Studies suggest it’s possible that every aspect of muscle activity – from oxygen uptake by the muscles, to mitochondrial functioning, to lactate build up, to the ability of the muscles to relax, to problems with the microcirculation – are present to some degree in fibromyalgia. Where it all starts is anyone’s guess.
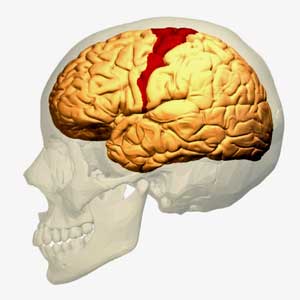
The motor cortex of the brain might be one place. Every time you pick up a pen, hit a key on a keyboard, or turn on your smartphone, the premotor and supplementary motor areas of your motor cortex plan the movement first. Then your primary motor cortex sends a message to the muscles to act. When a muscle cell gets fatigued, the motor cortex recruits another cell to pick up the slack. Plus the motor cortex also plays a role in pain inhibition pathways and connects to the insula – a sensory integration center and autonomic nervous system regulator.
As someone who’s been diagnosed with both ME/CFS and FM and who experiences pain and movement problems, I wouldn’t be surprised if the motor cortex is involved. If I overdo movement – engage in too much “exercise” (e.g. too much walking or something as seemingly innocuous as that), contracted muscles and pain are two of the first things I notice.
Researchers have long thought it’s the other way around; that the pain is shutting down the brain pathways involved in activity. Perhaps it does, but for me, the pain often comes second. The movement issue is paramount, and that suggests that some aspect of the motor cortex could be involved. There’s obviously more to my pain than that. I typically wake up in pain, for instance. Also, concentrating too much, oddly enough, results in muscle pain. There’s nothing like mild exercise to exacerbate my pain levels, though.
The Study
Motor Cortex Function in Fibromyalgia: A Study by Functional Near-Infrared Spectroscopy. Gentile E, Ricci K, Delussi M, Brighina F, de Tommaso M. Pain Res Treat. 2019 Jan 16;2019:2623161. doi: 10.1155/2019/2623161. eCollection 2019.
It turns out that you don’t have to do anything dramatic to get the motor cortex engaged. All you need to do is tap your finger. That’s what these Italian researchers had 24 people with FM do – tap their finger slowly and then more rapidly while measuring the activity of their motor cortex using functional near-infrared spectroscopy (fNIRS). fNIRS is a non-invasive approach which allows researchers to assess both blood flow and metabolic activity in the brain. The more active a brain region is, the more blood flow it will receive.
Results
The results were strikingly familiar. If we didn’t know otherwise, one would have sworn these were chronic fatigue syndrome (ME/CFS) patients. The motor cortex activity (oxyhemoglobin content) was similar between the people with FM and the healthy controls at rest during the slow tapping, but when asked to tap rapidly, the activity in the motor cortex of the FM patients faded (and so did their tapping ability).
The motor cortex of the FM patients didn’t have the metabolic wherewithal to keep up with the healthy controls. Essentially, these FM patients showed the same kind of hypometabolic (failure to perform) issues that have been showing up in ME/CFS. When put under increased energy demand – a demand, it should be noted, that simply requires one to rapidly tap one’s finger – the motor cortex of FM patients “poops out”.
This pattern of doing OK while at rest but fading when put under stress has been seen in people with ME/CFS during exercise, in their immune cells, their mitochondria, etc.

Pushing the motor cortex harder with the rapid finger tapping task appears to cause it to “burn out”
At first blush, the finding of reduced activity in the motor cortex flies in the face of the findings from transcranial magnetic resonance studies that found hyperexcitability, not somnolence, in the motor networks of FM patients. The authors believe, though, that this hyperexcitability may be an attempt to compensate for a sagging motor cortex; i.e. weak signals from the motor cortex cause the network to amp up its activity to try to decipher what’s going on. (That smacks of Bob Naviaux’s assertion that systems which are depleted of energy get “twitchy” and have trouble resting.)
Researchers have thought that reducing motor cortex metabolism could be a protective mechanism that shuts down motor cortex activity in the face of chronically activated pain circuits. That suggests, though, that the more severely ill FM patients would have more inhibited movements – and be “slower tappers” – but that wasn’t true.
Everyone with FM, whether they had a moderate or severe illness, demonstrated the same metabolic motor cortex defect in this study That suggested that the metabolic problems seen were not the result of overloaded pain circuits giving up the ghost, so to speak, and ordering the motor cortex to shut down in order to save themselves. Instead, the authors suggested that, “cortical motor dysfunction and movement impairment could characterize FM at its onset“; i.e. could be an integral part of the disease.
In fact, instead of pain severity being associated with finger tapping speed, fatigue – (which is more associated with movement) was. The more fatigue present, the slower the FM patients were at finger tapping.
After finding that fatigue was not associated with oxyhemoglobin status (motor cortex activity), the authors went a bit woo-woo and suggested that “psychopathologic traits” may come into play – a default response to any type of fatigue that can’t yet be explained physiologically.
A Chronic Fatigue Syndrome (ME/CFS) Connection?
The findings in ME/CFS belie that idea. Similar issues with the motor cortex and motor planning have been found – most of them dating back decades.
A 1999 study, which found a reduction in “premovement” brain activity and slower reaction times, concluded that the “central motor mechanisms” that lay the groundwork for an accompanying movement were impaired in ME/CFS. A 1999 study came to the remarkable conclusion that “an exercise-related diminution in central motor drive” was present; i.e. the brain was having trouble driving the muscles in ME/CFS. A 2001 study found it was harder to get the brain to cause the muscles of people with ME/CFS to react. Another study (2003) suggested that problems in “motor planning” are present.
Tying Movement to Pain
Our study…points toward an important role for brain motor control… consistent with previous findings of altered pelvic floor muscle activity. Our results are also consistent with several studies demonstrating the importance of the primary motor cortex for pain processing. Kutch et. al. 2015
Motor cortex problems may be present in other painful diseases. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is another mysterious and often debilitating pain condition which is characterized by persistent pain that can show up during urination, sexual activity and even just sitting. It’s another one of those “major healthcare problems” which is solely identified by symptoms and affects a pretty good section of the population (2-6% of males) and gets little funding.
