

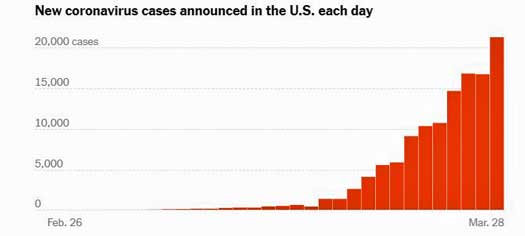
My, how things change. Less than a month ago, only 70 coronavirus cases had been reported in the country. Our president had just recently announced that the virus was under control. The sun was shining brightly.
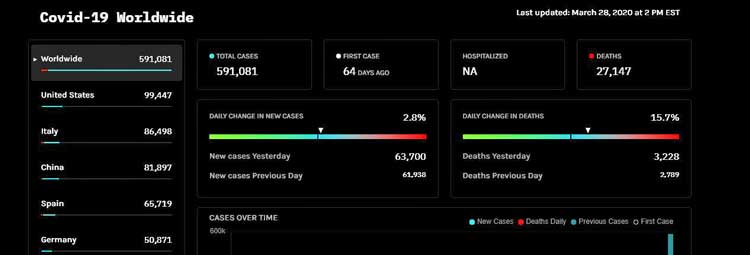
How naïve we all were. Just a month later, the U.S. leads the world in confirmed coronavirus cases (131,000) and will likely hit 200,000 cases in a couple of days. Italy has also passed China, and Spain will probably pass it tomorrow. By the end of the week, China – the birthplace of the pandemic – will likely be in fifth place.
Anthony Fauci today said the U.S. could experience between 100,000 and 200,000 deaths from COVID-19 with millions becoming infected. (Just over 2,000 people have died thus far in the U.S.). Dr. Birx , the leader of the coronavirus task force warned “No state, no metro area, will be spared”.
The U.S. has passed a bailout that makes the 2008 stimulus package look like peanuts, the stock market has fallen by 25%, and jobless claims are off the charts.
- Stimulus Checks: you’re on disability in the U.S., will you be getting a check from the U.S. government? How about if you’re simply not working? Check out the answer to that and other questions in How to Get On’s superb “The Disabled Person’s Guide to Stimulus Checks“.
Cool Tracker
Stat news has a neat tracker which shows the percentage of new cases a day. Cases in the U.S. the other day went up by 11%, and in New York City by 14.4%.
They increased in California by 34% but then dropped – get this – in Santa Clara Country by 60%! Why? Perhaps because almost two weeks ago, the Bay area was the first metropolitan area to go on a lockdown. (Again, with the availability of testing kits varying widely, it’s hard to tell what these numbers actually mean.)
Report From The Hospital
A gripping story, “I Am Hospitalized With the Coronavirus“, in the New York Times, demonstrates why we should all – healthy or sick – be ever so careful. The man, a healthy 45-year-old with no risk factors, came down with a fever (101-102 F), muscle aches and severe fatigue. A week later, after he began to violently cough and could hardly move, he went first to Urgent Care and then to the ER, passed an X-ray and blood oxygen test, and was sent home.
“I returned home in terrible shape, chest burning and wracked with chills, unable to do anything other than shudder under a blanket.”
The next day, his temperature spiked to 103.5 F and he was admitted to the hospital. He reported:
“The first night and day were a literal fever-dream of pricks, prods, scans and sweat. I floated in and out of consciousness and hallucinations as nurses drew blood from all over and gave me shots of blood thinner in my stomach, which became a daily routine.”
In two days, his chest x-ray went from mostly clear to looking like “some demented handyman had sprayed my lungs with insulation.” He has not had to go to intensive care, and on the 13th day of his COVID-19 saga, his fever finally broke. He is still in the hospital, but slowly improving.
Remarkably, nether his wife nor his daughter have gotten ill or even tested positive for the virus.
Ian Lipkin Talks
“It’s the most transmissible virus I’ve ever encountered.”
Speaking from his home, an obviously sick Ian Lipkin talked in depth with Dr. Oz about the coronavirus, how he believes he caught it, how his symptoms started (with a splitting headache), and what he believes should be done – including a coordinated nation-wide effort to stop the virus – something that will obviously only happen with leadership from the White House.
