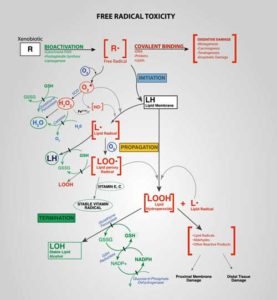
Studies suggest that oxidative stress could impact the brain, the muscles and energy production in ME/CFS. (By Dan Cojocari – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=46529393)
Indeed, energy production problems were not the theme of this study. Instead, an old “friend”, oxidative stress, was. Virtually every study that has looked for increased oxidative stress (free radicals) in ME/CFS has found it.
Free radicals have been linked, for instance, to reduced energy production in the muscles of people with ME/CFS. Plus, the membranes of the muscle cells – major targets of free radicals – appeared to be damaged as well.
Muscle biopsies have turned up evidence of oxidative damage as well. Plus a muscle gene expression found, guess what, altered expression of the genes involved in mitochondrial functioning, oxidative stress, and muscle structure and fiber type.
The higher levels of heme and three forms of bilirubin in this study suggested a general disturbance of the heme degradation pathway – reflecting perhaps an attempt to cope with high levels of oxidative stress. A disruption in the metabolism of vitamin E- an antioxidant – suggested that the antioxidant system in ME/CFS may not be up to snuff. All in all, the oxidative stress component appeared to be fairly well established but left a central question unanswered: why was it happening?
Oxygen, Oxygen, Oxygen
A further analysis of the metabolites provided a possible answer. Citing an anoxia metabolite set seen in this study and a past metabolomic study, an asphyxia metabolite in another study, and a similar metabolite profile in infants with hypoxic-ischemic encephalopathy (which is caused by oxygen deprivation), the authors got down to brass tacks and proposed that poor oxygen delivery was the tie that may bind these results together.
That was a nice conclusion given that low oxygen states produce enormous amounts of free radicals (oxidative stress).
The idea that energy and other problems in ME/CFS could derive from an inability to utilize oxygen properly seems to make more sense as time goes on. Oxygen utilization problems, after all, are certainly behind the early anaerobic thresholds reached during exercise in chronic fatigue syndrome.
In 2014, Vermeulen, a Dutch researcher, asserted just that. His massive ME/CFS exercise study (n=426) indicated that the energy production problems resulted from poor oxygen delivery to the muscles. Vermeulen was sure enough of that conclusion to plunk it into the title of his study (“Decreased oxygen extraction during cardiopulmonary exercise test in patients with chronic fatigue syndrome.”)

Cells gasping for oxygen – and turning instead to anaerobic energy production to produce energy – could be a core feature of ME/CFS.
Seven years earlier, Dr. David Bell, who, some years into retirement, seems to show up again and again, published a book called “Cellular hypoxia and Neuro-immune fatigue” positing that low oxygen levels at the cellular level were likely behind ME/CFS.
Since then, the evidence has been piling up. Health Rising recently reported on a condition call “stagnant hypoxia” found in POTS which occurs when the blood oxygen content is normal but the blood is flowing too slowly to deliver normal amounts of oxygen to the cells. The hyperventilation found in ME/CFS upon standing or during exercise (as Systrom has reported), could be a compensatory response to stagnant hypoxia.
The consequences of hypoxia in healthy people – fatigue, confusion, headaches and numbness of extremities – aren’t a perfect match for ME/CFS but are close enough to suggest that some form of hypoxia may be present.
For the past five years or so, Shungu’s findings have suggested that a similar scenario is occurring in the brains of ME/CFS and fibromyalgia patients. He believes that high levels of oxidative stress are constricting the blood vessels, impairing blood delivery and creating a hypoxic condition. That hypoxic condition wipes out the aerobic energy production process causing the brain to rely more on anaerobic energy production and produce the high brain lactate levels he has consistently found.
Recently, Ron Davis and SJSU researchers identified red blood cell deformation problems in ME/CFS that could easily impair microcirculation and oxygen delivery. The authors of that study, by the way, concluded that the red blood cell problems were likely caused by oxidative stress. The fact that methemoglobin – a form of hemoglobin that does not carry oxygen – was most closely associated with symptoms in one ME/CFS study suggested that red blood cell problems in ME/CFS could extend past deformability issues.
Several studies have also found reduced blood flow to the muscles in fibromyalgia as well as muscle abnormalities. An overactive sympathetic nervous system in FM may be producing a low oxygen (hypoxic) state caused by vasoconstriction (narrowing) of the blood vessels.
Many studies have suggested that oxidative stress associated mitochondrial issues exist in fibromyalgia. The latest suggested that the mitochondria in FM were in “hibernation” – perhaps a good place to be if a hypoxic environment is present.
Fundamental Processes Possibly Hit
The authors hypothesized that high levels of oxidative stress may be interfering with basic enzymatic processes involving coenzymes such as NAD+/NADH, FAD+/FADH and others.
Pointing to evidence of these problems from their past work, they asserted that, “dehydrogenase, transferase, and oxidase deficiencies are prevalent” and that a, “general imbalance”; i.e. a redox or oxidative stress issue – is affecting basic enzymatic processes in ME/CFS.
Interventions?
A big question with metabolomics, as with all the omics, is whether it will be able to deliver precise enough information for therapeutic intervention. The Hanson study – which pointed to a number of interesting findings, which jive with what we seem to be learning elsewhere about ME/CFS – was a case in point. The findings seem to make perfect sense, but the authors noted that the study was still too small to provide medical advice.