Researchers have learned, though, that people with CP/CPPS have problems with pelvic floor muscle control. These muscles support the bladder and bowel and play a role in urination, continence, and sexual functioning. A recent study found that the pelvic part of their motor cortex (the “pelvic-motor”) was communicating in a strange manner with the insula – a key integrator of sensory information.
The insula appears to relay the sensory information that the motor cortex needs to control the pelvic floor muscles. Plus the insula is involved in autonomic nervous system regulation – another issue in CP/CPPS (and FM and ME/CFS). Issues with the insula and reduced heart rate variability have been found in CP/CPPS, FM and ME/CFS patients).
Altered motor cortex excitability was recently found in myofascial pain syndrome (MPS)- another mysterious, poorly funded central sensitivity pain syndrome associated with FM. An inability to inhibit the motor cortex led to problems with the inhibitory pain pathways. One wonders if problems with muscle activation could somehow contribute to the tightly contracted muscles in MPS.
Stimulating Findings
Studies indicate that magnetic stimulation of the motor cortex is able to relieve pain and other problems in FM. A 2017 study of 20 people with FM found that transcranial random noise stimulation (tRNS) of the motor cortex resulted in a significant reduction in pain, depression, anxiety, and fibromyalgia impact (FIQ) scores, as well as a significant improvement in a variety of cognitive tests.
Another FM tRNS study resulted in reduced pain and mood improvement associated with changes in serum endorphin levels. A 2017 review of six studies concluded that tRNS was most effective in FM when focused on the motor cortex.
These studies suggest that the motor cortex may play a key role, not just in the movement problems in FM, but in pain, mood, and other issues.
The motor cortex – movement connection goes both ways. The use it or lose it adage applies to the motor cortex as well. If movements aren’t performed regularly, the pathways to produce them can be lost and must be relearned. Just as motor cortex activity is required for exercise, exercise actually boosts the health of the motor cortex. The fact that exercise boosts the health of the motor cortex may be one reason why mild exercise can be quite helpful in FM.
When physical damage to the motor cortex has occurred, therapists move limbs and have the patients use mental imagery to picture themselves moving to help restore movement pathways. Using visual imagery can even help with people who have trouble swallowing.
Conclusion
The activity of the motor cortex – the part of the brain responsible for planning and carrying out voluntary movements – appears to be impaired in both fibromyalgia and ME/CFS. Given that the motor cortex is also involved in sensory integration and pain modulation, it wasn’t that surprising to see motor cortex problems show up in diseases associated with pain and movement issues. It was interesting, though, to see hypometabolism and energy issues possibly showing up in the motor cortex of FM patients for the first time. A wide variety of mitochondrial issues have been found in fibromyalgia.
How important a role the motor cortex plays in FM is unclear, but studies suggest that a wide variety of muscle issues ranging from the motor cortex to the muscles themselves are present in FM. Other pain disorders such as chronic pelvic pain syndrome and myofascial pain syndrome also feature motor cortex problems.
rTNS magnetic stimulation studies suggest that stimulation of the motor cortex may relieve a wide variety of symptoms in FM.






As always, I wonder whether these motor cortex problems can “initiate” the cascade of problems that manifests in palpably distorted muscle tissue, logically explained by fascia adhesiveness.
Either the fascia is “adhesive” and the adjacent muscle fibres are “sticking” and ending up in knots and clumps, or there are nervous system signals independent of the motor cortex, keeping these muscle fibres in a tense state.
Biochemical dysfunctions could explain muscle tension independently of any “nervous system” signals – after all, rigor mortis is biochemistry, not nervous system signals.
The motor cortex “wimping out” suggests to me that it is not the intial cause – how can a lack of signals to muscles, result in them going inappropriately and permanently tight? I would accept that a dysfunction in the energy production mechanism results in very poor anaerobic and high-exertion function that creates toxic residues that cause adhesiveness; but this could be a dysfunction originating from anywhere in the system.
I’m inclined to still think that the motor cortex wimping out, and the heightened CNS, are both results of biochemically-originating dysfunctions in muscle tissue. The muscles simply cease to respond to orders as they are expected to, and both the CNS and the motor cortex rebel in different ways as a result. And the order may be: stuck muscle fascia; heightened CNS; motor cortex giving up.
I think discovering what precedes “stuck muscle fascia” is where the “cause of FM” is to be found. So far my best explanation is just that a combination of things tip the system into a vicious circle. Chronic stress makes the muscles inappropriately tense, fascia gets its fluid “squeezed out” over long periods of this tendion, and the fascia ends up sticking even when the muscles are consciously relaxed.
An elevated level of toxins that already increased the “sticky” property of the fascia fluids may be a factor. Many people contracted FM following an infection, and everyone with FM has a high level of at least one toxic element in standard tests. Many also had an accident or an operation; fascia being “cut through” anywhere in the body may set up a “protest reaction”.
I now believe that eastern medicine’s hypotheses about “flows” (in Yoga, Tai Chi, massage etc) are absolutely correct; there are “flows” throughout the body through the interstitial spaces between the fascia wrappings of muscles and organs. Blocked flows are deadly for human health. Mainstream medical science has been disgracefully incurious about this. Now researchers are belatedly describing the “interstitium” as a new “organ” – which is as important for medical science as discovering the continent of America was for geography; in my honest opinion.
As a laymen I have no idea how this really works but how about this – the motor cortex sends weak, intermittent or confusing signals which causes the muscles to kind of freeze up in confusion. Or the motor cortex doesn’t recruit enough muscle cells for a task causing the ones that are recruited to become exhausted, unresponsive and stuck?
Whatever is happening in FM and ME/CFS it appears the motor cortex – up in the brain – is part of it. Like you said it’s probably a combination of factors.
Hello Cort,
I am interested in this hypothesis, because it could fit with the cervical stenosis hypothesis of ME/CFS/Fibro.
In this 2018 study Motor Cortex hypoperfusion was detected in Cervical Spondylotic Myelopathy:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5769569/
Well that’s interesting!
Thanks
I start to believe that in both ME and FM an important part of dysfunction is overlooked: the difference in strength/health/endurance of individual cells based on their location along the blood stream.
In order to produce a useful output cells need sufficient access to energy (glucose, fat, ketones, amino acids), oxygen (for the aerobic part) and a “local environment” sufficiently free of waste products/toxins.
Now let us take what research learned in ME. Same things may apply in FM but insufficient (even less) research has been done.
* Many capillaries seem to be constricted a lot.
