

How close are we to understanding chronic fatigue syndrome (ME/CFS) when we can accurately predict how the greatest stressor of all – exercise – affects ME/CFS patients’ physiology? Nothing, after all, whacks a person with ME/CFS like exercise. That’s a nice question to ask.
We’re not there yet – the models being produced aren’t comprehensive enough – but the fact that they’re successfully incorporating important elements of two of the major systems (immune/endocrine) of the body suggests we’re on our way to teasing out major factors in this disease.
It’s taken an unusual approach to get to this point. It’s pretty clear that ME/CFS arises out of the dysregulation of numerous systems, yet too often researchers approach these systems as if they exist in isolation from each other. That approach, these two research groups – Gordon Broderick’s group based at the University of Rochester and Dr. Klimas’s group based at Nova Southeastern University – believe is a big mistake.
Hypothalamic Model of ME/CFS

The models were missing a vital element – sex hormones. Putting them in made the models much more powerful.
A possible model of ME/CFS explains why. This model proposes that a stressor, such as an infection, triggers one stress response system (hypothalamus/pituitary/adrenal [HPA] axis) to activate the other stress response system (ouch!) (the sympathetic nervous system or fight/flight system) to create a positive feedback loop (an ongoing disease situation) kept alive by an inflammation in the hypothalamus.
It makes sense that the hypothalamus might be involved. In fact, a couple of years ago, Dr. Bateman concluded that everything in ME/CFS could begin with inflammation in the hypothalamus.
Located deep within the brain’s limbic system, the hypothalamus is tasked with maintaining the body’s internal balance – or homeostasis. The link between the endocrine and nervous systems, the hypothalamus produces the hormones which regulate the other hormones in the body. It directly regulates:
- Heart rate and blood pressure (autonomic nervous system)
- Temperature
- Fluid and electrolyte balance
- Appetite and body weight
- Glandular secretions in the stomach and intestines
- Production of substances that influence the pituitary gland to release hormones
- Sleep.
Inflammation in the hypothalamus could be sustained by increased levels of just three cytokines (interleukin (IL)-1b, IL-6, and tumor necrosis factor-a (TNF-a). Put all these factors together and you could have the ongoing inflammatory/hormonal/autonomic nervous system mess that is ME/CFS.
Adding Women into the Equation
That HPA axis/immune model works, but it has a glaring hole – women. It can’t explain the much higher ratio of women who have ME/CFS. It’s possible women’s greater susceptibility to infection and/or autoimmunity plays a role, but even then, any model of ME/CFS has to take into account women’s complex hormonal situation.
In fact, researchers have for years shied away from including the complexity that women’s hormones add into the equation. So few female mice studies were being done at the NIH, for instance, that in 2014 Francis Collins mandated that NIH-funded mouse studies include female mice.
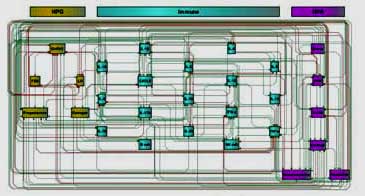
However, there’s been no shying away with this team. Instead, they’ve embraced the complexity of the female hormonal system and its manifold effects in a way no one else has done. The new model incorporates the HPA axis (corticotropin-releasing hormone [CRH], adrenocorticotropic hormone [ACTH], and cortisol), the hypogonadal [HPG] axis (estrogen, follicle-stimulating hormone [FSH], GnRH 1, luteinizing hormone [LH], and progesterone), norepinephrine, dopamine and immune factors (IFN-g, IL-1a, IL-1b, IL-2, IL-4, IL-6, IL-10, IL-13, IL-15, IL-17, IL-23, TNF-a, B-cells and NK functions).
It may be the first of its kind. It may be that no other model has so comprehensively incorporated sex hormonal findings into its results. The good news is that it appears to have worked.
The Big Test
Once they had their model, they gave it the big test: could it predict what happens in ME/CFS before, during and after exercise?
Leveraging Prior Knowledge of Endocrine Immune Regulation in the Therapeutically Relevant Phenotyping of Women With Chronic Fatigue Syndrome. Matthew C. Morris, PhD1; Katherine E. Cooney, BSc1;Hooman Sedghamiz, MSc1; Maria Abreu, PhD2,4; Fanny Collado, RN2,4; Elizabeth G. Balbin, MSc2,4; Travis J.A. Craddock, PhD2,3;Nancy G. Klimas, MD2,4; Gordon Broderick, PhD1,2,5; and Mary Ann Fletcher, PhD2,4. Clinical Therapeutics/Volume xxx, Number xxx, xxxx
This study collected blood 10 times before, during and after (up to 24 hours) a maximal exercise test in 88 women (43 ME/CFS / 45 healthy controls). It measured frequencies of B cell (CD19+) and natural killer (NK) cell (CD3-CD56+) populations, cytokines (IFN-g, IL-1a, IL-1b, IL-2, IL-4, IL-6, IL-10, IL-13, IL-15, IL-17, IL-23, TNF-a) as well as estrogen (estradiol) and progesterone (at 4 time points).
Success!
This finding suggests that the set of candidate mechanisms embodied in the endocrine immune circuitry model offer a framework for accurately reproducing the immune response to exercise. Authors.
The methodology is way beyond me and the authors do note several issues including a lack of data for some elements of the modeling, but a remarkable thing happened: the model was able to predict what happened to the immune/endocrine systems in ME/CFS both during rest and exercise. Consider how difficult it must be to characterize the complex interactions in these systems at rest – something no one else to my knowledge has done – and then to throw in the huge impact that exercise must have on these systems – and get that right as well. This is clearly a robust model and a remarkable achievement.
The model found, if I’m reading it right, no less than 26 different ways to tweak these systems so as to arrive at an ME/CFS-like state. All the simulations, interestingly, involved “widespread endocrine dysfunction”.
Immune Hits
The exercise part of the study found that exercise does, indeed, whack the immune systems of people with ME/CFS quite hard: the levels of almost half the immune markers (IL-1b, IL-2, IL-4, IL-5, IL-6, IL-13 as well as the NK cells) were significantly altered in the ME/CFS group.
As noted above, the immune test results suggested the group’s crazily complex models were right on; i.e. the modeling actually predicted perfectly the immune findings the testing revealed at rest and were within 5% of the immune findings during and after exercise.
Sex Hormones
The modeling predicted the women with ME/CFS would have significantly higher estrogen levels (p<.002) at rest and throughout the exercise study – and they did. It also predicted transient up-spikes in progesterone would occur and a trend (p<.07) towards elevations in progesterone was found as well.
Predicting the Future
Once they knew the modeling worked, the researchers pushed it to predict what the heck else was going on in ME/CFS. It turned out that the immune and endocrine dysregulations found were just a prelude: the modeling also predicted that a host of other markers (ACTH, cortisol, estrogen, GnRH1, IL-17, IL-23, LH, and TNF-a, IL-1a, B-cell activation, CRH, and dopamine levels) would be thrown off by exercise.
The models suggested, then, that exercise was massively altering many areas of ME/CFS patients’ physiology – which would come as no surprise to anyone with this disease. All in all, the model/exercise study indicated exercise was triggering the following processes in women with ME/CFS.
- Inactivation of the HPA axis
- Overactivation of the HPG axis
- Heightened sensitivity to inflammatory stimuli, driven primarily by three cytokines (IL-1b, IL-6 and TNF-a) (particularly in the brain).
The model also predicted that:
- Inflammation in the brain is present (via the upregulation of chemokine (C-X-C motif) ligand 8 and IL-23).