There’s no answer to the oxygen delivery dilemma – if that’s happening in ME/CFS and FM – yet but some approaches may provide some help. Anti-inflammatory diets and antioxidants have been used in ME/CFS for years to try and tamp down inflammation and the wear and tear of oxidative stress. Shungu reported at the last IACFS/ME conference that NAC was helpful, and Dr. Klimas will be exploring ways to reduce inflammation and oxidative stress with her new Gulf War Illness grant.
The fact that phenylephrine, by getting more blood (i.e. oxygen) to the brain, virtually stopped orthostatic intolerance in its tracks in a small ME/CFS cohort seems all the more notable given these findings. Dr. Julian Stewart suggested that some way has to be found to get more blood to the brain.
Blood volume enhancers, stockings, drugs (Mestinon, Florinef, Amytriptyline, propanolol, midrodrine) may be helpful as well. Staci Stevens of Workwell suggests deep breathing exercises to get more oxygen into one’s system.
Mestinon, which affects parasympathetic nervous system functioning, enabled one ME/CFS patient to exercise for the first time in decades.
Blood flow enhancing devices such as the Hummingbird and the Avacen devices described in these pages might be helpful as well.
Hyperbaric oxygen therapy (HBOT) is controversial but one Isreali study got good results in fibromyalgia.
Migraine – A Disease to Keep an Eye on
Migraine, with its blood flow issues and high prevalence in ME/CFS and fibromyalgia (and the recent breakthroughs in treatment), is certainly a disease to keep an eye on. In fact, a recent study broke new ground when researchers were able to trigger migraines – something they’ve had difficulty doing – by subjecting migraineurs to low oxygen conditions. Doing that caused their blood vessels to dilate – which the phenylephrine study suggests may be happening in ME/CFS – and their brain lactate levels to mount – something which is happening in ME/CFS.
The Next Study
The authors noted that the study’s small size (52 participants) relative to its wide scope (over 800 metabolites) probably made it impossible to distinguish all the important metabolites in ME/CFS.
The authors reported that
“Future work in which a larger and independent cohort is analyzed and compared to other fatiguing illnesses will likely increase the prediction confidence and will reveal whether plasma metabolomics may serve as a reliable tool for objective identification and monitoring of ME/CFS patients.”
Translation = we need bigger studies – with more disease sets – not an uncommon conclusion.
Conclusion
The largest ME/CFS metabolomics study to date highlighted metabolites involved in energy production, oxidative stress and low oxygen conditions. While the study was too small to be determinative it did point an arrow in a familiar direction with its suggestion that low oxygen conditions are creating massive amounts of oxidative stress which are affecting energy production and possibly disrupting fundamental enzymatic processes in the body.







I just asked my Doctor about my Oxygen levels she checked in Office saying nothing wrong there it was 98% & fine. A paper recently Published
on a Girl with (HATS) Hereditary Alpha Tryptasemia Syndrome she responded to Omalizumab injections a Case study back in November 2018 in Annals of
Allergy, Asthma & Immunology she was even taken off the medicine for a short period her symptoms came back & back on the medicine now. It
mentions autonomic dysfunction & mast cell issues & anaphylaxis
Interesting that medicine maybe worth a trial.
As for you, yes I’d expect ME/CFS patient oxygen levels to be within the normal in arterial vessels heading towards cells. But where the abnormality appears is the venous (return used blood) to the heart is abnormally high in oxygen. Meaning the cells in the body didn’t uptake enough oxygen for the production of energy.
Interestingly I had an oxygen monitor and found when sitting still for more than 10 minutes my oxygen tended to drop to 95% (which is still considered ok)
but had I just walked in a doctor room it would also be at 98%.
Even so I tried a portable oxygen machine for about 6 weeks, but no benefit whatsoever. In fact I stupidly fell asleep with it turned on and woke up in extreme pain. Extra oxygen for prolonged periods is dangerous.
Anyway research is showing the problem is in the cells not utilising the oxygen provided. As we do provide enough. Although those bedridden must learn good breathing techniques. As part of normal deconditioning is reduced oxygen supply due to the lungs not fully inflating enough. 5 Deep breaths held for several seconds once an hour helps maintain the lungs and in turn higher oxygen levels can be maintained.
This video is cued to start about a minute before the researcher talks about abnormally high oxygen levels in venous blood returning to the heart. It’s basically an unused oxygen problem, not a lack of oxygen supply…
https://m.youtube.com/watch?v=FMaKfv8peww&t=643s
Thanks Brendan for the link to the video 🙂
No problems with oxygen supply in the arteries that’s for sure. If it happens it’s happening in the capillaries I guess. Even then the oxygen supply in the blood may be just fine – if I’m reading it right – and this is very complex – it’s just not getting through. Interesting about the O2 in the venous circulation. I hadn’t heard about that. I would think that Systrom would have some data on that.
This information is fascinating, thanks! Perhaps if we learn to pause between every breath & breathe through the nose to increase nitric oxide (& open the blood vessels) there may be a breakthrough. I practice breathing techniques, but now feel the answer may lie in correcting a habituated ‘tense’ breathing pattern throughout normal day to day life? I
certainly find myself holding or constricting my breath regularly, especially in social situations & spiralling from feeling ‘too tired to breathe.’ Many thanks for this ‘aha’ information! 🙂
Any thoughts on using 35% food grade hydrogen peroxide orally ?