* Confusing information exists about blood vessel over-dilation but it is not clear what blood vessels are involved or that it is even proven to be a typical thing for this disease. So I’ll (conveniently) only consider capillary over-constriction.
* Blood is a liquid where the flow reduces far more dramatically when capillary diameter decreases compared to what would happen if water was pushed with the same pressure trough the same vessels. The RBC having about the same diameter or greater diameter then these smallest capillaries are the main culprit.
* The RBC having about the same diameter or greater diameter then these smallest capillaries makes it key important for the RBC to be flexible so that they can be squeezes fairly easily in a more tube like shape with smaller diameter in order to have sufficient blood flow in these smallest blood vessels.
* ME and FM are supposed to be characterized by high levels of oxidative stress. In ME it has been found that RBC are far less flexible then in healthy controls, causing them to be more spherical and hence reduce blood flow in these finest vessels even more then in healthy people.
* In ME and FM blood volumes far less then average are often found. Low cardiac output is also a common finding.
* In blood vessels where the hydraulic resistance rises quickly with reduced flow speed, reducing the total cardiac output increases the percentage of blood that flows through the larger vessels at the cost of a decrease of blood flow in the smallest vessels.
* Potential additional effects like auto-immunity against blood vessel epithelial cells or temporary ischemia are likely to scar these finest capillaries further and create less smooth and more blocked vessels further reducing blood flow in these finest vessels.
When combining all of these, the ratio of blood flow in the finest capillaries as a percentage to the total blood flow is likely to be a *lot* lower then in healthy controls. I wouldn’t be surprised if it were shockingly bad for the worst affected (micro)areas at the worst of times.
If so, we end up with a situation where both the amount of blood a cell can use and its contents are totally different dependent upon if a cell is located in a less affected area next to a larger blood vessel or located in a more affected area next to a very narrow capillary.
The first cell would see blood flow comparable to healthy persons. As researchers found by examining tapped blood (that is conveniently tapped from large blood vessels) glucose and oxygen content are often higher in ME patients and CO2 is really low.
The second cell would see blood flow going down to a trickle. To be clear, one such very narrow capillary would feed many cells in a row. Blood flow too all of them would be roughly similar.
*IF* the capillary would start as a side vessel of a large vessel (a simplification for easy of explaining the idea) then the first cell would see blood rich in oxygen and glucose and low in CO2 coming in at a trickle. This cell would have reasonable access to all what it needs and levels of waste would be moderate. That is, moderate as is seen in tapped blood from ME patients.
However, as the blood streams along the small capillary cells would take nutrients (glucose, oxygen…) out of the blood and dump waste products (CO2, lactate, ROS, uric acid…) into it.
That means that along the small distance of the narrow capillary, blood composition changes dramatically from nutrient and oxygen rich and relatively clean to very poor in nutrients and oxygen and very polluted.
If cells further along this capillary are starving (and nearly dying) for food and oxygen one can’t expect them to just let all of this food and oxygen untouched in the blood can one?
Cells down the line of these narrow capillaries would be so starved of oxygen and glucose that they start to go massively anaerobic, pillage remaining amino acids in the bloodstream to strip a molecule of glucose out of them (and dump uric acid in the blood), produce plenty of ROS… hence would become even more polluting as they are starving and have to get what they need to survive.
When this capillary would end up again in a larger blood vessel returning to the hart then the very polluted blood would trickle in the larger blood vessel. If there goes very little blood in the capillary then very little will come out at the other end as well.
Now this blood in the larger blood vessel would have a far greater flow. All cells located near this large vessel would have access to a far larger amount of blood per cell then these previously discussed. This blood would stay rich in nutrients and poor in waste.
So a large volume of relatively clean blood rich in glucose and oxygen and low on CO2 would mix with a really small volume of really dirty blood poor on glucose, amino acids, oxygen… and rich in CO2, lactate, ROS…
Mixing this would give an end result that looks surprisingly clean. It would be relatively clean blood rich in glucose and oxygen and low on CO2 and with “moderate” levels (compared to seen in the narrow capillaries) of lactic acid and ROS.
That could easily puzzle researchers tapping blood from the large vessels and seeing that that ME patients blood is rich in glucose and oxygen but somehow the body can’t seem to use it as the returning blood remains rich in it and poor on CO2.
Now in order to produce a useful output cells need sufficient access to energy (glucose, fat, ketones, amino acids), oxygen (for the aerobic part) and a “local environment” sufficiently free of waste products/toxins.
According to the previous hypothesis cells near the larger blood vessels would have good (even rich) access to energy (glucose) and oxygen and bathe in “relatively” clean blood. They would be able to produce ample energy.
Equally according to the previous hypothesis cells further down along the most narrow and restricted blood vessels would see very little energy nor oxygen and would bathe in blood polluted with very high levels of lactic acid, CO2, uric acid, ROS…
For sure very low levels of energy and oxygen would let energy production of these cells nosedive. Very high levels of waste products like lactic acid and CO2 should further hamper energy production. I also have found many information that strongly suggests that key enzymes for both aerobic and anaerobic energy production are strongly inhibited in an environment with high oxidative stress.
In this reasoning, it is very clear that the amount of energy produced by both of these cells is utterly incomparable. Estimating that the latter could could produce less then a tenth of the energy of the former is a fair guess IMO. Yet, these two cells may be located less then a single millimeter apart.
If so, that would mean that nearby muscle cells have entirely different capabilities to react to a brain command / nerve impulse. Muscle work done by cells in the same fiber would vary widely. Muscle work done by cells in adjacent fibers would vary hugely.
Some (parts of) muscle fiber would barely contract, while others would show near healthy contraction. When having adjacent fibers contracting at totally different percentages, the entire structure becomes very deformed and mechanic tension becomes huge.
Just to get the picture (trying to do my best to explain in a foreign language):
* Imagine a square frame for a wooden house to be build.
* Imagine it to be 2.5m by 15m.
* Ideally the frame has 2 wooden beams of 15m and 2 outside beams of 2.5m to form the rectangle and 5 beams of 2.5m to be placed in parallel of the other two beams of 2.5m to complete the structure.
* The 7 2.5m beams are numbered from 1 to 7 from left to right and have to be used in that order.
Now due to some problem the beams aren’t exact size. The beams are:
* The two 15m beams are perfect.