Want more Lipkin? Ian Lipkin has been through the virus wars. He’s someone you can trust. Vincent Racaniello is another trusted figure in both the ME/CFS and the viral field.
- Check out Vincent’s hour long talk with Ian Lipkin here. (Thanks Ron)
The Bateman Horne Center on the Coronavirus and ME/CFS
The nicely rounded, calm, and reassuring discussion from the BHC in their recent videocast about the coronavirus is about what you would expect. Nothing is downplayed, nothing is overplayed – you just get information can trust. Dr. Bateman, Dr. Vernon and Dr. Yellman talked about coronavirus research, staying safe and medical issues. It’s well worth watching. Just a few highlights are below.
Like Dr. Klimas (below), Dr. Bateman stated there’s no need to seek medical care or testing unless your symptoms get severe. When asked how to differentiate your symptoms from COVID-19 she said if you have the virus you will know.
As more treatments become available, testing for the virus will become more important as a positive test will open the door to them, but for right now – if you just have normal flu-like symptoms, there’s no need to get tested, and as Dr. Klimas points out (below) a good reason not to.
Dr. Bateman emphasized that drug treatments are rapidly becoming available. At least 14 possible drugs are being tested and 20 different companies are working furiously on a vaccine. The medical profession is moving faster on the coronavirus than it has ever moved before.
Are ME/CFS patients at high risk? We don’t know but it’s safe to assume yes. The immune dysregulation in ME/CFS may reduce, particularly early in an infectious process, the ability to fight off infections.
Should we be taking measures different from the general population, then? Probably not. Everyone with ME/CFS or not, though, should be taking this virus very seriously. Dr. Vernon noted that a recent study indicated that a 30% (or more) alcohol solution is enough to stop the viruses ability to replicate. (Hand sanitizers typically are 60% or more alcohol.)
- Here’s another take from Rutgers on ways to kill or disable the virus which suggests using higher amounts of alcohol and being sure to leave them on the surface for 30 seconds. Vinegar is not believed to be effective.
Dr. Bateman also talked the need for advanced care directives and end-of life-wishes, which, she noted, are important for everyone with a chronic illness.
Dr. Bateman presented a hopeful picture of researchers, drug companies, industry working together at a furious pace to understand this virus and produce treatments for them. Both Dr. Bateman and Dr. Vernon believe the virus may actually be a boon for research beneficial for ME/CFS – and produce new treatment possibilities.
The Bateman Horne Center has created a COVID-19 resource page which includes a variety of documents including one that should help doctors better manage an ME/CFS patient’s case with COVID-19 (medical considerations letter):
- Medical Considerations Letter — In the event you become acutely ill, the Medical Care Considerations Letter serves as a guiding resource for outside medical care intervention. The intent of this letter is to provide care professionals with further information about your illness of ME/CFS and/or severe FM to assist them with their medical intervention decisions.
- COVID-19 and ME/CFS/FM Frequently Asked Questions: Drs. Bateman and Yellman answer specific questions about COVID-19 as it relates to ME/CFS/FM.
-
Suzanne D. Vernon, PhD, addresses: SARS, CoV-2, and COVID-19: A scientific overview of COVID-19, infection/transmission, testing, and treatments on the horizon.
- Advance Care Planning: The Advance Care Planning document provides guidance for establishing advance care directives and POLST documents.
The BHC also provided a link to this video of how germs spread:
Dr. Klimas Talks
Dr. Klimas added some more advice to the video she produced a week or so ago.
Regarding Going to a Testing Center – “Right now there are not enough tests to test everyone, and if you are not very sick they are sending you away. If you go to a testing center, and you are sick enough that they tested (fever, cough, flu-like symptoms), then there is one chance in 4 you will be positive, but if you go to a testing center you also have 1 chance in 4 of standing next to someone who is positive and coughing virus in the vicinity.
So go get tested if you think you are too sick to stay home, that is if you are short of breath (can’t hold your breath for 10 seconds), are flu-like and have a higher than your normal temperature.