I totally agree that CFS is related to not getting enough blood flow to the brain or utilizing energy correctly. When I take Adderall it improves drastically bit as soon as it wears off I crash. I need help and want to live my life. This condition is only getting worse the older I get. I have had it my entire life. There has to be a way to increase my low blood pressure without drugs so I can function.
Great article! I would love to participate in a larger study.
Is anyone looking at using hyperbaric oxygen therapy (HBOT)?
Or glutathione?
I’ve tried! It’s a difficult thing to get insurance to cover.
Liposomal glutathione is an easy option, but many of us do not tolerate it.
https://www.pureencapsulations.com/liposomal-glutathione-2783.html
I tried an oxygen machine for 6 week but it didn’t improve me at all.
This video is cued to start about a minute before the researcher talks about abnormally high oxygen levels in venous blood returning to the heart. It’s basically an unused oxygen problem, not a lack of oxygen supply…
https://m.youtube.com/watch?v=FMaKfv8peww&t=643s
That’s my guess as well. It’s oxygen that’s not being used.
HBOT is controversial but an Isreali study got good results in FM = https://www.healthrising.org/blog/2015/06/26/new-age-fluff-or-real-treatment-fibromyalgia-hyperbaric-oxygen-therapy-study-opens-eyes/
Hi Cort – we are getting access to a HBOT shortly for our ME teenager (with largely neurological symptoms). What is controversial about it. We were encouraged by the Israeli study.
Hi Rose,
Only controversial in the sense in that aside from that study it’s not validated and is a brand new approach. For myself I certainly don’t see any harm; the only question is if it works and if it does work, if it sticks.
Good luck with your son!
Hi Rose,
how did HBOT work for your son?
I’m thinking of trying it myself.
I’ve been using 200mg/day s-acetyl-glutathione by mouth (bioavailable form of glutathione) for about 5 years now. I’ve also done glutathione IV pushes once a week for 3 months, HBOT therapy, and ozone autohemotherapy for 3 months…..all without any benefit.
I’m not surprised. I think those things work for some but I don’t think they are the answer for most. My guess is that they’re not getting at the root
hi i am trying to increase glutathione with non denatured whey protein concentrate. also I use turmeric. it is helpful for mental health too. I hear some get side effects from NAC.
Cort, I’m just sitting here thinking how very intelligent you are. We are so fortunate to have someone with your smarts to be able to, year after year, break down all these different areas of research into something that is comprehensible to those of us with less than a scientific grasp of things.
Thank you.
Ditto, ditto, ditto.
Cort’s communications are consistently informative, intelligent and upbeat.
We are all lucky.
Wonder how it compares with extremely atrophied people. If oxygen/metabolic/hibernation problem also shows up in atrophy, it could be just another symptom of CFS rather than the cause. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6176399/#!po=34.0000 in particular seems to confuse CFS with atrophy and therefore prescribes exercise.
“it could be just another symptom of CFS rather than the cause.”
I agree. While very high amounts of oxidative stress and poor oxygen delivery can create a vicious circle, most of us should be able to snap out of it over time and with sufficient rest IMO.
I believe many of us have additional weaknesses or problems creating a constant stream of “new” problems adding new oxidative stress to this vicious circle making it much more difficult to snap out from.
Think of chronic infections or immune problems, gene mutations, gut issues… all able to deliver a constant high production of “new” oxidative stress. In such case, tackling this disease should target both the vicious circle and the remaining strong sources of oxidative stress.
Still, I believe that the very strong body-wide chronic oxidative stress thing is causing plenty of our symptoms including energy production inhibition. It may just be not the final cause of it.
Great blog Cort!
I love seeing more research going into this direction!
Remember when you asked me if some of my ideas (NADPH/Pentose Phosphate Pathway route strong upregulation in ME, hyper body wide oxidative stress, body wide reduction of heme stocks (RBC + mitochondria), damaging heme by ROS producing even more ROS being a potential dominant feed back loop closing the vicious ME/hyperoxidative circle…) could be backed up by metabolics research? This looks like hitting close.
Unlike the authors I do not believe the high amount of free heme is mainly due to poor heme degradation by the liver but rather by the very high rate of heme damaging by oxidative stress. Oxidative stress is known to be very damaging towards heme. Damaged heme must be removed out of the body. One step in that is moving it from the cells into the bloodstream in order to reach the liver. So much damaged heme equals plenty damaged heme in the blood. Heme detoxification may be impacted on top of it.
“Krebs energy cycle – alpha ketoglutarate – pop up was rather stunning. Oddly, though, the levels of alpha ketoglutarate were higher, not lower, than in the controls- something the authors did not attempt to explain.”
Makes total sense IMO http://www.science.co.il/hi/pub/1995-NADPH-production-steroidogenic-mitochondria.pdf:
“isocitrate + NAD(P)+ + H+ –> a-ketoglutarate + CO2, + NAD(P)H”
=> It’s one of the few pathways in the Krebbs cycle that can shift from producing NADH to NADPH. NADH produces energy and tends to increase oxidative stress. NADPH production is anti-oxidative by producing the basic building block needed to recycle all important anti-oxidant glutathion.