* The supposedly 2.5m beams are from left to right 1.8m, 2.8m, 2.3m, 3.1m, 1.9m, 2.2m, and 2.6m
* You are NOT allowed to change their length.
* You have to nail the end of each beam perpendicular to the 15m beams so you can’t cheat by placing the 7 beams outside of the plane formed by the 15m beams. What I mean is: you have to use the length of the beams you have been given.
=> Now imagine the incredible bending the top beam has in the final constructing just in order to fit the poorly sized individual beams.
=> Now just imagine how much mechanical stress this imposes on the individual beams.
=> Now just imagine how many nails you need in order for the structure to not just detach and disintegrate.
=> Now just imagine how bent the supposedly 2.5m beams are.
=> Now just imagine how those “C-shaped” supposedly 2.5m beams are nearly snapping in the middle.
Now that is “total uneven ability to contract nearby muscle cells and fibers to the same degree” explained in plain speak. Our FM muscles.
* That curls and knots our muscles.
* That contracts our muscles a lot as something that is bend, curled and knotted becomes thicker and shorter.
* That produces plenty of pain.
* That produces such short knots that these are pointy and sharp cutting like the point of a steal knife through fascia and other muscle fibers alike. Remember that small curved objects are very hard even if they are made of soft materials. Think of the “cutting” water of a water saving shower with tiny drops versus the soft drops of a luxurious rain shower.
* That outspoken bending, curling and knotting throughout the muscle acts like deforming a spongy to squeeze out the water so it could disrupt fascia liquid contents too.
* That causes our many cramps, as cramps are in effect uneven contraction of muscles.
* That causes our blood vessels in the muscles to be subject to much mechanical stress, squeezing some vessels to have them nearly closed off further impacting blood flow (both reducing and making even more uneven). Some tissue may become necrotic.
* That makes coordinating any movement a computational disaster. None of the muscle fibers respond as hoped. Any movement becomes a tormented attempt to move well. Efficiency of movement is very low and friction is very high. Even small movements cost far more energy then they should.
* This unpredictable outcome of what movements result from the brains commands requires both very difficult guessing how to get a movement done beforehand and continuously measuring, monitoring and adjusting brain / nerve commands each tenth of a second. Now that is both sensory overload and firing up the sensory brain part and the motor cortex into exhaustion very quickly.
* This exhaustion is only in part avoidable by moving few and very slow.
* Even when moving few and slow, an exhausted brain plus these “unruly” muscles make for very poor motor control further damaging / exhausting muscles, making the problem worse for the brain again.
* The above two points are exercise intolerance and PEM due to exercise in essence.
For the brain (ME), I recently described something very similar. If each individual brain cell responds with “random” strength then result and efficiency of thinking and other brain activity will go down the drain.
Let me be clear: I don’t say this is FM nor ME. But I see a very good reason why relatively low levels of average problems can become far more challenging for functionality if they contain plenty of variation in strength/health/endurance between nearby cells. It kinda makes an underlying base problem much much worse then one would believe at first guess.
Finally there is a link to what Ron Davis and Dr. Naviaux reported.
* When you put a healthy cell in ME blood, it behaves as an ME cell.
* When you put a ME cell in healthy blood, it behaves as a healthy cell.
If the “ability to perform” of a cell is indeed dependent on amounts of energy, oxygen and waste then let us take a look at this:
* It’s hard to reason that ability to perform does not depend on energy and oxygen to a large degree.
* But when taking blood from a large blood vessel both healthy and ME blood contain sufficient energy and oxygen.
=> Therefore the impact of the factor “waste” must be *huge* on the functioning of both healthy and ME cells.
=> That could be an argument to say all the things I wrote in the previous comments are useless, in the end it’s just the special thing in the blood that makes the difference.
=> But I disagree: each factor impacts energy output: energy in blood, oxygen and waste. They multiply.
=> If this is correct, then the research of Dr. Naviaux and Ron Davis is a *very* optimistic version of reality. If they used blood tapped from a large blood vessel then they did put the cell in an environment that had way better access to energy (glucose, amino acids, fat…) and oxygen and saw way lesser concentrations of this “waste” factor hampering the cells immensely more then cells living at the end of a very narrow capillary.
If it were possible to tap blood from the end of these very narrow capillaries in sufficient quantities and put the cells in that blood, results and chances to observe might be magnified like being put under a microscope.
It also might shed light onto another thing: the according to Ron Davis very unusual thing that ME patients cells seem to throw very hard won ATP out of their cells. That is according to him an act of desperation.
Now if I get it correct they did study ME cells seeing a “moderate” environment, meaning they had good access to energy and oxygen. They still saw the “waste” factor to let them behave like ME cells.
Now if the body “knew” about the problem at hand, it would figure out (in my hypothesis I explained above) that cells seeing the combination of good access to energy and oxygen but seeing this “waste” factor would mean that a large number of cells in the body down the blood vessels are likely to see a combination of insufficient energy and oxygen and masses of toxic waste.
In a “normal” body this could mean (certain) death. Short term death like plenty of people who don’t survive another day like in sepsis. Massive ischemia and (if one still lives) even worse re-perfusion damage.
That’s one thing that I’ve been wondering about so often. People with sudden blood loss no worse then still having the amount of blood we live with day by day die. People with sepsis often die the same day while ME is often compared to some form of chronic sepsis. People with diabetes lose limbs and sight when untreated and eventually die due to damage by poor blood flow, yet plenty of us have so little clear permanent damage that could easily convince any doctor we are truly very sick. In short, our disease seems to be impossible. Many doctors see it as an excuse to ignore or abuse us. I however sometimes wonder what keeps us alive.
Now let’s go back to the inflexible RBCs having to go through the most constricted blood vessels. Many of these vessels will be side branches of larger vessels. I sometimes wonder if any (significant number of) RBC go through these vessels at all.
It’s like having a flood in the street with water running down the street and being 1 foot high. In the rainstorm a lot of big ruble is dragged along with the water. Now suppose that some of that ruble were intact dinner plates (the ones to put food on). If they pass a sewer then, when the plates would be well fitted they could be pushed through the grate of the sewer hatch. As they are heavier then water they would have no problem to sink in the sewer. Yet near all plates will “bounce” against the grates, temporarily blocking the feeding of water to the sewers, and then float further along the street as it’s so hard to get them through that narrow hatch.