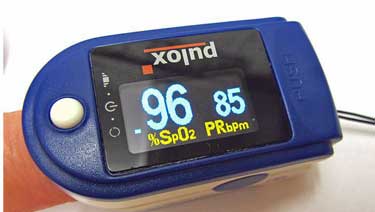
Pulse oximeters tell how much oxygen is in your blood. They’re cheap and easy to use (but don’t try right now to get one on Amazon.)
Pulse Oximeter – At our local ER, the screening test is a O2 saturation test. Normal is 96% to 100, but if it’s below 92% you will be short of breath and you will be admitted. So if you have a pulse oximeter or oxygen saturation meter (they cost about $30 at the local pharmacy or online), or you download the app on your smartphone, you might reassure yourself enough to treat this at home.
(Cort’s Note: I was unable to order a pulse oximeter from Amazon – which seems to be having considerable problems with deliveries (several Prime orders were going to take a month). I was able to order one quickly from Walmart, however.)
Prognosis – Much like the flu, you won’t be better as quickly as healthier people – plan for 4- 6 weeks but if you get sick bear in mind that the worst part is usually the first week.
Use Grocery Delivery – Take the STAY AWAY FROM PEOPLE advice seriously, using grocery delivery, washing your hands obsessively, taking care about who comes in and out of your home. This is really serious, and it’s way better to never get it than cope with what to do once you have it.”
If You Get Sick, You Probably DON’T Have COVID-19
This is still flu and cold season, after all. As both Dr. Bateman and Dr. Klimas noted, for all the worries about coronavirus, most people seeing a doctor with cold symptoms do not have the coronavirus – they apparently have a cold or the flu bug that’s still going around.
The COVID-19 Tracking project indicates that just 16% of the 735,000 people tested in the U.S. have tested positive for the virus. With an expected 10-15% false negative rate that suggests that about 75% of the people with flu-like symptoms – and probably more since it’s still not that easy to get a coronavirus test – probably just have a cold or the flu.
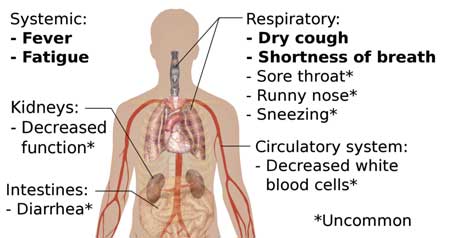
Symptom check – note that in about 10% of coronavirus cases, abdominal symptoms occur first and the virus can produce a vast number of symptoms.
The Gist
- In just a month, the U.S. has gone from about 50 confirmed cases of coronavirus to 135,000 and should hit 200,000 in a couple of days. Over 20,000 people tested positive yesterday.
- Reporting from his sickbed, Ian Lipkin calls the virus “the most transmissible” he’s ever seen, calls for more social distancing and an organized nationwide effort to stop the virus from spreading.
- A 45-year-old healthy reporter describes his hellish experience with the virus which landed him in the hospital.
- Testing levels have increased dramatically with the U.S. now testing over 100,000 people a day.
- A COVID-19 symptom tracking app is available for people in the U.S. and Canada that could help identify outbreaks and the spread of the virus.
- Comparatively low mutation rates provide more hope for an effective vaccine.
- In-home and other antibody tests are on their way. Antibody tests will give us a much better idea of how far the virus has spread and how lethal it is.
Could a Dose-Response Curve Help?
A dose-response curve would indicate if you got a small dose of coronavirus whether you would have a lesser chance of becoming ill – or vice versa. If someone with the virus sneezes all over you do, you have a better chance of getting really ill?
Nobody knows yet, but it’s possible. A 2004 SARS study found that people with low initial levels of the virus in their nose/throat area did better while those with higher levels had a high mortality rate. Joshua Schiffer, a clinical virologist at the Fred Hutchinson Center, has stated that nasal viral loads in this virus have generally tracked well with disease symptoms and progression.
One researcher noted that that “makes immunological sense, because the interaction between the virus and the immune system is a race in time. It’s a race between the virus finding enough target cells to replicate and the antiviral response aiming to eliminate the virus. If you give the virus a head start with a large dose, you get higher viremia, more dissemination, higher infection, and worse disease.”
That all suggests that good social distancing might help in reducing the loads of the virus, if we do come into contact with it, to more manageable levels.