- Dysregulation of the female hormone system has impacted the blood brain barrier, allowing immune cells to infiltrate the brain (Jarred Younger is exploring the possibility of an impaired blood/brain barrier now. A blog on that is coming up.)
Neuroinflammation
Neuroinflammation is clearly a topic Dr. Klimas is very interested in, and the paper suggested that the Epstein-Barr virus and/or mitochondrial issues in the hypothalamus in connection with mast cell activation could be causing it.
With Jarred Younger and another Univ. of Alabama at Birmingham researcher (McConathy) diving into a large brain imaging study, and with Ron Tompkins at the Open Medicine Foundation-funded Harvard ME/CFS Collaborative Research eager to use Harvard’s brain imaging facilities, and with Dr. Klimas apparently interested in modeling neuroinflammation in ME/CFS, we will hopefully learn much more on the role neuroinflammation plays as time goes on.
Context Matters
We often think of the body in terms of test results or data points, but single data or even multiple data points are hardly able to capture what’s going on in a complex, dynamic system like the body. The upshot of modeling studies like these is that more traditional studies, with their focus on identifying “abnormal levels” of a factor, may be missing something vital: biological context.
Several ME/CFS studies, for instance, which appeared to fail to find evidence of significant alterations in female hormone levels, may be missing the forest for the trees. These studies didn’t account for the outsized roles some female hormones could play once put into the altered endocrine-immune networks the models suggest are present in ME/CFS.
The fact that the models put forward in this paper suggest that overactivation of the HPG axis (estrogen, progesterone, etc.) is a major driver in women with ME/CFS indicates how stark the differences between a systems and non-systems approach to ME/CFS can be.
Predicting Responders
The modelling efforts – which can assess how all kinds of inputs will tweak ME/CFS patients’ systems – are also being used to predict the effect of different drugs – in this case, Rituximab and Ampligen.
We know that Rituximab didn’t work out in ME/CFS and Ampligen is still up in the air – and the computer simulations largely bore this out. They suggested Ampligen would either work really well or have no effect at all. A return to health, however, occurred in only a “small fraction of the simulations run”.
The Rituximab simulations did not suggest the drug would return ME/CFS patients to health, but that it might help ward off some “highly pathologic states” and offer a partial remission for a small number of patients.
The modeling effort also predicted which type of patient might benefit from each drug. It suggested, for instance, that ME/CFS patients with a certain biological profile (low levels of IL-1a, IL-17, and cortisol, intermediate levels of progesterone and FSH, and high estrogen levels) might benefit from Ampligen, while those with another biological profile (low norepinephrine, IL-1a, chemokine (C-X-C motif) ligand 8, and cortisol levels; intermediate levels of FSH and GnRH1; and high TNF-a, LH, IL-12) might benefit somewhat from Rituximab.
The potential of these kinds of models – to create personalized drug treatment regimens for people with ME/CFS based on their particular endocrine, immune and autonomic nervous system results – is clearly huge. That’s a good thing, as the authors noted that the great heterogeneity found within ME/CFS (“the multiple disease phenotypes”) presents real challenges.
Dysregulation, Not Irremediable Damage
The good news was that, again if I read it right, the models suggest that ME/CFS is a disease of altered endocrine-immune regulation, not overt or irredeemable damage (i.e. to the basic circuits). Those circuits have just been, as the authors have asserted in the past, pushed into a kind of altered reality. The circuits are functioning abnormally, but are not in themselves damaged: they just need to be pushed, prodded, and enticed back to normal functioning.
Conclusions
For me, this is all fascinating stuff, but what I’m really excited about is the process underway.
It’s encouraging that the newer, more extensive model rested on – and ultimately validated – their earlier, less sophisticated model; they’ve had it right since the beginning. It’s more than impressive that their models are actually able to predict the changes that occur when people with ME/CFS are put under the greatest stressor ever – exercise.
The model suggests people with ME/CFS might be able to be moved from an unhealthy state to a healthy one.
Even more encouraging is the fact that these models are being used to predict helpful treatments which are now being tested. It seems too much to ask, quite frankly, that any treatments at this stage will be a complete success. I would never expect the answer to come tomorrow, but a 30% improvement would be life altering – and if they can get that far, that success should lay the foundation for more.
Plus, even in the worst-case scenario (were the current drug trials to fail), the results will be fed back into the program and help them produce better, more accurate models of this disease. So long as Klimas, Broderick, Morris, Crawford et. al. can continue to get funding – and Dr. Klimas just got a major GWI grant – they should be able to keep improving their models of GWI and ME/CFS. (Next up, there appear to be models which incorporate mitochondrial metabolism, mast cells and neuroinflammation.)
There’s no doubt that this is cutting-edge stuff, with all that implies – potential breakthroughs as well as considerable risks. Consider how powerful, though, it would be to be able to put your immune and hormonal results into a computer and have it spit out a series of possible treatments that might shift your health back towards normal.







So I guess that the poor fish are not the only ones downstream of environmental estrogens problems. I love how these articles illustrate the hard work that is being done, finally, and also the big picture of learning how our body systems work together.
Impressive research! And to me it sounds very likely.
Hope!
Thanks for translating this into more comprehensive language. ?
Jay Goldstein was talking about the Limbic system and CFS 25 years ago! He was so far ahead of his time.
Wasn’t dr. Straus (R.I.P.) who predicted that the HPA was the cause of ME 25 years ago?
No kidding! The Limbic Hypothesis in 1993 – which I just got a copy of. Dr. Cheney also talked about the limbic system way back when as well I think.
These findings also seem pretty much in line with the hypotheses of people like Dan Neuffer (‘CFS Unravelled’ and ‘ANS Rewire Programme’) and Ashok Gupta (‘Amygdala Retraining’). They’ve been saying for years that some people are recovering via programmes that aim to reset these dysregulated stress responses and the resulting self-perpetuating cycles (or cascades) of multisystem dysfunction seen in ME/CFS
Hi Cort, Elizabeth
I have written two comments on https://www.healthrising.org/forums/threads/potential-linking-fm-mast-cells-sleep-deprivation-food-intolerance-exercise-intolerance-and-me.6217/, comments number 11 and 12. I did my best to keep things as simple as possible.
There I make a hypotheses that excessive levels of excitatory chemicals like glutamate in the space between brain neurons could cause a runaway over stressed and over excited response.
Finding ways to calm that response down would help to break this vicious circle. Pacing, yoga, mediating and the neural techniques could all be things that could help to break this vicious circle down.
One note: most of above mentioned techniques also lower (nor-)adrenaline levels. And those increase the energy supply to the brain. That may explain why some people who depend on elevated (nor-)adrenaline levels to keep their brain somewhat functional may experience poor results with some techniques like meditation and yoga. Other issues have to be solved first then IMO.
So did Dr Majid Ali, and Teitelbaum, and Goldstein some 20 years ago. Shame all this was neglected.
Cort, any thoughts on what’s happening with us men? Do we also trend to higher estrogen levels?
I don’t know what these groups think about men but we are simpler, low testosterone is part of the equation for some and If I have it right the etanercept/mifepristone combo being tried in women is not believed to be a good fit for men.
Thankfully Dr. Klimas has completed a major exercise study focused just on men and those results are surely being integrated into their modeling studies. The GWI modeling has been focused on men as they were the most affected. Altho more women than men have ME/CFS – a lot of men do – and I expect we will hear more on men and ME/CFS in the future.
There is a little known and rarely discussed syndrome called ‘P.O.I.S.’ that has symptoms very similar to ME and almost exclusively affects males. Hormones must surely play a part in that syndrome.
That is absolutely outstanding news for us patients!!! The potential benefits of this approach are plenty and the shear scope of it is hard to imagine.