Producing as quickly as possible alfa-ketoregulate from D-isocitrate by firing this route up enzymatically produces more anti-oxidant. It also pulls back along the chain (cis-aconitate, citrate, pyruvate). That decreases the overload on pyruvate ME patients have (metabolics, Naviaux). When pyruvate gets too high it gets toxic and must be converted in large amounts of lactic acid and puts a heavy load on the liver for recycling to both to glucose.
The next logical step the body could take would be to decrease Alpha-ketoglutarate dehydrogenase. That would make sure high amounts of Alpha-ketoglutarate would not push strong conversion rates into succinyl-coa as that would produce copious amounts of energy and NADH plus added oxidative stress.
Now that would create a massive stockpile of Alpha-ketoglutarate if this stuff wasn’t used or decomposed somewhere else. Lucky for us, it’s an anti-oxidant further increasing the anti-oxidative power of this hypothetical process:
en.wikipedia.org/wiki/Alpha-Ketoglutaric_acid:
“Antioxidant
α-Ketoglutarate, which is known to be released by several cell types, decreased the levels of hydrogen peroxide”
Also of interest:
http://www.jneurosci.org/content/20/24/8972
with title “Inhibition of Krebs Cycle Enzymes by Hydrogen Peroxide: A Key Role of α-Ketoglutarate Dehydrogenase in Limiting NADH Production under Oxidative Stress”
Note: this is the enzyme producing succinyl-coa from alpha-ketoglutarate. Being low on that means more alpha-ketoglutarate and a blocked Krebbs cycle producing less oxidative stress and energy.
Another interesting one:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3169906/
with title “α-Ketoglutarate dehydrogenase: A mitochondrial redox senso”
and content:
“Treatment of mitochondria with H2O2 results in reversible inhibition of KGDH due to glutathionylation of the cofactor, lipoic acid.”
=> That’s another part of my “massive oxidative stress blocks energy production willfully” hypothesis. Glutathionylation is glutathion or oxidized glutathion binding to key enzymes. This action can either make an enzyme more potent or inhibit it. As levels of glutathion and reduced glutathion and their ratio are a very strong marker of oxidative stress, it stems to reason that this mechanism is strongly modulating energy production / the Krebbs cycle in function of oxidative stress.
So far I did find other points in the Krebbs cycle and in glycolysis where glutathionylation plays a key role. That makes our very strong downregulation of energy production look an awful lot like a drastic protection mechanism against rampant oxidative stress.
Nice to note: this Krebbs downregulation mechanism is REVERSIBLE! Just have to get rid of massive oxidative stress ;-).
As to fast converting to anaerobic energy production upon exercise I still remain a firm believer that it is actually a very strong shift from glycolysis and Krebbs towards the Pentose Phosphate Pathway. The PPP shares 2/3 of its pathway with glycolysis making it very easy to mistaken a very strong upregulation of the PPP for a very strong upregulation of glycolysis. But the PPP produces plenty of NADPH, an essential ingredient for both fighting oxidative stress and firing up our immune system. And both seem to be all over the place in ME.
As to poor blood volumes, flow and cellular oxygenation levels. Yes I believe they are damaging to our health. And they do produce plenty oxidative stress creating a vicious circle. But I believe they prevent even worse longer term oxidative stress. Firing blood volumes and flow up a lot may trade short term improvements for long term risk IMO. Medically unblocking the Krebbs pathway may do the same for most patients IMO.
“Now that would create a massive stockpile of Alpha-ketoglutarate if this stuff wasn’t used or decomposed somewhere else.”
I’ve been searching for a more precise pathway then “has anti-oxidative properties”. I found one:
http://www.hindawi.com/journals/bmri/2018/3408467/
“Alpha-ketoglutarate (AKG)… …AKG acts as an antioxidant agent as it directly reacts with hydrogen peroxide with formation of succinate, water, and carbon dioxide”
=> Now that is nice!
* Increasing AKG *can* increase NADPH production *if* the NADPH pathway is chosen instead of the NADH pathway. That increases glutathione recycling plus immune system strength.
* AKG can directly scavenge H202 and permanently remove it.
* One of the end products of AKG is succinate, another intermediate of the Krebbs cycle. The direct production of succinate from AKG and H202 just “happens” to bypass the syccinyl-coa step, a step producing NADH, energy and oxidative stress.
=> That’s three birds in one stone when it comes to reducing oxidative stress. That seems to be no coincidence. Unfortunately it leaves us with fewer energy :-(.
=> As another advantage, it uses no “real” oxygen in a sense that it just uses an oxygen atom from plentiful available H202 rather then needing to get oxygen from the RBC. That makes poor oxygen availability a bit less bad.
See also the big picture on en.wikipedia.org/wiki/Krebs_cycle
That’s an impressive command of metabolic biochemistry, dejurgen 🙂
“I love seeing more research going into this direction!” The problem I have with all these studies is that they are either not controlled or very small with healthy people as control. I wish they would do a larger authoritative study with similarly atrophied people just to establish there indeed is metabolic problem unique to CFS.
Likewise, I wish they would hurry up and replicate Younger’s and Nakatomi/Watanabe’s brain imaging studies to establish the brain inflammation as a fact. The brain inflammation has to be there; you can’t otherwise explain paranoia that Bruce Campbell reported, or whole slew of other neurological symptoms.
Once we have established all the facts — metabolic anomaly, brain inflammation, microglial over-activation, etc — we can start figuring out which is the cause and which is the symptom. Not only that, we are likely to have a biomarker for CFS if any of them becomes established and testable.