COVID – Radar – Coronavirus Symptom Tracking App Available
Doctors and researchers at Harvard, Massachusetts General Hospital, King’s College London and Stanford University, working in partnership with ZOE Global Ltd., reported that they’ve created in four days what usually takes three months: creating a SARS-C-V-2 symptom tracking app that will help researchers and policy makers track COVID-19 outbreaks more quickly, determine who is at most risk, and understand how fast the virus is spreading in any area.
All they need are participants – lots of them. Two million people in the U.S. and the U.K. (it’s limited to those countries, but they hope to release it to other countries soon) have already signed up. It takes about a minute to do the questionnaire.
Check out the U.S. site here for links to download it on Google Play and the Apple App Store.
Testing, Testing…
Testing levels are reaching new heights with with over 100,000 people being tested each of the past two days, and a grand total of 735,000 tests done.
Abbott Labs announced it will be sending out 50,000 test kits a day starting April 1st. They will operate on Abbott’s toaster-sized ID NOWTM platform which can be situated just about anywhere.
Lipkin Team Develops Tests to Identify “Coronavirus Carries”
Research suggests a lot of Typhoid Marys; e.g. Coronavirus Carries” are out there unknowingly spreading the virus. In fact, Jeffrey Shaman at the Columbia University Mailman School, found that people with mild, limited, or no symptoms were the driving force behind two-thirds of all documented infections. Finding them and getting them off the streets is crucial.
Enter Ian Lipkin and his team at the Columbia Center for Infection and Immunity who have, not surprisingly, developed an “ultra-sensitive, highly accurate, and quickly scalable” SARS-CoV-2 test that can do what other tests cannot: pick up infections in people with very low viral loads; i.e. people who are asymptomatic. They report this test, called the C-3 Test, is “already proven to be effective and accurate” and can reduce both false positives and false negatives.
The crisis has caused the Mailman School to get creative and begin raising money through an unusual online fundraising effort.
“This independent fundraising effort which, while unorthodox, fills an immediate need even while the lab continues to pursue grants and other more traditional funding sources.”
The group expects FDA approval soon but the test kit will not be available commercially initially or outside the New York City area. The upside is that Lipkin and his team have been using the test to evaluate treatments for COVID-19, the disease the virus causes.
Antibody Testing – A Missing Piece Emerging
“If we can get this antibody test mass-produced — and I know they’re working on it right now — and put it into commercialization really quickly, this could be a game-changer for the whole pandemic.” Laurie Walsh Pulitzer prize-winning author of The Coming Plague.
“Of all the data out there, if there was a good serological assay that was very specific about individuating recent cases, that would be the best data we could have.” Alex Perkins, epidemiologist at the University of Notre Dame.
Antibody tests are a crucial missing piece in our battle against the virus. In contrast to the current PCR tests which determine if the virus is present, antibody tests tell us whether someone has developed an immune reaction to the virus; i.e. if they’ve been infected with it at some point in the past. Only with antibody tests will we understand just how far the virus has spread. Only then will we know how lethal it is, how worried we should be, etc. Only then will we be able to develop truly accurate models.
In an interview with New York’s James Walsh, Laurie Garrett, the author of “The Coming Plague”, explained how antibody tests can help.
“One of the things we would love to know right now is how many people who have had pneumonia since January were actually COVID cases? … If we were suddenly seeing a surge in hidden pneumonia cases since mid-February, that would tell us we’re in deep, deep doo-doo; that this thing is like Italy; that we’re going to suddenly skyrocket and our hospitals are going to be overwhelmed.
But if, by contrast, the same number of cases are found in the historic samples going back to the first of January, that would tell us, ‘Okay, it’s gradually unfolding, we don’t have to go down to lock down every single person in New York, we may be able to flatten the curve.’ And that makes a big difference in terms of how drastic our policies need to be.”
Antibody tests do have downsides, though. Because someone has to have developed antibodies – which can take some time – they can’t be used to determine who is infected or not. On the other hand, they are cheap to produce, are easily scalable, and can provide ultra-fast results – often in just 15 minutes. Doctors are well versed in using them as well.