Thanks out of the bottom of my hart to Gordon Broderick’s group and Dr. Klimas’s group for doing the cooperative work and to Dr. Bateman for building the foundations this work seems to rest upon. Also thanks to anyone who ever in any way made this possible by contributing their efforts, research and thoughts in the past.
While not being a cure, it will help make finding them and understanding what our disease actually is so much easier. And it will give all doctors and psychologists in the world a clear mathematical underpinned way to see what drives this disease, transferring it from the domain of the impossible to the domain of the confirmed. That may be the greatest thing these brave researchers could offer us for the coming years!!! Combined with the findings of Drs like Davis, Naviaux, Younger and many others this announces the final and inevitable death’s roar of the psycho-social school. Hurray!!!
There is still plenty of work to be done to actually beat this disease, but adding models of mitochondria, mast cells, gut working etc. could provide such success that it reinvigorates the field of medicine as a whole. This sounds very much like a domain where future Nobel price winners will be born from.
I’m also delighted to see indeed that much of the disease seems to be in the system rather then in damaged processes, cells and organs. That opens possibilities to regain more of our health as understanding on how to reverse this ill system functioning.
Thanks for bringing us this joyful news Cort!
https://www.healthrising.org/forums/threads/potential-linking-fm-mast-cells-sleep-deprivation-food-intolerance-exercise-intolerance-and-me.6217/#post-35555
Please note this thread/blog. Some of us have been working on our hypothesis for quite some time. For me about 15 years of making connections with MCAS, POTS, EDS, FMS, CFS.
There has been alot of water go under the bridge and happy to see doctors taking note to some of our own observations and hypotheses. This has been my goal, over the years, for those who could take my hypothesis and either prove me right or prove me wrong.
There has seemed to be an estrogen dominance issue and that could apply to men too. I have asked both POTS and CFS guys if the balance between estrogen and testosterone has been checked. Even if estradiol is in range, if testosterone is low. . .the balance is off. That possible could be one reason for a few men having predominantly female illness.
MCAS, is another interest of mine. Mayo Clinic got to know me well when I wouldn’t let up that there was a connection with POTS and mast cell issues. They even called in an expert from MN when I insisted there was a connection. The book of information I presented to them was well read, underlined and tab marked by them. Thankfully now, all POTS people are looked at for MCAS. (Strong connection to endometriosis,MCAS and estrogen dominance. )
I have also felt that Glutamate was a key issue with the sympathetic responses and dysfunctions we all experience. There seems to be issues between Glutamate and GABA and pathways not working properly. As you can see by the linked thread/blog —- dejurgen and I are hard at work linking this to many, many functions/dysfunctions with our illnesses. Along with this is dysfunction with dopamine.
With my subset type of POTS and my bodies response to the many “self experiments”, I found that not all of us need to vasoconstrict. With me, I’m better to mildly vasodilate. Of recent there has been more studies showing this to be true for some. And the connection to too thick blood and APS and other too thick blood factors coming in to play. (For me Factor 8 and Collagen Binding.)
And there also seems to be issues with Calcium Channels. We have yet to look into this. But there seems to be a connection to glutamate and calcium channels and seems to be connections to MCAS and POTS and FMS. (Another self observation hypothesis. )
Mitrochondrial dysfunction and genetic inherited issues another huge factor. My recent “self experiment ” is trying to work around all my Mitochondria Complement Factors (yes, 5 of them) having very low to near no function. That’s a work in progress.
Some of us have been working for years and being rather bold in our hypothesis trying to find answers for not only ourselves but the masses. There is HOPE on the horizon. So very happy that these fine doctors are in the process of either proving it right or proving it wrong. Let’s hope right, and we will be closer to solutions. In meantime, I’ll continue to tweak, explore and “self experiment “.
But we have to get to the core……my hypothesis still stands as to bottom line…..Autoimmune dysfunction and Inflammation. But finding the best “purple bandaid” for the symptoms of the core issues….. still on that quest.
Issie
These models are so complex that it seems wild that they would actually work out but they seem to be. Besides getting the exercise right in this paper and pretty accurately predicting how Rituximab and Ampligen would fare they accurately predicted how the animal model of GWI would respond.
Dr. Klimas expressed an interest in MCAS years ago and that is apparently the next area of the immune system to include in the models. The mitochondria were mentioned as well and her treatment protocol aims to turn down the fires in the brain – the neuroinflammation.
Correction on mistyped word……that would be issues with Mitrocondria Complement Factors. This was found in the study done by Drs. Light in Utah. It was a study between families and those if us with multiple family members having CFS, FMS, POTS etc. Even though my sister and I share similar genes, the function of mine was much lower. Meaning I had much more dysfunctional expression, at that moment, than she did. I was told I was in lowest 20% of function of all CFS patients in the study. So trying to tweak this and see if there is a way around the apparent lack of energy in the powerhouses of the cells —- mitrochondria.
There is more than one part to this very complex puzzle. But it’s nice when they all come to light and there are things that can be done for them.
Again, issues with getting this right……Mitrochondria Complex. 🙂
There, now you can look it up easier to see dysfunction with mitrochondria complexes.
Issie,
Thank you for your willingness to share your discerning ideas and conclusions. You draw such great ‘connect the dot’ pictures using words.
Cort, I trust the day will come when so many can put so much behind themselves, and not just look to the future with hope, but experience the good results in theirs and families’ and friends’ lives.
Thank you for reporting this amazing news, and keeping us all in the loop.
Welcome……hope we all benefit! When I have such severe ” brain fog”, it’s a wonder that anything makes sense……but I’ll keep trying.
Speaking of brain fog…..that’s another important issue……hypoxia and lack of blood flow and oxygen to the brain. Very common in POTS, but also proving to be important in ME/CFS.
Issie
I found Viagra to help get blood flow in the brain. It soothes the heart too.
Interestingly I originally used it for my relationship and found my PEM was much lower or didn’t happen at all. meaning the blood flow in the entire body was helped by Viagra.
More interesting is that bodybuilders also use either Viagra or Cialis for faster recovery from training. Meaning they can exercise the same muscle groups the next day.
They prefer Cialis but unfortunately there appears to be no cheaper generic for it yet.
It’s not a cure, just a slight help, but this last year has been an improvement, especially after 30 years with the last 3 years being mostly bedridden and couch bound
Anyone with ME taking the likes of Viagra ought to wear medical grade 3 or 4 compression stockings to keep the blood pressure up when sitting or standing)
I also take antihistamines and anti inflammatories before or immediately after exertion of any type that I‘m sure will trigger PEM
Interesting! A UCLA researcher started a study on Viagra in ME/CFS years ago but it was either never finished or never published. I didn’t know about the bodybuilders. Niacin can be helpful for me.
I know some docs were RXing Viagra, even for women, to see if it would help ME/CFS. There are however over the counter things that can assist with this too. Things that improve blood flow, vasodilate and thin the blood. (Not all of us tolerate niacin and the “niacin flush”, especially if there is MCAS issues.)
There will be more information added to dejurgen and my blog/thread (noted above) as we get it more sorted and referenced material compiled.
@Brendan – if blood flow is a possible issue you might be helped by a PEMF mat. PEMF increases blood flow, in fact some forward thinking ED clinics are incorporating into their treatment offerings. I use a PEMF mat for 20 minutes every morning and it’s significantly helped various issues that I think are all down to blood flow, and the myriad of things poor blood flow affects in the body.
(I don’t know if it’s ok to recommend stuff to folks like this, apologies if not)
I’ve run into body building info on many paths while researching CFS. There’s decent scientific knowledge out there in the body building community & it seems like they’d be worth talking to concerning some areas in CFS research!