“The brain inflammation has to be there; you can’t otherwise explain”
I’ve missed the ball big time with thinking that alpha-ketoglutarate could pull forward conversions of citrate and pyruvate, depleting pyruvate stocks. But the alternative is very intriguing.
Aconitase is a key enzyme converting citrate to D-isocitrate. See en.wikipedia.org/wiki/File:Citric_acid_cycle_with_aconitate_2.svg
It’s used in 2 steps so any inhibition must have extra strong effect on inhibiting the Krebbs cycle.
Now see this: the first effect of low doses of oxidative stress is to inhibit aconitase activity a lot, and it is happening before (at far lower oxidative stress levels) the supposed major modulater of the Krebbs cycle (alpha-ketoregulate dehydrogenas).
See http://www.jneurosci.org/content/jneuro/20/24/8972.full.pdf
“t isconcluded that (1) aconitase is the most sensitive enzyme in theKrebs cycle to inhibition by H2O2, (2) at small H2O2concentra-tions (#50mM) when aconitase is inactivated, glutamate fuels theKrebs cycle and NADH generation is unaltered, (3) at higher H2O2concentrations ($100mM) inhibition ofa-ketoglutarate dehydro-genase limits the amount of NADH available for the respiratorychain”
and “Decrease in the glutamate content of nerve terminalswas induced by H2O2at concentrations inhibiting aconitase.”
Now see the outright baffling curve about aconitase activity on figure 1: it tanks the first two steps of the Krebbs cycle very very very deep with even light amounts of oxidative stress! That may help clarify why even low grade brain inflammation can be so utterly crippling.
In the body, the glutamate can fuel a fairly big part of the Krebbs cycle, see second picture in en.wikipedia.org/wiki/Gluconeogenesis because it does not need to pass through the aconitate depending citrate steps.
Good thing: the glutamate to alpha-ketoglutarate step can produce a piece of NADPH as well.
For the brain, things seem to be rather dire however. Information or even consensus on what passes the BBB well is limited. But glutamate and glutamine appear to be only travel it slowly. The best ones at passing the BBB are the essetial amino acids like leucine, isoleucine and valine. Only the latter 2 seem to enter the Krebbs cycle at the wright insertion point (not needing the citrate steps).
Problem is: removal of ammonia.Their seem to be limited “typical” ways to reduce ammonia out of the brain, but all I did found so far seem to be limited in speed to do so. And ammonia pile up in the brain is rather poisonous and causes edema (brain swelling by water retention, relation to high CBF pressure?).
Just removing it by binding it to a product of glucose or pyruvate and ammonia and transporting that through the BBB ain’t working well either as the produced amino acid would be non essential and those seem to be pretty poor at passing through the BBB.
I did find one theoretical way to remove ammonia easily through the BBB and it is by conversion of citrate to malonic acid to barbituric acid that travels well through the BBB and reaction speeds seem to be OK. That pathway is however never studied nor observed and needs to be nontoxic or be very quickly broken down. However it is “weird” that the barbituric acid would be rather easy to produce and pass through the BBB while also decreasing the citrate pile up in the Krebbs cycle.
Another possible one is internal conversion of glucose or pyruvate to glutamate in the brain to alpha keto-glutarate. I can find no pathway so far and it would be inefficient too but at least keep nitrogen/amonia balance fine.
last ones:
citrate is a prime blocker of glycolysis and low NADPH is a prime enhancer of the PPP in parallel to glycolysis.
So direct glucose (the main brain fuel) is inhibited in all pathways (Krebbs, glycolysis) but the NADPH producing PPP. But that is providing rather low amounts of energy, even less then glycolysis per amount of glucose!
Beta oxidation of fats is problematic as well as it’s product acetylcoa needs to get trough the citrate roadblock too.
It seems that our brains have to work *very* hard to get any source of fuel at all! No wonder they are hit so hard with even low grade inflammation. Don’t know where ketones enter the Krebbs cycle. I’d bee VERY interested to know if someone can help me out on this one. I’d guess they might enter at the keto-glutarate point as that is a ketonic step as well. Would explain a lot about ketogenic diet being helpful for many in ME, epilepsy…
Problem with ammonia probably solved. It popped into my mind that Iosine, one of two components in Isoprinose, is a ribose structure incorporating nitrogen. And Isoprinosine is the one drug that helps me the most.
Isoprinosine aka Immunovir aka en.wikipedia.org/wiki/Inosine_pranobex:
“Inosine pranobex (BAN; also known as inosine acedoben dimepranol (INN) or methisoprinol) an antiviral drug that is a combination of inosine and dimepranol acedoben”
There is also quite a lot of information on its neuroprotective action and action on the CNS. As the drug is administered orally I suppose it has to pass the BBB quite well.
en.wikipedia.org/wiki/Inosine:
“Adenine is converted to adenosine or inosine monophosphate (IMP), either of which, in turn, is converted into inosine (I)”
So in order to get Inosine, inosine monophosphate (IMP) will do. en.wikipedia.org/wiki/Inosinic_acid:
“Inosinic acid or inosine monophosphate (IMP) is a nucleoside monophosphate.” See the picture, it contains plenty of nitrogen.
en.wikipedia.org/wiki/Inosinic_acid:
“The inosinate synthesis is complex, beginning with a 5-phosphoribosyl-1-pyrophosphate (PRPP).”
en.wikipedia.org/wiki/Phosphoribosyl_pyrophosphate:
“Phosphoribosyl pyrophosphate (PRPP) is a pentosephosphate. It is formed from ribose 5-phosphate by the enzyme ribose-phosphate diphosphokinase.”