Suggesting many narrow capillaries see (nearly) no / never RBC passing through them yet both the capillary and these cells around it survive longtime seems to be an absurd thing. Yet somehow it may be the most logical thing to be happening.
How might such thing be possible? Cells that see sufficient food and oxygen but are alarmed by this toxic waste “know” that some friends will be in deep trouble. So they produce “disaster relief aid packages” and they send them to them. Now these packages could be ATP.
Cells need them more to survive then either glucose/fat/ketones or oxygen. And they (IMO) are far smaller in diameter then RBC. So putting out part of their precious won ATP would be like war victims liberated a month ago sharing their small supplies for sending them to the people just liberated yesterday. Putting them on the blood stream would be both an efficient and inefficient process in order for the “gifts” to reach “the most needy cells”.
It would be efficient in a way that it is the only reasonable way to get ATP to them by just dropping packages on the bloodstream.
It would be inefficient in a way that much ATP would go to other places then desired. But then again those cells creating “aid packages” wont be the same as taking advantage of other cells “aid packages” so they’ll have a chance to remain in the blood stream till they reach a place of true needs.
That way of transporting energy would have another nice advantages: the now really deep lack of oxygen in those very narrow capillaries should reduce ROS production of these cells. Give them some catalase and they may even extract a little oxygen out of the hydrogen peroxide rich blood they see.
Combined the above with trickles of glucose (for mainly anaerobic energy) into these vessels and they might have sufficient energy to survive.
Not having to destroy and rebuild stuck/damaged/narrowed capillaries would have another main advantage. Destroying capillaries is a very inflammatory process (and increase risk to auto-immune problems IMO). Just as cleaning up ischemic damage to these vessels and destroying and cleaning up death cells next to them. We very likely don’t need that. And we can’t afford the cost to rebuild.
ME research indicates that cell death (and renewal) is very low in ME (the hibernation effect). That doesn’t logically goes together with very very poor blood flow we seem to have. Except if we have a special survival technique (please NIH fund money to learn from us how to survive lethal injury and threat ;-)).
Maybe this “sending of ATP packages” into the bloodstream (my words, Ron Davis said only out of the cells but into the blood seems the most logical place to me) links to another enigma in ME: what is causing the immune system to be seemingly on constant high alert in ME?
Well, if you send plenty unprotected ATP packages down the bloodstream, bacteria and other pathogen could feed, grow and multiply (very) rapidly on them. That would both deprive the most needy cells from their basic needs to survive as it would allow pathogens to grow rapidly out of control.
It’s like sending huge carts of food to needy zones in the example of wartime aid above. Without some extra military patrols on the streets it would attract bandits from far and wide.
This is my favorite hypothesis of why our cells are not getting the energy they need 🙂
I’m struggling to follow but your hypothesis is plausible. What if the toxicity in the further reaches of the capillaries is simply because of proximity to the toxicity in the interstitical space itself? The further away the circulation gets from the fascia, the better it is protected from the toxicity. I have been wondering for years whether the blood is very successfully self-regulated, and maybe other parts of the biology are very well-regulated, but the “interstitial space” is kind of a dumping-ground for these other self-regulating, self-purifying systems. As those “Interstitium” researchers two years ago said, “standardized tests of the interstitial fluid could have major diagnostic power for a wide range of conditions”. Maybe mainstream medicine is up a blind alley constantly testing the blood, which actually successfully cleanses itself of many of the vital “indicators of dysfunction”, leading the doctors to tell people “there is nothing wrong with you”. What they really mean, is “what is wrong with you doesn’t show up in your blood”.
Hair tissue mineral analysis is another subject with which I am familiar. The mainstream belittles it like they do for so many “alternative” approaches – and yet if I supplement based on what the HTMA says about me, I get better. The mainstream says; “you are not low in magnesium” because according to them, my blood is not low in magnesium. But I might be suffering debilitating cramp attacks daily, and HTMA says I am dangerously low in magnesium; and when I supplement with enough magnesium that the results in the HTMA come up to normal, I am cramp-free.
So all the “mainstream” does is destroy my confidence in them and strengthen my belief that more is known about many aspects of wellness, by the alternative practitioners. The whole hypothesis about the Insterstitium suggests to me that mainstream medicine owes a massive apology to the Yoga practitioners and the Chi Gong and other eastern ones that work with “flows” in the interstitial spaces between muscle tissue. Those modalities have been onto something all along.
Hi Philip,
Over time I hope to illustrate my ideas with pictures but for now there is so much to learn and so few usable time and energy.
I think that the individual ME/FM cells producing toxins is a fair assumption. Even if the fascia are full of toxic stuff then it has to come from somewhere. That can be external (absorbed through the gut for example) or produced by the cells suffering. In many cases it will be from both origins I believe.
If the cells themselves produce toxic stuff then the highest concentrations of that type of toxins should be located near the cells themselves. The fascia should see lower concentrations of them. That doesn’t mean low however. Diffusion is a process that doesn’t support to have higher amounts of diffused chemicals away from it’s source then at the source itself.
With “the highest concentrations of that type of toxins” I mean that for example if the cells produce excessive lactate then that chemical will be higher near the cells producing it compared to further away from it. For toxins like mold spores gotten into the body into the lungs toxins will be highest in the lungs. That is, if no other process then diffusion is involved.
In that view, toxins produced by the cells will accumulate downstream the smallest capillaries. Compare it with two identical industrial zones using process water along a water stream. All factories take in water at the upstream side of their plot of land and dump it after use on the downstream side of their plot of land.
The first industrial zone is located near a big river with plenty of water flow. The second industrial zone is located near a small creek with few water flow.
It’s not hard to see the water in the creek will become much more polluted compared to the water in the river if the factories dump identical amounts of waste. When dumping in the big river the toxins will become much more diluted. It is also easy to see that when you travel the industrial zone along the creek downstream that the water will change from clean when entering the zone to more and more polluted as it passes along more factories.
How much toxins will leak into the interstitial zone is very hard to estimate. How well they will be removed is near impossible to guess as there is hardly done any research down about interstitial fluid flow. It stems to reason that the concentration of toxins will be somewhere between the very/extremely high concentration at the end of the narrowest capillaries and the concentration of the major blood vessels. So that leaves plenty of room for it to be pretty high and help pollute the zones that have good blood flow.