I also used viagra and have had some side effects. The blood flow was better but then i get very warm upper body and dizzy, also i get panic attacks so i don t use it anymore. But i think it can help for some patiënts ME and POTS either.
Issie,
In one of your comments below, you mention over the counter things that improve blood flow, vasodilate and thin the blood. I get the “niacin flush”, so would love to know which OTC things you would suggest.
If the “niacin flush” doesn’t bother you, it has alot of benefit. It can lower cholesterol for one thing and is very good for circulation and blood flow. Helps moods that this can be deficient in with some neurotransmitter dysfunction/mood disorders. With time, that flush should diminish as you get your levels up.
But, it actually causes me anaphylactic responses with my mast cell issues.
I use herbs such as ginger, gingko, turmeric. Also enzymes to help with this.
This is such great news, thanks for always sharing new information in such an understandable way, Cort. I’ve seen three ME/CFS specialists so far and only one has looked and my adrenals, hormones, etc. I only started his protocol a week ago so I can’t say if it helps, but I am hopeful. I’m grateful for all of the research being done and believe we are getting closer to treatment.
Thanks – good luck and please let us know if it helps.
In Dr. Klima’s modeling; what about menopause? I often notice there is an age cut-off in many of these studies, as if ‘older’ people are destined not to function well. I’m not all that ‘old’ but I am in menopause. So did the study mention anything about us? After all, people with ME/CFS do age…
Regardless, this is very exciting! I do wonder if it becomes a practical tool for accessing treatment, how will all of us be able to get into the model?
Another random question, what is happening with the water hyssop? Are they testing that?
Issie, I’m with you in trying to figure out the interactions of all my body systems (and self experiment)–especially since you are a part of the ‘dazzle’ (zebra herd). You claim to suffer from brain fog, but you seem to express yourself quite well to me! I can’t wait until the mitochondria modeling comes out!
Thanks again Cort, for the review!
Kelly, I’m curious about your protocol. Do you mind sharing?
My Stanford treatment has so far been a very small daily dosage of Abilify (dopamine agonist) plus some suggestions for additional supplements (such as magnesium etc.). I was doing really, really well–and then I crashed and am now limping along. Thought part of my improvement was my addition of Pycnogenol, which has since run out, so I just bought more to see if it helps again. Still haven’t ordered the water hyssop. Must not get too excited to try to many things! One at a time…
Yes, unfortunately, I’m a zebra. For those who don’t understand what this means, we have Ehlers Danlos. The mascot for Ehlers Danlos is a zebra. “Just because you hear hoof beats, doesn’t mean it’s a horse. It could be the rarer zebra.”
I’m glad my thoughts come across cohesively. Sometimes, it seems to be a bit of a jumble. (At least my mind will feel that way.) But I’m a deep thinker and my brain is usually in over drive. My friend and research buddy – dejurgen, tells me to slow the information down. I’m overwhelming him with the speed and diversity of subjects. Yet, it all ties together and to me it’s just different facets of a whole. Pieces all coming together. His ability far exceed mine as to putting the science together and explaining it. And him being younger, maybe his stamina will allow him to follow through with my/our hypothesis.
Yes mitrochondria is one piece of the puzzle. Not the whole picture.
And as for being older and past menopause, yes I’m there with you. This has been a lifelong thing for me dating back to preteen years. I haven’t given up trying to find answers and I’m certain they won’t forget us “older” (in body…..not mind) ones.
Issie
I have ME and EDS and the EDS (obviously) runs in my family. A great grandmother of mine was diagnosed with fibromyalgia (probably misdiagnosis for EDS), and she was reported to say she felt better after 70 than ever before. So there’s hope for aging.
Forgot to comment on your dopamine “experiment “. I’m tweaking with a natural supplement for this. (Among other things ) and I found that I had to, after a few days, cut back to 1/3 of what I was taking. Seemed to build up and start backfiring. Now with the cut back, I’m back on track.
Issie
Hi Nancy,
My protocol includes hydrocortisone for my adrenals, a prescription for sex hormones (biest, progesterone & testosterone) based on my test results and a number of supplements (thymic protein, a few sleep aids and NAC. I’m also on hepapressin and Valcyte, prescribed by other doctors.
The Valcyte has not seemed to work, after 7 months but I am going to stick with it for a full year.
I’ve only had 3 injections of hepapressin and it takes about 10 weeks to know if it helps – seems like there’s a 50% chance it will.
If I remember correctly, I hope I am, the ME/CFS clinical trial Dr. Klimas is conducting (which was filled) was for post-menopausal women. If sex hormones play as big a factor as these teams think they may then menopause will certainly have to be taken into account.
As someone noted, I think, the temporary remissions that some experience during pregnancy certainly seem to implicate the changing hormonal milieu.
Good luck with your Stanford treatment.
Cort, thank you for the article. Is anyone exploring whether women who have a tendency to PCOS also have a tendency to ME/CFS? Both came along closely for me, and link the sex hormones into this multi system thinking. I understand PCOS can be asymptomatic in many women (PCO without the S).
Christina, there is a connection to MCAS and PCO(S).
Jubilant- as soon as I saw the heading of today’s email I knew we would be beyond impressed. Jubilant is more like it!
Has anyone, amateur or professional, tracked the issue of the estrogen in our drinking water? For instance, people who do – and don’t – have certain levels of estrogens in our drinking water. So-called water treatment facilities for most communities do not (cannot?) remove estrogen when providing “treated” water to their communities. A surge of estrogen in our drinking water has developed since women have been taking birth control pills and then constantly peeing out estrogen, only for the estrogen to be ignored in the water treatment process.
But not _everyone’s_ water has this problem. Any public health studies, population studies, or geographical studies?
Note of caution- simply taking testosterone has resulted in massive coronary attacks… but I wish we had an easy option to reset our hormonal balance.
Thank you Cort for this wonderful news and thanks to the devoted and insightful researchers!!!
I’ve been ill with ME/CFS for over 30 years and the most helpful medications for me all had to do with hormones (hydrocortisone, fludrocortisone, desmopressin, throid T4 & T3; and then added estrogen, and progesterone during menopause). Everything else I’ve tried has either not been helpful, or made me worse. If I remember correctly, women with ME often feel much better during pregnancy, but then revert back to previous illness after delivery. I wonder if any researchers who are interested in studying hormones have looked into why those women seems to go into remission.
I only have a hypothesis based off my own observations and what happened with me and connections to estrogen being a factor in my family with cancer with my dad. (He had prostate cancer and that predisposes daughters to gynecological cancers.)
Having had a complete hysterectomy at a young age, and trying to stay off replacement hormones for 6 months due to endometriosis, when I tried to go back on something — everything we tried wouldn’t work. Progesterone gave me hot flashes. (MCAS at play here.) And estradiol made me feel awful and didn’t help hot flashes. Herbs didn’t help. And I was told until my body was supposed to naturally be through menopause, I’d have hot flashes. I was only 36. And this was my 8th abdominal surgery. So, we found compounded estriol to work. This is the estrogen highest with pregnancy and it is a breast cancer preventative.
So maybe…..the type of estrogen is what is key here. There are 3 types.
Things get out of balance with all the xeno estrogen we are exposed to daily. It’s in our foods, plastic, medicines etc. Men are exposed as are women. I was told by a Functional doc to quite eating chicken, unless it was organic and hormone free, estrogen is pumped into chicken to make it grow larger and faster. You eat that meat and you are eating estrogen. How many do this daily, thinking it’s better than red meat. But with red meat and dairy there are added hormones that imbalance the system.
I could go on a rant about diet and things done with chemicals that make us unwell…..but, you get the point. Only we can control what we eat and put into our bodies. But it will take this for us to get “healthier”.