And finally en.wikipedia.org/wiki/Ribose_5-phosphate:
“Ribose 5-phosphate (R5P) is both a product and an intermediate of the pentose phosphate pathway.”
=> Puting glucose into the PPP produces among others much needed NADPH and R5P on the side.
=> It seems under oxidative stress very few energy producing pathways are left, a main one is converting the few amino acids that get through the BBB for use in the Krebbs cycle. Problem is getting rid of very large amounts of ammonia waste near permanently.
=> That seems to be solved by producing Iosine, a drug that has multiple good properties for ME patients and *very* likely can cross the BBB well enough. It has neuroprotective propertiesm antiviral ones, immunomodulating ones and anti-oxidant properties. And it can (very likely) remove fair quantities of ammonia waste too.
One has to love the inguity of the body :-).
This is so very interesting. Since becoming ill with ME, my main way of describing how it feels is: “it feels like every cell in my body is deprived of oxygen”. Especially my brain. This is a great summary of the research in this area and I found myself reading it and saying “yes! yes! yes!”
Oh my gosh Anna Kerr.. I’ve said that for the last 20 years that I’ve battled CFS! “Oxygen isn’t getting to my organs
.. Especially my brain.” How can we know and feel that? Yet I do. In my first and worst 5 years, I even experienced an overpowering sense of going crazy, and I even had out of body experiences. ..like my brain was on the fritz.
What do we do? My levels have been in the mid 80s sometimes at night as low as 78 but get up to around 94 when I’m moving but cannot move but for seconds because I’m severe
Cort, I am 100% convinced that are only hope is Hyperbaric Chamber treatments. Who can I contact with my information to get them to do a study on this so the FDA can aporove it and insurance will cover it and we can all get better someday?
Hi Lil, i’d love to hear more about the hyperbaric treatment you did. i’m on twitter at RivkaTweets
Too few oxygen supply does cause oxidative stress. Too much oxygen does cause oxidative stress as well. It tends to be worse then a bit too few oxygen. In an ideal world we sit in between.
Having increased heme in our blood, be it due to more production of damaged heme or due to slower removal from the body by the liver, does increase oxidative stress.
That probably does increase our vulnerability to oxidative stress, shifting the optimum between too few or too much oxygen towards lower values. As such these “too low” values of blood flow and cellular oxygen may be closer to optimal then we would like them to be.
It also adds another potential reason as to why we hyperventilate. Free and or damaged heme has not much use as far as we know except waiting to be recycled. When it comes in contact with oxygen then it does easily produce oxidative stress. One way to reduce the contact between free heme and oxygen in the blood is by having the RBC hold tight to their oxygen molecules.
This can be done by using the Bohr curve: low amounts of CO2 in the blood significantly decrease the release of oxygen by the RBC. As such, free heme passing by a RBC is less likely to snap oxygen from this RBC, leaving the heme “unable” to produce extra oxidative stress. The downside is that this also means poorer oxygen delivery at the cells. But knowing that free and damaged heme can produce vast amounts of oxidative stress I’ll stick to “hyperventilating” (in order to reduce blood CO2 to “too” low levels).
The Bohr and Haldane effect in combination with hyperventilation (physical problem not psychological) low Vo2max, bloodflow and problems with red bloodcells is the core problem in ME. If we can tackle this we know the cause of this disease. ME looks like anemia and sepsis to me.
“ME looks like anemia and sepsis to me.”
Maybe it (better: a large subgroup of cases) looks like sepsis plus anemia caused by this sepsis.
For many other cases I guess you can replace sepsis by serious immune problems. Both cause the need of a massive immune response costing plenty of NADPH and glutathione.
The NADPH is needed in order to create ROS used as a weapon by the immune system. Glutathione is needed to clean up the ROS that didn’t hit the “bad stuff” and prevent ROS from hitting and damaging our own cells. In order to recycle this used glutathione, another big stash of NADPH is needed.
When that happens too much for too long a time both NADPH and glutathione are deeply depleted, leaving us with very poor defenses against oxidative stress.
As a desperate defense against that NADH/energy production is willfully inhibited to the max in order to reduce generation of oxidative stress. Reducing oxygen “usability” by among others hyperventilating (low CO2, Bohr effect) / poor blood flow… energy production is further decreased on top of inhibiting key enzymes, further decreasing oxidative stress production.
Decreased availability of oxygen further decreases the prime substrate of ROS: oxygen, further decreasing oxidative stress *on average*. I add on average as low oxygen conditions do increase percentage of oxygen converted to hydrogen peroxide.
Note however: *percentage* of oxygen converted to hydrogen peroxide. Having for example only 60% of oxygen available / consumed but producing twice as high a percentage of hydrogen peroxide sounds really bad, but that still is only 0.6*2 = 1.2 times the normal hydrogen peroxide production or a small increase of 20% *at the mitochondria*.
That increase of 20% ROS production at the mitochondria in this hypothetical example may be less than the reduction (by having poor oxygen availability) of ROS produced by the increased (as seen in this study) free and damaged heme in the blood.
Also note: it is increased heme in the blood *per volume* in this study. We’re generally short on blood volume.