But you raise another point. If Ron Davis says that ME cells dump ATP out of their cells then it can be just as much into the interstitial fluid as into the blood vessels. If this ATP was dumped as a energy source for those cells starved of oxygen then the interstitial fluid might play a (big?) role into providing the ischemic cells with essential amounts of energy to survive. A good condition of the fascia and good “activation” of the fascia (small regular movements that pull fluid in an out of this sponge like structures helping diffusion of nutrients and toxins alike) may determine how well one can recover from exertion and disease like FM/ME. That is for the non-brain part at least as the brain seems to lack this interstitial fluid or at least fascia IMO.
When it comes to “blood comes more polluted near the end of the capillaries downstream” I recall both your (IIRC) and my experience: when doing light bicycling well within the envelope (resistance free electric bicycling for me) our breathing needs go well below that of in rest. That is a very remarkable feat!
Now light bicycling is an excellent movement to reduce blood pooling in the legs. This helps “flushing out” stalled blood out of the leg muscles and temporarily increases the amount of “active blood volume” for the entire body helping to push the smallest capillaries open increasing blood flow in them a lot, “flushing them out”.
That may be a hint that our “hyperventilation” may not be “wrong”. It looks like an indication that CO2 levels at the end of the smallest capillaries get excessively high and bicycling helps to improve blood flow there and hence to reduce these excessively high CO2 (and very low O2) concentrations.
Hyperventilation, or having very few CO2 and plenty of O2 in the blood at the entrance of these capillaries would improve oxygen provided to the cells near these capillaries and removal of CO2 produced by them to a fair degree. It would alleviate their burden with double digit percentages. Yet, due to the small flow into these capillaries compared to the flow in the larger blood vessels it would seem as if our cells are refusing to make use of the O2 in the blood (as the “depleted” blood from the smallest capillaries is diluted so much).
As to Chi Gong movements they also provide excellent static and dynamic muscle loading, taking away strain from the muscles, tendons… Working that out with you, if you were up to it, is one of the things I wish to do some day. But so much to do, so few usable time…
Again, these studies compare FM/CFS patients with healthy controls. But how do they compare with severely atrophied or aged people? The motor cortex problem seems to be similarly present in the aged people: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3184350/
Looking at what our cells can produce on average can be misleading. To make the idea clear with another example, think of a dog sled in Alaskan snow.
* With 4 snow dogs, the sled can ride fast.
* With 8 snow dogs, the sled can really race.
* So using 6 good snow dogs plus an elderly snow dog and a tiny Chihuahua dog having 8 dogs in total should produce ample of power to pull the sled at fast speed.
* In reality, you might be able to get 5 minutes of moderate speed out of it. Going faster or doing this moderate speed for longer and you either run the elderly dog and Chihuahua over with the sled or you’ll pull them behind the sled badly injuring their legs or bellies or backs depending on how they are pulled by the sled.
* So a good sled pilot will and must purposely reduce both intensity (speed) and duration of the ride a lot even if 75% of the dogs provide more then enough power to pull the sled far and fast.
That is IMO a good analogy to ME/FM under this hypothesis. It just gets a bit worse if also the output of the “good” cells is reduced to a level that would only support prolonged moderate activity.
The hypothesis would help solve another mystery. It looks like our cells produce plenty of toxins that need to be cleaned out of the blood by the liver. It also needs to neutralize heavy amounts of oxidative stress. And it needs to recycle large amounts of pyruvate and lactate back to glucose (as anaerobic energy production produces them in toxic quantities).
That would mean our livers work 24/7 at a double or even higher pace compared to the liver of a healthy person. But in a healthy person the liver is already a major source of energy consumption. Thus our livers seem to be the only organs that work continuously in heavy overdrive while the other organs are in near constant hibernation. How does that work out?
My best two ideas so far were:
* It is the really high amounts of oxidative stress that turn down the functioning of aerobe and anaerobe energy production. As the liver is a / THE major production plant of anti-oxidants the liver is seeing far less oxidative stress and can work at a high rate.
* The liver is located near the center of the body. That means it is at optimal temperature for blood flow (cold blood becomes thick and reduces flow) and it is impacted little by gravity / POTS unlike head and legs. But the same can be said of the hart and lungs and they seem to be impacted by this disease a lot.
I’d now transform the first idea in “It is the really high amounts of toxic waste (including oxidative stress) that turns down the functioning of aerobe and anaerobe energy production. As the liver is THE major production plant of detoxification the liver is seeing far less toxins and can work at a high rate.”
This is the power of modeling: if the model would be correct, the toxins that inhibit energy production would be ones that are filtered out by the liver. That is still a large list but narrows it down a lot at the same time. It may be one toxin or a combination of toxins.
Also, if the model is correct, it suggests that the capillaries in the liver are far less constricted or in a dire state then those in the rest of the body. That would further help to narrow down the list of main toxins turning down energy production.
The toxin or combination of toxins would have chemicals in them that reduce blood flow and do so mainly by narrowing or damaging blood vessels. Other toxins could be present as well.
Oxidative stress and markers of it are such potential toxins, but their may be other ones that are processed by the liver too.
Hi Dejurgen, this is a slow reply but wanted to say thank you so much for your ongoing generous knowledge sharing, your creative ideas about our illness, and the kind support you give to members of this forum. I really enjoy your comments and find them very thought provoking.
Here’s some more food for thought!
I liked your latest hypothesis but can’t help but wonder why we don’t all have Raynaud’s or necrosis if our microcirculation is so badly affected?
I don’t know a lot about FM but I do know that in 2003 Pashke found elevated endothelin 1 in FM patients (endothelins being peptides that constrict blood vessels and raise blood pressure and with elevated levels often being found in autoimmune illnesses). Researchers at Dundee University replicated this finding in FM patients but also found that endothelin 1 is normal in ME/CFS patients. The same university found that ME/CFS patients have a prolonged acetylcholine response when acetylcholine was applied transdermally to the skin. This did not occur in FM patients, and so I think that both findings may provide hints to the origins of the two illnesses or at least highlight some differences.
In regards to ME/CFS, obviously, researchers are now taking more interest in the Japanese and German findings of auto-antibodies against muscarinic / adrenergic receptors, which have a clear acetylcholine connection, but given these antibodies are only found in about a third of us and have some cross-over with POTS, the waters are still murky. I’m not sure this is where I belong.