My having questioned “treatments”, of symptoms of what I consider as being the bodies compensation to something far worse going on. Has allowed me to form my hypothesis. I found that treating “symptoms” is sometimes more detrimental. We have to get to the “core” and see if those “symptoms” are actually of benefit. If they are of benefit……WHY. and if WHY leads to a good reason that can be moderated……then we have a solution. No more symptom.
Your Dr is misinformed about chicken.
In the US chickens are not allowed to be fed any hormones, unlike beef and lamb. They can be given antibiotics though so organic is always best when you can afford it. https://www.businessinsider.com/no-hormones-chicken-poultry-usda-fda-2016-3
That makes me happy to hear. Since if I eat meat, I tend to prefer chicken as I digest it better. But dont like all the antibiotics used. Since I have antibiotics resistance issues. That’s not a good thing either.
Hubby pointed out to me when I told him what you posted, that they feed chicken’s lots of soy and that would up their estrogen levels. So maybe being more modest still a good thing to do with it.
Next to estrogen, the body also modulates the immune system during pregnancy. As a baby growing in the whom is in effect “a foreign body” with foreign DNA, tweaking the strength of the immune system downwards may be a logical thing to do during pregnancy.
Chances are however with this supposed lowering of immune strength during the pregnancy, that it can go any direction after the pregnancy. That includes keeping part of the improvement, getting a strong flare as the immune system wants to catch up or maybe even a strong surge in “pro-depressing” chemicals as that is a known effect of the immune system getting in overdrive. Personally I experience these mood depressing effects when I have likely massive mast cell degranulations.
What got me interested in some aspects of system theory, and biochemical systems, was the realisation that a blood test reading is both a time and spacially averaged reading. It tells you little about the dynamics, for that you need serial readings at least. I am always interested in reading about these kinds of models.
Sometimes even the timing of hormone releases has an impact, and levels can be misleading.
Our daughter was a surfer, zumba dancer, yoga devotee, skater and snorkler and full time teacher until she developed ME/CFS.
Here is her account (and that of another) of a fast and remarkable remission of ME/CFS symptoms with Tamiflu.
1. Friday working around kindergarten kids, lots of sneezing.
2. Felt my throat swell on Saturday
3. Sore throat Sunday; it was definitely red around the edges.
4. Nose running sneezing on Monday but went to work,
5. Worse on Tuesday, the sickness coming on strong
6. Wednesday so bad I had to stay home from work and was laid out so I called Kaiser,
desperately askimg for Tamiflu.
1. Prescription came so took two on Wednesday.
2. Thursday stayed home just to rest but started to feel better.
3. Friday. Was able to go to work on Friday. Felt tired but not bad enough to stay home.
4. Saturday was able to go to the Professional Development Workshop. just a little bit runny nose and sneezing and a little bit of fatigue.
5. By Sunday I was well enough to go surfing.
6. This is a remarkable improvement compared to my normal fatigue, body pain and brain fog.
7. Monday had plenty energy went to work, Surfed after work.
8. Tuesday felt so good with great mood & so much energy that I went to work, did errands and went surfing after work. I haven’t done this in years.
a. Called my mom to tell her because I’ve never felt this great in years and it was so shocking because the only thing different I did was the Tamiflu.
1. Dosage: took two pills on Wednesday, Thursday, Friday, and one pill on Saturday, Sunday, Monday, & Tuesday.
2. By Wednesday I was fatigued again. Back to my normal,
3. By Thursday the fatigue returned and back pain with body pain came back.
4. By Friday fatigue returned with body pain and brain fog
a. . Had to take a nap after work both Thursday and Friday could not meet my friends to surf.
I cannot explain to why Tamiflu (generic) works and why it works so quickly and so well, yet I have never felt that good in over 2 to 3 years. It even helped me to be in a better mood. I had the normal amount of energy I used to have in the past where I could work, do my errands, and surf all in one day.
I would very much like to try a 30 day supply of Tamiflu (generic) twice a day to see if it helps with ME CFS. Certainly it did something great since I’ve never felt that good in years.
And I’m not the only one see the link below.
https://cfsremission.com/2017/03/17/temporal-remission-from-tamiflu/
(I also heard from the Open Medicine Foundation and they knew of a case of remission with Tamiflu, but I am not sure if that is another case or the same case we found on the internet.)
I looked up veterinary use of Tamiflu and found that it is used for viral infections of both dogs and cats, but these viruses don’t have the enzyme neuraminidase which Tamiflu inhibits in flu patients.
It turns out that neuraminidase is an important enzyme used by pathogenic bacteria invading through the protective mucous barrier of the GI tract. This invasion through the mucous barrier is biochemically similar to the budding of virus from the cell membrane and oseltamivir is able to inhibit invasion of intestinal bacteria into the bloodstream.
Since it is not likely that any doctor would prescribe Tamiflu off-label for ME/CFS, I have done some more research and found that a herbal product called Sha Ren has the same ability as Tamiflu to inhibit neuraminidase. It has tested well against Tamiflu for flu control. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5859433/ It is called Amomurn villosum Lour in this study.
I wondered if anyone who read Health Rising has had a similar experience with Tamiflu.
I’m actually doing retrovirus and biofilm treatments with an herb and enzymes and finding improvement. Also works on pathogens.
Had quite the detox happening and caused lots of issues as things started coming out of tissues, organs, veins etc. But I’m sure it is making a difference. (I had some lipomas since my 20s that are almost completely gone.)
This is a very likely piece to the puzzle.
Amazing story Betty!
Thanks for passing that on. I’ve never heard of Sha Ren before. Has your daughter been able to get more Tamiflu?
Betty, I am so happy to hear that your daughter has such a positive experience taking Tamiflu. Unfortunately, my recent experience was not the same. I began having the classic symptoms of the flu (fever, cough, sore throat, increased body aches…) and immediately went to doc. Even tho the rapid flu test came back negative, the doc prescribed Tamiflu because he said flu test can return false negatives in early course of flu illness. I took the full course of treatment prescribed. While I recovered completely from the acute illness over the course of the next 2 weeks, I have not had any improvement in my ME/CFS at this point, 6 weeks later. Maybe because I had different acute illness than your daughter? Or that I am more significantly affected by ME/CFS (90% couch bound)?
Tamiflu is for viruses so if your CFS was precipitated by a bacteria or rickettsia (like Lyme disease) then it wouldn’t help you. Have you been tested for Lyme? If you’ve been sick for a while it likely won’t be positive anyway. Have you ever taken antibiotics? Many people with CFS, Parkinsons, MS,etc have been “cured” when given antibiotics because they actually have Lyme disease.
I have Post Lyme Syndrome (and a whole cart more) and antibiotics didn’t help me. I was on low dose Doxycycline for years. Just made me antibiotic resistant to it. The herbal I’m doing is helping this too and it is an antiviral thing too.
Hi Betty, would you share what you know about Sha Ren, effects, dosage etc. As I have never heard of this. Thanks.
Cort – do all the docs read this blog that are working on this in some fashion? I have a hypothesis that needs to get in the hands of all the docs to see what their thoughts are on IF this theory may perhaps fit into all or most of the categories as a potential causative factor.
Hi Leslie,
I don’t know how many doctors read the blog. I do know that some do but don’t know how many. I would certainly be interested in your hypothesis.
I agree, I’d like to hear it. I know of about 7 doctors who regularly read the blogs here.
Ok, let me try to finish the dreaded income taxes, and will then post my thinking, will be a few days tho, hate to keep you waiting but want to also provide the reasons why I think this could be a factor in it.