Having a partial moderate oxygen starvation and really low blood volumes and flow as a last line safety mechanism sounds desperate, but many forms of sepsis are lethal in less than a day. So it may be worth it for long term survival and it seems it could work.
I love that you keep us abreast of all that science is doing to try to help us. You put it in language that makes us able to understand some of the nuances involved, without dumbing it down.
I still keep up with most studies, but my WOOHOO has simmered to a ‘that’s interesting’ over the years. Where I was sure that a miracle cure was around the corner and I would have my life back, I am now content that there’ll be answers for those younger, or not yet afflicted …. some day.
Keep up the great work!!
Maybe issues with nicotinamide salvage, caused by NAD levels dropping below a critical threshold, leads to ME/CFS by initiating a pathological steady-state level of AMPK activity.
Oxidative stress leads to damage of DNA and cell membranes and repair of this damage depletes NAD. This would lead to AMPK activation which leads to NAMPT upregulation which stimulates to nicotinamide salvage pathway to replete NAD levels back to normal. Apparently this salvage pathway critical in humans to maintain appropriate levels of NAD. However if NAD levels are too low, one cannot make sufficient ribose to support this salvage pathway. This results in an increase of nicotinamide levels in the body which further activates AMPK. High AMPK activity leads to more ROS due to an increase in fatty acid oxidation. When NAD levels drop below a critical point, one can becomes trapped in a vicious ROS cycle until a new steady state of (high) APMK activity levels is reached.
A pathological steady-state level of AMPK activity would explain a lot of the findings in the metabolomics studies done in the last couple of years, including high levels of alpha-ketoglutarate (as AMPK activation leads to downregulation of dehydrogenases) and disregulation of the kynurenine pathway.
Theoretically, inhibition of AMPK and/or avoidance of AMPK activators with support of the nicotinamide salvage pathway (via an appropriate combination of supplements which will depend on the biochemistry make-up of an individual) may help break through the pathologic steady state of high AMPK activity that may be causing the symptoms of ME/CFS.
Whoa- a biochemist to the fore – you even got increased alpha ketoglutarate in there! Thanks Moira. I was lousy in chemistry but I think there’s been quite a lot of thought around NAD (I think it was NAD :)) over at Ron Davis’s lab. I remember some very intricate diagrams 🙂
Cort, I just wanted to add my thanks to all the work you do to help us to understand what is going on with Fibro and CFS. My wife has one or both and suffers terribly. But at least your blog helps us to understand that research is going on and to try and find hope in that. Thank you and God Bless.
Thanks Roy. Having got this around the age 20 it’s a personal journey for me. Thanks to the community’s support I am able to keep digging in, trying to find out what’s going on and continuing to report on it. 🙂
I want to add my thanks as well; this blog is an important part of my life, it allows me to be optimistic. I’m about to send a check to help support it.
Extra : I would love to read a blog about Dr Nath’s NIH study, an update on how it’s going. Cort, didn’t you interview him?
A 1969 (!) paper on ATP and red blood cell deformability:
https://dm5migu4zj3pb.cloudfront.net/manuscripts/106000/106038/JCI69106038.pdf
Gee it seems we are quite close to understanding this wretched disease! Close but no cigar! Who is going to finally solve the riddle?
Isn’t Fluge looking into the blood vessels?
Yes. One of the substudies of the Rituximab trial involved the blood vessels as I remember.
This is nice evidence to have in my pocket, supporting the hypothesis by which I’ve managed my illness for 28 years: that my cells were not uptaking O2 or nutrients effectively.
Sadly, it was only 12 years ago that I began O2 monitoring along with my HR monitoring, and discovered that my blood O2 randomly drops into the 80% range and has gone as low as 66% under workload (on a treadmill during an early heart test).
This drop happens fast when under load and slowly when sitting around, but it always drops eventually. Like my brain has forgotten how to recognize a drop in blood O2 and call for more. When that happens eventually my adrenal hormones surge to try to keep me alive, which burn through available cellular energy and leave me tired-but-wired.
Now I have a portable O2 concentrator (set only at 1.5 l/min) which immediately repairs the situation when my O2 drops, and has considerably reduced my adrenal surges as well as improving my cognitive-function time on a task.
I still hit the wall of cardiac hypoxia if I walk too quickly or get stressed, and still get PEM/aching muscles if I outstrip my muscles’ anaerobic threshold but I can stay on my feet at a slower pace for longer with O2 stabilizing blood levels than without.
Now that I’m on Mestinon I’ve noticed another increase in my muscles’ ability to do more and recover between exertions without triggering the deep PEM I used to experience. It’s not a miracle cure for me but it’s definitely improved my ability to function on the Activities of Daily Living that are my form of endurance athletics.
This aligns well with the work that Bob Miller is doing at https://www.nutrigeneticresearch.org – he is finding significantly higher genetic variants in chronically ill lyme patients relating to the heme pathway, NAD+ production, and a few related pathways that in his words, create a ‘perfect storm’ of free radicals and oxidative stress. If you find this interesting, worth looking at Bob Miller’s posters of his findings at https://www.nutrigeneticresearch.org/research/ as it lays out a lot of these biochemical pathways…
Of course it is. I wrote on PR nearly a year ago how ALA took me from 5 to 8 on bells scale.
It MUST be taken on empty stomach 4 times a day. 500mg each time. Works well with carnitine.
There is a systematic detox block in us that prohibit mito function.