My interest was piqued following the latest NIH conference when Ron Davis (?) mentioned that they were investigating vasoactive neuropeptides. It looks like Griffiths University has been interested in these molecules for some time. Staines provided the following description:
“Vasoactive neuropeptides are widely distributed in the body particularly in the central, autonomic and peripheral nervous systems and have been identified in the gut, adrenal gland, reproductive organs, vasculature, blood cells and other tissues. They have a vital role in maintaining vascular flow in organs, and in thermoregulation, memory and concentration. They are co-transmitters for acetylcholine, nitric oxide, endogenous opioids and insulin, are potent immune regulators with primarily anti-inflammatory activity, and have a significant role in protection of the nervous system to toxic assault, promotion of neural development and the maintenance of homeostasis.”
Brenu put together the following hypothesis in 2010:
http://iacfsme.org/PDFS/Brenu-Pathomechanisms-Spring2010-7-30.aspx
Given this illness so often represents a system wide loss of homeostasis it wouldn’t surprise me at all if the illness affects a system-wide communication pathway, and probably a poorly understood one like nitric oxide or neuropeptides.
It looks to me, based on Brenu’s hypothesis, that disruption to VIP would strongly feature gut motility / leaky gut issues and disruption to PACAP would strongly feature loss of blood-brain barrier integrity and neuropathic pain. Both could potentially result in significant ATP / mitochondria issues and system wide loss of balance that are aggravated by stress and exercise. This line of enquiry and its potential for treatment opportunities excites me.
Hi Debsw,
Thx for the kind words. It pleases me that I am not writing for an empty room. I do realize that many readers of HR dislike my hard to read and long comments but that is nothing a “collapse comment” function couldn’t solve. I wouldn’t have been able to read nor understand any of what I write now just a few years ago.
In the comment section I launch many new ideas and it kinda helps structuring my thoughts. Structure is still a major issue for me since getting ill, at least compared to when I was healthy. And I get many good comments like yours helping me to point me in new and unexpected ways. So it’s helping myself as much as I hope to share ideas with other patients. I’ll have to dive into your information deeper before I can say anything useful about it. Absorbing new information and turning it into usable resources can take weeks to months.
“but can’t help but wonder why we don’t all have Raynaud’s or necrosis if our microcirculation is so badly affected?” is still a big question for me, but it is in the same line as “how can so many of us live with such low blood volumes that do kill healthy people that loose so much blood to a point they have only as few blood as us within less then a day?” So these questions are part of what may lead to answers.
As written in other comments in this blog, I start to wonder if we don’t have alternative routes for cell survival. It is assumed all cell must have their oxygen supplied by RBCs or die. Anything else is considered impossible. Yet, on a microscopic scale this may make the least of sense.
I can imagine there must be many larger blood vessels (size just above big capillary) that have a side vessel that is a significant smaller capillary branching out at an 90 degree angle.
Now I learned that in healthy people RBCs are larger in diameter then these smallest capillaries. Only the flexibility allows them to easily pass through the capillaries.
Now let us even assume a healthy person. Suppose a RBC is heading towards this “crossing point”. It now still flows in a larger blood vessel, that has a larger diameter then the RBC itself.
Now it is very close to this small side capillary with a diameter smaller then itself and perpendicular to itself. It has a motion and velocity along the direction of the larger vessel and thus perpendicular towards the smaller capillary.
What are the chances this RBC all of a sudden will make a very quick (I heard blood flows at speeds of several meters per second so passing a capillary with diameter 1 mm would take less then 1 millisecond!) turn and go to this perpendicular vessel where it has to deform itself to squeeze itself through? Physics (flow and kinetic energy of the motion of the RBCs) tell that this is *very* unlikely IMO.
So that leaves us with four options:
A) bends and splittings in blood vessels must be very precise and symmetrical in order for each splitting to get sufficient RBCs; the smallest deformation will mess this up a lot.
B) RBCs are ridding so much bumper to bumper that they are in continuous collusion; seems reasonable as they represent about 40% of blood liquid volume; this however should require modeling blood as something totally different then a liquid in order to understand how it flows in small vessels; also, for kinetic energy of these bumps to be sufficient to change direction of one RBC so much this would require really large differences in individual RBC speed
C) An “active mechanism” uses energy to drawn in RBC into these smallest capillaries
D) The last part of a millimeter to the cell doesn’t require a (or much) RBC to pass into the capillary next to it.
D) might sound insane, but at such small scale the simple process of oxygen diffusion (plus unidirectional blood flow) may go sufficiently fast to provide a significant amount of oxygen and remove a significant amount of CO2 so that the cell still has a reasonable supply and removal to them. Having theoretically no need to have plenty of RBC passing through them would increase flow of (RBC light/free) liquid a lot through them as that would flow way more easy through these small vessels then blood with normal RBC content. That in turn would increase supply of dissolved oxygen, glucose and CO2 removal even if amount of dissolved oxygen per milliliter is really small. (We are supposed to decent from aquatic organisms that can live on dissolved oxygen in water so our individual cells being able to live on that is not that farfetched).
At this very small scale, with blood composition changed, cells dumping ATP into the bloodstream (and maybe into the fascia liquid as a bypass building upon Philips ideas) may actually be the normal rather then a very exceptional thing for cells to do. It would be easier for our cells to do this (on a larger scale then in healthy people) if this happens already on a very small scale in healthy people IMO.
I believe combining option B) and D) and clinically investigating plus physically modelling and simulating it with supercomputers might reveal a rather unexpected behavior of blood and especially RBC flow that would improve knowledge of it to the benefit of many more diseases then just ME/FM.
Physically I just can’t feel that the current model of “all blood has equal composition and RBC content no matter the scale” makes sense.
Note: it’s on a bit larger scale, but when I was at worst I did bleed very few from wounds and was very pale in color about everywhere. Now that I improved I start to “bleed better” when I have a wound and the larger amounts of blood present make my wounds heal better too. But the thing I noticed so often is that the inner side of my hands has a “red/white(pale)” pattern with red and white dots lying next to each other varying in size between 0.5 and 2mm in diameter. Anyone recognizing this pattern?
I think this is the same thing as I described but on a bigger scale:
* varying zones between 0.5 and 2mm in diameter with either good or “poor but sufficient” blood flow.