Issie,
I, too, am “post Lyme.” Please let me know which herbal is helping.
Thanks!
Lisa
Lisa w. I linked to info on comments here. Its. Lomatium. Good link listed, see comment. Helps Lyme and virus. What I’m reading is most stay on it 18 months. I didn’t get the dreaded rash as I made sure my liver was in good shape before starting. Did have to go down on my dosage as I got reflux initially.
Great reporting as always, Cort. And as always, great comments as well. Seems like every week the picture grows clearer, though at the same time I know everybody is waiting with bated breath for the research to turn up more concrete treatment solutions.
Personally it took me years of living with moderate ME/CFS before I understood the mechanism of PEM. I thought, like many of us, that exercise was important to my health, so I regularly subjected myself to crash-inducing exertion, blind to the cause-and-effect cycle of PEM.
Right now I’m staring out the window at some beautiful waves breaking on the beach and wondering if it will ever be safe for me to go surfing again without risking a crash. I know for some people just being able to get out of bed and walk around the house, but if this research is indicative of a rising tide that could lift all our boats, there is cause to be hopeful.
I will say that midodrine (part of Dr. Teitelbaum’s treatment) has been a real asset for me in terms of everyday exertions. I also find that Viagra and DAAVP, though prescribed for different symptoms, have side benefits of increasing blood volume and blood flow. Earlier this year I was suffering from regular OI episodes almost every time I stood up. Thankfully that particular symptom rarely affects me anymore.
Thank you for sharing this information it is reassuring that so much research is being carried out. In the UK we are do far behind.
I have had undiagnosed ME/CFS since my early 20’s. I was treated for depression many times and antidepressants definitely helped me. I was finally diagnosed in my mid to late 30s and have been on high dose venlafaxine since then, I am now 50.
I definitely believe that hormones play a big part in this illness as during both my pregnancies, 1 a twin pregnancy, I was incredibly healthy. So much so that I commented afterwards that I should just be pregnant permanently. I suffered severe PND both times.
A significant symptom of my depression has always been extreme exhaustion, so much so that I was bed bound. My sleep cycle has also been knocked off, when not working I only come to life around 2-3pm.
I am currently seeing a rheumatologist and for the first time am having more than just routine blood tests, so it will be interesting to see the results. I hope to be referred to an ME specialist in the UK but all they seem to offer is pacing etc.
I am encouraged to see research that is looking at the body as a whole as this illness is not straightforward.
Hazel
It sounds like your circadian rhythms are off. I was sent to a sleep study center and instructed to keep a sleep log. The doctor took one look at it and said “We need to reset your circadian rhythm using light therapy.” I had to get up and sit outside in the sun every morning at 7 AM. After years of horrendous insomnia I started sleeping at night, instead of being awake all night to finally fall asleep during the day. Now I use a light box during the winter. Wouldn’t be without it.
I’m happy to hear some talk about the “sickness behavior” signalers, TNFa, IL-1 & IL-6! Sickness behavior, unfortunate as the name is, does a good job describing the symptoms we endure. I’m sincerely surprised that there’s not way more talk about it within our community.
Since it’s a common endpoint for all kinds of sicknesses (we often experience the same symptoms from different pathologies: bacteria, viruses, injury, etc), it wouldn’t be surprising if we end up finding that many CFS/FM subtypes end up endlessly triggering it from a wide variety of immune entry points. It seems like “sickness behavior” would be a typical place to start a discussion about CFS/FM pathology.
IL-6 is both a pro-inflammatory and anti-inflammatory cytokine. It’s also a myokine (muscle signaler), released during exercise and, for some types of exercise, it’s released more gradually over the next few days… Which suspiciously corresponds with the payback period we can get after overexertion. There are IL-6 variations depending on the cells that produce them… There’s muscle cells, fat cells & many immune cells that make it & some of those reside in our brains.
Here’s general IL-6 + exercise info from 2002: https://pdfs.semanticscholar.org/5bb8/dacbcf6455587f3a05f440d9e5c6edbd44cb.pdf
There’s also the fast route of sickness signalling: the vagus nerve… The neuro-gut connection.
Here’s a good summary (maybe required reading?!) of Sickness Behavior by Dr. Robert Dantzer: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2740752/
Is Dr. Dantzer on Cort’s and the CFS community’s radar?
Thanks Stephen for the links and the SB explanation.
I haven’t looked him up lately but Dantzer is definitely on my radar. I think he even spoke at an ME/CFS event some time ago.
Thanks for sharing Stephen. My specialist and I are currently discussing the role that pro-inflammatory cytokines are playing in my illness, particularly IL8.
@MKW. What is a PEMF mat and where did you get one?
Thanks, kate
More great studies…not done on FM.? Men with FM…also SOL.
PEMF mats run about $1000. for full body, I think.
Thank you Cort for sharing this study. Sounds like a Klimas / Broderick / Cortene collaboration would be a match made in heaven.
I haven’t met a lot of ME/CFS patients during my 25 years with this illness but I am always struck by how many of them I would describe as artistic, gentle and sensitive. Not all, but many, including myself. According to Elaine Eron who studies highly sensitive persons, sensitivity is a genetic trait that can be found in approximately 20% of most species (COMT / MAO polymorphisms?). Apparently it has an evolutionary advantage. If there is a food source, the non-sensitive will descend on it rapidly while the sensitive hang back to assess the situation. This wariness can cause the sensitives to miss out if food is scarce but is more likely to save them from a predator. In many ways I am downright easy-going and cheerful but I recognise this hypervigilance and jumpiness in myself. Even if this trait has not predisposed me to this illness, I know it certainly uses up plenty of precious energy.
ME/CFS has been a slow burn for me. I may have always had it but it first made its presence known after a number of traumas in my teen years and a subsequent viral infection at 18 which left me bed-ridden for a number of months. I got better but every year thereafter I would pick up at least 10 respiratory infections. Then the mysterious gut symptoms set in and despite losing a lot of weight the only reassurances the hospital specialists could give me was that I wasn’t dying. For all of my 20s the illness was mild and undiagnosed. One doctor after another wrote my extreme fatigue off as depression and I had no choice but to carry on until eventually years of mismanagement meant that the illness deteriorated to a point where I could no longer work in any capacity and I eventually got diagnosed.
It is actually an example from my years with undiagnosed mild ME/CFS that has always made me suspect that my illness stems from the limbic system. I live in New Zealand and in my early 20s I went on a 10 day wilderness hike with friends, which looking back seems very ambitious but I didn’t understand PEM then. I was ok on day one but on day two, 2 km from the next hut, my legs turned to jelly and I couldn’t walk any further. At the time nobody, including me, could explain what happened, but now it is pretty obvious that I crashed. We waited an hour until I could walk again and then I shuffled the rest of the way to the hut. I needed to rest the following day while my friends climbed a mountain, but I was fine on proceeding days and by day 10 in nature I experienced the sort of wellness and unbounded energy that I haven’t experienced since. The most interesting thing though is that in theory I did a lot of things wrong – we lived on packet noodles for 10 days and ate next to nothing in the way of fresh fruit and vegetables. I certainly wasn’t pacing myself either. However, in that beautiful, timeless place my central nervous system rapidly relaxed into the speed of nature, which is periodically violent, but is mostly unhurried and rhythmic. We walked, talked, laughed, ate and slept. There was no other stress and I have never been more well in my adult life. My nervous system appears to be incompatible with modern life.
Sha Ren , Amomum villosum, is a herb grown in China. It has been tested and found as effective as Tamiflu.
I have only been able to find it in powder form from China and am not sure about dosing, but I plan to order some and give it a try.
It will be interesting to see if it has the same effects that my daughter and the reporter below experienced.