Hi Greg I’ve just started on ALA and will order the Carnitine. My doc in Aus knows nothing about ME/CFS and Fibro but at least he’s trying. He even attended an infectious diseases seminar in Portland (USA). He didn’t tell me what dose to take so will follow your advice. Thanks for sharing.
I had some good results too, although NAD+ has been the best thing for me.
I have nad+ here never gave it a proper trial. What dose u take mattias.
Peach please pulse ALA. Need to take a day off it every 4 or 5.
Funny I find ibrufen good as well for fatigue. No idea of mechanism but must be suppressing cytokine production or something.
Does IVIG or rituximab address this low oxygen uptake issue? Or is the immune system totally unrelated to this proposed cause (low O2)?
It’s possible that the immune system is attacking receptors that open/close the blood vessels. The immune system appears to be attacking several aspects of the cardiovascular system in POTS so it’s entirely possible that it’s doing something similar in ME/CFS.
Unfortunately, the Rituximab trial failed.
“Unfortunately, the Rituximab trial failed.”
Either that, or the placebo (IV saline) proved to be a success.
Given previous reported success of long term IV saline administration I would hope someone would at least redo this IV saline study on a larger and better controlled scale.
I’d prefer it to the IV saline that came with the Rituximab administration to be successful over Rituximab any day given IV saline will very likely have less severe side effects then Rituximab. It’s cheap and unpatentable as well.
Interesting stuff. Patients can list a whole lot of similar and some different symptoms, but like others I have experienced the main clinical picture to be some sort of oxygen starvation, muscles, lungs and brain – whole body on a cellular level. Why? No idea.
It’s much like having a low level of oxygen at hand, for instance 20% of the ideal 100%, given at your best. The more you use during the day, the harder the payback is. That’s even when trying to walk a short distance and very gently. You will probably reach PEM by late afternoon/night and pay hard the following day(s), meaning – lay flat to load oxygen/not expend for a while. Then you do the same all over again..
I can’t count the evenings when I’ve read/told a story for my children at bed, and have had to stop cause there are literally no oxygen left for speaking. That is extremely absurd, but that is what’s it’s like when severe shortage of oxygen.
Cort,
Once again thank you for your continuous contributions in disseminating data and keeping us abreast of ongoing research and developments! Is it just me that thinks oxidative stress, metabolic traps, and ? on ? are symptoms rather than causations of this disease? Why isn’t more research dedicated to pinpointing the etiology of the illness? Is there anyway you can post a future article on the importance of re-examining ME/CFS cluster outbreaks ?? I think there are many clues and ?s that may exist in getting closer to finding the true culprit that caused or is still causing our illness by studying the history of cluster outbreaks whether in a scientific, journalistic, or historical approach.
Paul Cheney has been talking about oxygen issues for years. His lecture from 2013 talks about oxygen and oxidative issues among other things. The video with a full transcript is available on the Paradigm Change website at: https://paradigmchange.me/wp/cheney/
THIS is why my supplements work (down to the mitochondria in my cells)!!! I freaked when I read this!!! I studied every single ingredient in detail and took notes and compared strengths and dosages of ingredients before I started my supplements (14 per day). I also compared it with other brands and vitamins. I believe I was on track and this is a huge reason I was able to get almost back to my normal quality of life! I’m completely blown away by this research by OMF doctors, scientists, and researchers! Wow! Exciting stuff!!! I wish so badly that I could speak to someone doing research. I know I’m only one of so many, but I feel like my story might be helpful.
Jamie, can you list your supplements? Thank you!
Jamie can you share the supplements you are taking with us and dosages? Thanks! ?
Hi I take an all Natural Activator that reduces Oxidative Stress and Inflammation in our body’s by 40% in 30 days and will increase our Glutathione by 300% Naturally within 120 days.This Activator, Activates a pathway in our body that makes our own antioxidants. I will start on the other Activator soon that Activates our pathway to our Mitochondria To enhance Mitochondrial Function.One of these Activators have at least 24 peer review studies on Pubmed.If anyone is interested in learning about how to biohack your health back using nutrigenomics please send me a message and I be happy to send a information link 🙂
I tried out this polyphenol mix recently for the antioxidants:
https://www.amazon.com/Country-Farms-Energizing-Polyphenol-Antioxidants/dp/B0777C3N81/ref=cm_cr_arp_d_product_top?ie=UTF8
After two doses, I had extreme brain fog, headaches, horrible sense of depersonalization, burning on the sides and back of my head, lop sided head pressure, weakness, etc. It’s possible reductive stress may have been the trigger for me.
I’m curious if anyone has improved their symptoms by correcting physiological breathing issues? I’ve struggled with CFS for 20 years and I’ve tried many different treatments with limited results, including expensive IV treatments, HBOC, peptide injections, etc. I was talking to my dentist and he pointed out that I have a forward neck position and that I may have some sort of upper airway issue. I’m sure I do, as my breathing is shallow. If I move my lower jaw forward and out of the way, I’m able to take a full breath and I feel my brain calm down. I also had a tongue tie, which he fixed earlier in the year. But, my oxygen issues only improved slightly with that procedure. My dentist mentioned the work of Dr. Robson and oral systematic balancing. He doesn’t offer the appliance, but he suggested that I look into it. He said that many people have reported their CFS symptoms resolving or significantly improving after using this appliance to correct their breathing. I’m curious if anyone has tried this appliance or taken any other corrective measure to improve their breathing that has helped. Thank you so much!