* within this 0.5 to 2mm diameter zones of poor blood flow I imagine sub-diameter zones with either “poor but sufficient” and “too poor according to classic blood/RBC flow ideas”
Their could also be another process at work:
These zones could be alternating. A (micro) zone with too restricted blood flow could accumulate waste products like CO2 and lactate to such extent that these vessels dilate enough to temporarily improve blood flow enough to provide these zones a quick shot of energy and oxygen plus waste removal and then revert back to constricted once these blood vessel dilating waste products are flushed out.
There are plenty options IMO for poor blood flow and restricted vessels to behave quite different at the sub millimeter scale then at a larger scale. Remember that when talking about ischemic damage / diabetic damage and the Raynauds thing you pointed to medical science is referring to larger scale (and more constant problems like sticky blood with diabetes) problems.
This last idea about “micro zones of alternating blood flow” would make a potential model for tingling:
Tingling often is a feeling of having an area of weird sensations whereby small zones feel like changing continuously in feeling/sensation.
That maps onto this “micro zones of alternating blood flow” model.
* the zones are small like sand grains or dust.
* blood flow to the nerves into these zones changes possibly fast enough to make it difficult for the nerves to generate a steady signal, hence “a change in local sensation”
* expansion and contraction due to adjacent dilation and constriction of nearby capillaries may provide sufficiently fast changing mechanic pressure to be observable as a real physical tough sensation
This would also align with the typical winter feeling (before I got ill) when getting from the cold into the warmth of the house:
* hands and feet (and nose and ears) tingled a lot due to this fast change in temperature
* hands and feet (and nose and ears) are the body parts where amount of blood flow changes a lot when going from cold to warm
* above gives similar sensations and it at the very least coincides with a strong change in blood flow near the nerves
Pins and needles is also a known concept. Again we speak about very small sized zones with precisely located strong sensations. It *feels* however not always as these pin sized points change as fast in sensation as with tingling. Maybe a difference in blood flow over the length of the nerve could be sensed as a strong difference in mechanical pressure along the nerve fiber (like what would happen if a needle cut into the flesh).
Also being stung by nettles gives both a tingling and stinging feeling. Nettles are known in alternative medicine to affect blood flow a lot. Around the zone of being stung this variation could be very strong.
Both the tingling and pin’s and needle feelings are common in ME and probably in FM too (I have them combined, hard to attribute to one of them), as very likely are blood flow problems.
That would allow for our nerves to send “real” touch sensations to the brain even if our nerves were in perfect health. Damage would add on top of it. All of that would create large sensory load to the brain that cannot be ignored / cut off / left unprocessed by the brain as it would represent REAL and correct sensory touch information that correctly provides information about real mechanical stress (and near impossible to compensate for changes in very local blood flow).
Dampening or ignoring these signals from the nerves would be no less then just dulling our touch sense. The brain has to process this information and decide it’s “just” tingling or pins and needles and not react too much to it. Doing so all day round must put a heavy sensory load to the brain, lighten up plenty of zones in the brain and contribute significantly to exhaustion.
Maybe it could be even that what cannabis does: dulling our sense and hence both reduce pain and brain overload? I haven’t got experience to tell if cannabis indeed dulls the (touch) senses.
Really interesting ideas dejurgen. So impaired micro-circulation or “something” in the plasma might be blocking oxygen diffusion in the interstitial fluid and it isn’t being delivered to the cells that need it? Hence we need to produce more energy from non-mitochondrial sources – i.e. glycolysis -than someone healthy (as per Lawson’s 2016 findings). Makes sense. Not sure if you saw Steve’s comment below but he provided a link to Ron Davis’s impedance findings and their significance in terms of a biomarker. He is finding that our immune cells cannot respond to stress – they cannot maintain homeostasis in saline. At some stage, surely all of the jigsaw pieces have to come together to make a complete picture? Fingers crossed the critical missing piece turns up really soon.
“So impaired micro-circulation or “something” in the plasma might be blocking oxygen diffusion in the interstitial fluid and it isn’t being delivered to the cells that need it? ”
I’d reword that too:
“So locally and maybe temporarily impaired micro-circulation might be blocking oxygen distribution by classical means of delivering it by RBC through the tiniest capillaries.
This might cause the need to make (?more?) use of other means of energy and oxygen delivery to local spots such as glycolysis, distribution of oxygen dissolved in blood, ATP dumped outside cells to provide energy to those cells starved the most, diffusion of energy and oxygen sources and removal of metabolic waste through alternative channels like the interstitial fluid”.
“a link to Ron Davis’s impedance findings and their significance in terms of a biomarker.”
I hope it works out for (near) all ME patients as a reliable bio-marker. If I get it correctly the group of patients were the most severally affected and 20 is still not that big a number. It may need some additional phase1, 2, 3… trials before it is commonly accepted?
The test being very cheap would be a very attractive point and it would aid a lot to finally banish the the psychobabble.
I wonder what would make the impedance go up: reduced surface area of the cells due to the membrane being less flexible due to oxidative stress, changed amounts of iron in the cells,…? Knowing that would help a lot.
I think the surface area may be a reasonable candidate as wetting the cell may help to deform it. would be observable under a good microscope if it happened.
Hey Cort
Thought you might want to see this
https://www.meassociation.org.uk/2019/04/stanford-biomarker-for-chronic-fatigue-syndrome-identified-30-april-2019/
Great post Cort. One thing to consider is that sensory and motor cortices should not be considered seperately. Typically the same impairments you mention in the motor cortex will also be found in the sensory cortex. The insular also plays a pivotal role in sensory processing.
In ME there are clearly major disturbances in sensory functionality, so I am resistant to getting too focused on the motor cortex in isolation.
I refer you to some great work with trans cranial magnetic stimulation by Dr Siobhan Schabron in Sydney. She shows people with low back pain can be impaired in their ability to maintain stability of excitation in the motor cortex. Although that was back pain, I expect people with ME or FM are similarly affected.
Cheers
Yes! Thanks so much Paul. I agree completely – the motor cortex is just one part of the picture. I was surprised to see how connected it was to other parts of the brain.
And yes, although, I didn’t mention it – the best evidence for the role the motor cortex plays in pain comes from people with low back pain. 🙂
Are you in pain and have these other problems, Pain, Anxiety, Stress & Inflammation Relief | Promotes Sleep & Calm Mood.
but you are afraid of getting addictive. to pain pills. after double ACL surgeries, I was giving a string of pain pills. I was close to being addicted. then I found Natural Hemp Gummies 3000MG.
on Amazon.if you want more details please click the link below.
https://amzn.to/2JjPAK2