” The doctor who diagnosed the influenza immediately put me on Tamiflu, which I took for ten days. I honestly felt better on Tamiflu than I have in years. By day three of the Tamiflu course, after being bedridden and sick with very high fever and some of the worst body pain I’ve ever experienced, I was full of energy and strength. I did things that haven’t been possible for me for years, like take down and pack up the Christmas tree and lift the entire thing and haul it out to the street alone. I traveled across the country alone with my daughter and felt fine the next day, even energetic the same day I got home, unpacking and doing more chores. I even wanted to exercise. This level of physical exertion would normally have me, at best, resting for a few days to recover. More than likely they would result in a full week of pain and exhaustion before finally returning to “normal bad”.
And it’s experiences like this that make me want to strangle anyone mentioning ”deconditioning” -it is so NOT what we live with. Deconditioning does not account for a sudden (albeit temporary) return to life anymore than it accounts for sudden jelly legs.
https://barlowherbal.com/blogs/blog/the-super-natural-power-of-lomatium
Here is the antiviral Im using. A huge word of caution…….it can cause a horrible rash the first time using if the liver isn’t detoxing as well as it should and it will come through the skin. I did liver support things before starting this and did not get the rash.
It is being used for Chronic Lyme and is helping others to eliminate post Lyme, hard to eliminate symptoms. (Most that I have read about, try to stay on this 18 months.)
So far, so good. I did have to cut back on amount that I was using as it seemed to give a bit of reflux. But, its tolerable and I do see benefit.
I also use enzymes. The fungus found in my Blood and thyroid biopsy (and known to cause tumors and gotten from mosquitoes) has been shown to be virtually eliminated with Serrapeptase. I use an enzyme with this in it at mid day and then Serrapeptase at night. These can also give a bit of reflux. So pay attention to your body. My Functional doc wanted me to use them 3 times a day, but that was too much for me.
It’s an interesting exercise. I’m not sure of its value though. It seems rather indeterministic a modeling that may allow the researchers to see what they want to see. An abstract model with 28 variables calibrated with measured values for 17 of them leading to top 26 competing numerical model leaves too much wiggle room, imo.
Their finding of “a potential etiology for ME/CFS in which sustained HPG overactivation may permit the initiation and maintenance of peripheral inflammation potentially leading to low-grade neuroinflammation” is a real interesting one though. I always thought that PEM is caused by peripheral inflammation from exercise translated into neuro-inflammation by hypothalamus. The question I have is whether this was the result they wanted to see and their bias sipped into their modeling.
I have long believed that “eradication” of the polio virus (like the removal of a violent dictator) opened the playing field for more and new enteroviruses and bacteria, and together with vaccine(s) and environmental insults hitting our immune system this has upset the whole apple cart in some people.
Has anyone looked closely for mutation(s) of the polio virus?
Don’t know if they have are not, but my problems started at age 8 after either a polio or measles vaccine. My sis and a male friend and I all had vaccine at same time. So sick afterwards we couldn’t lift our heads from a pillow or walk, we had to be carried. Missed most of whole school year with this. Had enlarged lymph and fevers, the whole time. None of us were well from then on. All of us later DX with CFS and FMS.
I do believe that part of the effect of vaccination might be: the temporary increased load on the immune system.
Vaccinations are meant to trigger and train the immune system. You wrote before on HR that you have very low IgG values. That would likely mean that you have difficulties fighting off even a moderate infection or immune challenge.
It also seems you have difficulties in producing (mitochondrial) energy. That may compound and increase the problems of fighting of an immune challenge so that even a minor immune challenge could trigger a cascade of undesired events.
As far as I know, the safety of vaccines is only tested on healthy people. That is far more ethical, but they do not have the challenges to fight off even small immune challenges like we do. It’s known in the ME community that for example getting a flue vaccination can cause quite a (often but not always) temporary deepening of our diseases. I certainly experienced that multiple times very clearly.
Maybe there should be more research done on the effect of vaccination on the weaker and elderly. Maybe other doses or spreading a single dose over several injections spread over multiple weeks would be more optimal, or not using combined vaccinations increasing the size of the immune challenge should be considered.
Vaccination subject is a highly debated and sensitive subject. I have very strong opinion about them based on my history. Also, my dad got a very bad and debilitating case of Guillain Barre from a flu shot. This has happened to people also with pneumonia vaccine.
I do have Hypogammaglobulinemia and very, very low IGG levels. I have to be very careful, as pneumonia I was told would kill me. (However, I did survive it back in my 20s.)
So those of us with weakened immune system and also genetic inherited dysfunction ….. are very susceptible to “problems” that others don’t experience.
Also having mitrochondrial dysfunction (powerhouse of the cells) doesn’t help in fighting illness or stresses.
Issie, thank you, your story led me on a search across the web. I learned a lot. Guess where I ended up! https://www.healthrising.org/blog/2017/05/23/doctors-hyde-amy-browns-m-e-enterovirus-story/
I love this website.
Debs, I remember that now, even commented on that blog. Interesting that this fits my history so well. And only now are we treating virus with me. And doing it with over the counter herbs. Let’s hope this works.
Thanks for the link and reminder.
Steroid hormones (glucocorticoids, mineralcorticoids, androgens, estrogens and progestagens) have so many interconnected pathways in the body, and, some of these can convert from one to another depending on what is needed. They regulate immune functions, inflammatory processes, metabolic processes, fluid balance, stress, among other things. Maybe we should have endocrinologists looking at ME/CFS?
I have often thought that the stress response is a big trigger for ME/FM, not the only one, but even the chronic, day-to-day stresses we all face could be a significant (and overlooked) underlying trigger. I still believe that some of us are wired (genetically or otherwise) to be more susceptible to stress.
I feel that my type A personality kept me from backing off when I pushed too hard with exercising, working, and not paying attention to my body (including the COMT gene that someone mentioned). I also feel that my introverted nature was a factor – the outer world (outside my own head) was a more stressful place to live than that experienced by extraverts. Someone mentioned being “sensitive” – that is another way of describing it too. We are more sensitive to sensory input (sound, odors, etc.) – the outer world is more overwhelming to us. Introverts brains are wired differently than extraverts. I am guessing that as we learn more about genetics the differences in genes will explain personality types a lot more. I am looking at a lot of my genes and am continually surprised at how accurate the information is as they relate to both physical and behavioral issues.
I think it’s great that patients are have their own theories and are looking for answers. Maybe out input will be read by the researchers and make a difference!
As several people in this blog commented on there being a link between people with ME and being highly sensitive, stressed, OCD, a tye A personality… I hurried up with writing the connection between these and ME/FM patient’s brain bathing in excitatory chemicals like glutamate.
It can be read in https://www.healthrising.org/forums/threads/potential-linking-fm-mast-cells-sleep-deprivation-food-intolerance-exercise-intolerance-and-me.6217/ in comments 10 and 11. I did my best to make it relatively easy to read and understand.
The short summary of it is:
More sensitive and “committed and caring” people are more prone to ME. But ME IMO makes people more sensitive too and increases the strength of senses. It goes both ways.
Being more sensitive at a young age may even bea hallmark of having smaller underlying health weaknesses that cause these persons to have heightened senses.
In that sense, being highly sensitive could be both a personality trait and an early indicator of energy production issues.
The comment also links these ideas to the recent over training symptoms blog, to sensory overload and to ME brains “lighting up like a Christmas tree” under scanners.
Correction, comments 11 and 12.
I’m one of those “sensitive and caring” persons and also one who needs to learn to channel a fast firing, rapid thinking brain. Trying to tame down hyper processing in the brain and lower glutamate is a challenge. But, it will help to avoid crashes. Please note the excellent information dejurgen just added to our blog/thread. It will be enlightening.
Not sure if this is relevant since I’m not clear which classes of hormones these studies are looking at. However, I recall that Jonas Bergquist did a study looking for steroid hormone dysregulation in ME (https://www.omf.ngo/2017/12/19/update-jonas-bergquist/).
From memory only one of the hormones met the 0.05 test for significant affect; however, other hormones were lower (but didn’t meet the 0.05 test).
“Dr. Bergquist screens for steroid levels and has found a decrease of pregnenolone with non-significant downregulation of most other steroids. Pregnenolone is a neurosteroid produced in the mitochondria.”
https://let-me.be/e107_files/downloads/the_me_global_chronicle_-_30_-_20181222.pdf
https://www.lifeextension.com/magazine/2007/11/report_pregnenolone/Page-01
And while glutamate is critical for normal learning, too much excitation by glutamate over time can damage neurons—in fact, overstimulation, or excitotoxicity, by glutamate is thought to be one of the underlying factors in neurodegenerative disorders such as Alzheimer’s disease.16,17 What makes pregnenolone so important in this context is that it seems to trigger the NMDA channels18,19 through a mechanism that is independent of glutamate,20 which in turn may account for the observed neuroprotective effects of pregnenolone on brain cells
You reminded me about pregnenolone moderating NMDA and increasing memory without affecting glutamate. It also is mother (or first) hormone in line of other neuro hormones and could affect all of them. Including adrenal hormones.
Thanks for the reminder!
Thank you to Francis Martin, Issie and Dejurgen for the great information you have posted since I did yesterday. I so wish we all lived close together so we could sit down and discuss these things. Seems like we are on the same track with a lot of it.
My current state of hypervigilance is not serving me well now in my older years. Back when I was young it was helpful to be a driven, Type A, but not now…it just causes insomnia and as Issie said, a fast-firing rapid thinking brain that has to be tamed. My “baseline” for sensitivity/overstimulation is so high now. I can’t have caffeine or even chocolate. I have tried decreasing glutamate containing foods, and now have included high oxalate food restrictions as well, but haven’t noticed any improvements. Will keep trying, though.
You may try addressing mast cell issues. That helps me alot.
I also found I had to eliminate grains and nightshade vegetables as they cause more pain. Limit lectin (some legumes are worse for me than others.) There also appears to be connections to salicylic acid foods.
Issie, I have been (mostly) eliminating grains and nightshade vegetables. When I stopped eating wheat/gluten I started eating more quinoa (technically a seed) and polenta for some carb’s (I don’t like animal protein all that much and feel better on a higher carb diet). Found out that the polenta (corn meal) and the quinoa are both very high in oxalates. I don’t eat tomatoes because of bladder pain, and rarely eat potatoes. I am familiar with lectin and try to avoid those foods too. So, now I’m supposed to eat gluten and dairy free, low lectin, no nightshades, low glutamates, and low oxalates. Pretty depressing when you try to plan meals for two people! What do you eat?
It’s not easy. We can’t do it perfectly, but if you do it mostly……it seems to help. I try to add things back, at times, to see if my body has recovered enough to handle certain things. With mast cell though, I could be okay one time and not okay the next. So it is always a gamble.
I had Lyme disease which my body was “handling” OK until I got bitten by another tick and got Anaplasmosis. Thanks to the VA I wasn’t treated for six months and after my 28 days of Doxy within 3 weeks I “flared” again. I did this 1 week in bed, 2 weeks recovering and 1 week feeling pretty OK for a year and then all of a sudden I was sick all of the time. I was diagnosed with ME/CFS/Fibro/POTS/PEM etc as well as Lyme by an outside Lyme Dr. I had a hysterectomy in my 30’s and was on Estrogen until mid 50’s when I tried to stop. That didn’t work. So I’ve been on tiny doses of estrogen, progesterone, (and for a while testosterone) cream, based on bi-annual bloodwork to keep in range. (I’m wondering if stopping the testosterone, because every Dr had a fit about it, precipitated the CFS?) I have tried periodically to stop but have to start again due to side effects but this year I finally succeeded in Aug. In July I was taking it every 2-3 weeks and the middle of the month I started taking 100mg (increasing to 300mg each) Vit B1 and 100mg Riboflavin. By day two I’m noticing the overwhelming fatigue is gone and I’m not in pain after doing any “work”. The next time I used the Est/Prog was 4 weeks later and I felt poorly for the next 3 days. That was the last time I used it. I also stopped the Vit B1/riboflavin for a week in Aug to see if it was really helping and I started to get more fatigued again. I also felt like I had a “bug” in mid Aug for about a week so maybe that helped kicked my body back into gear. It’s hard to say with the Lyme, EBV, etc in the background and the other supplements (including LDN) I’m taking, what exactly made the difference but when I get tired now it’s more feeling sleepy tired not the mitochondrial fatigue of the past two years. I’m slowly increasing my exercise still being aware of my pulse so as to not overdo it but I know for sure the POTS is better and I do not have the same muscle pain from working as before. I still have many Lyme symptoms and wish I could sleep and not have nerve pain and cognitive issues but it appears the ME/CFS is gone or in remission. I’m pretty sure that the hormones had a big part to play in the ME/CFS type symptoms I’ve had for 2 years. Thank you Cort for your great articles because reading them is when the light bulb came on about women vs men (ie estrogen) and seeing “Thiamine Deficiency Disease” made me try it since it’s a safe supplement. Keep up the great work!
There have been many helped with B1. If you look at what symptoms are connected to it being low…..you see a lot of POTS symptoms described.
This is a really old thread on B1 with a nice reference to an article on B1 that we had on a POTS forum.
https://www.dinet.org/forums/topic/22120-b-1-dysautonomia-autism-mito-miagraines-issues-with-wheatmilksugar-problems-with-glutamates-heavy-metals-epigenetics/comment-205673?ct=1570326006
Here is another older article on Dysautonomia and CFS with connections to low B1.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2644268/#!po=2.27273
With Lyme symptoms, I too have Post Lyme Syndrome. The antiviral herb I talked about above is helping. Along with enzymes.
Thanks for the info. I bought some lomatium as part of Buhner’s viral protocol but haven’t used it yet. I’m using Cistus Incanus tea and that has helped. I’ll switch to the lomatium now that flu season is approaching. I used the Serrapeptase for over a year when I first got sick but it didn’t seem to do anything. Interesting about the fungus from mosquitoes. Do you have that link by any chance?
Chronic Fatigue and Immune Dysfunction Syndrome (CFIDS) (The Plot Against Asthma and Allergy Patients, F. Ravikovich, 2003).
https://drive.google.com/file/d/0BxK4lGunsmzgSjFvcC00MUlGRG8/view
https://www.thehistamineconnection.com/
Bayard T. Horton, I find your links very interesting. I have said many times that until I started addressing MCAS, my POTS didn’t get better.
Does this book tell you how to address over histamine response without histamine blockers? I’m trying to not take them now and addressing with herbals. Not completely successful yet. But being off them more than on them is helping in some ways as for brain fog.
Yes, it is the leitmotiv of the book, F. Ravikovich used immunotherapy with subcutaneous histamine (histaglobulin, histamine dihydrochloride and gammaglobulin) to treat classic allergies and diseases he called functional encephalopathies such as CFS.
https://bookos-z1.org/book/5262975/5ff148
Having read this I believed it was realpy enlightening.
I aappreciate you spending soe time and energy to putt
this content together. I once agaikn find myself personally spending a significant amount of time both reading and commenting.
Butt so what, it was still worth it!
Very interesting. Can you please link the original study?