The FDA has yet to approve an antibody test, but they are coming…
The Make-Your-Own Antibody Test Idea
A new recipe could offer labs an alternative to waiting for or buying commercial tests. Virologists at the Icahn School of Medicine at Mount Sinai recently posted posted a preprint (non-reviewed journal article) describing a SARS-CoV-2 antibody test they’ve developed, and a detailed protocol for replicating it.
This is not for home use. The researchers hope that other labs will use the protocol and get Icahn the data they need to quickly assess how accurate and specific the test is.
Identifying people who have recovered from the virus, and are effectively walking around with their own anti-SARS-CoV-2 drug in their blood in the form of effective antibodies, could provide a boon for seriously ill patients. Donating their SARS-CoV-2 antibody-rich serum could provide a lifeline for critically ill patients.
Commercial Antibody Tests Coming…
- Henry Schein, a Melville, N.Y., company, said its Standard Q Covid-19 IgM/IgG Rapid Antibody Test, takes a pinprick of blood and can provide results within 15 minutes. The company said it expected to have at least several hundred thousand tests available by March 30 and “significantly” increased availability beginning in April.
- Mologic, a British company that’s produced a cheap ($1) 10-minute antibody test, began sending it out to laboratories for validation. If all goes well, it expects to have the test validated by June.
- A mail-in antibody test that works like a pregnancy test could also be in the U.S. in June if the FDA approves a test used in China.
Many other manufacturers are creating antibody tests.
Treatment
Slow Mutation Rates Ease Concerns
One of the reasons we rarely have a spot-on flu vaccine is that flu viruses are mutation machines. They change so rapidly that it’s difficult to get a fix on them. The H1N1 virus that caused the deadly 1918 flu pandemic morphed from a pretty innocuous flu virus early in the year to a deadly flu virus in the summer. That’s probably not going to happen with this coronavirus (which is not a flu virus.)
Genetic mapping indicates the virus is not mutating rapidly – which is very good news for vaccine makers.
Unlike flu viruses, coronaviruses have a “proofreading mechanism” which reduces their rate of mutations. Studies thus far indicate that SARS-CoV-2, the actual name of the virus, is mutating at about 1/3rd to 1/2 the rate of a typical flu virus. The mutation rates, thus far, don’t appear to have affected the virus’s transmissibility – which it already gets an “A” in anyway – or its lethality. As one expert put it – when something’s working, why change? The virus is spreading like mad. It’s doing very well – there’s no pressure on it yet that might make it change.
The fact that it’s a comparatively large, complex virus probably gives it an extra leg up in dealing with our immune systems, but the fact that it’s not changing very quickly suggests that: a) a vaccine developed for it will probably work quite well; and b) if you do get sick and recover, your immune system will probably quickly wipe out the bug the next time you’re exposed.
Check out GISAID – an open source database – for all the virus sequences that have been charted.
Treatment Takeaways
- Susan Vernon, PhD, reports that a 30% or more alcohol solution kills the virus.
- Dr. Bateman and Dr. Klimas note that most people who are sick now do not have the coronavirus; they mostly likely have a cold or the flu that’s going around.
- Neither recommend getting tested unless your symptoms are severe (trouble breathing, high fever, etc.). If you do have severe symptoms, don’t try to manage them on your own. Call your doctor.
- Dr. Klimas noted that a cheap pulse oximeter can give you an idea of how your lungs are doing. (92% or below is a cause for worry – and a call to your doctor).
- Dr. Bateman reported that treatments are coming, warns patients to be careful but remain calm.
- The Bateman Horne Center provides a variety of handouts for people with ME/CFS/FM (see blog).
- Remdesivir results should start coming in during April. Several other drug results are expected by June.
- Antibody-rich plasma has been approved for use in seriously ill patients.
- Hydroxychloroquine (Plaquenil) – the NIH has turned its back on this generic drug and is not funding any trials. The few Plaquenil drug trials under way are being funded by New York State and philanthropists.
- Other drug trials involving monoclonal antibodies and arthritis drugs that are moving forward quickly are being funded by pharmaceutical companies.
Drugs
Many drug trials are underway. Most appear to be finishing up around June or later, but some, including the possible star of the bunch, Remdesivir, will be finishing up in April.
Remdesivir
The great drug hope – Remdesivir – is a drug which targets RNA polymerase – the enzyme RNA viruses like the SARS-CoV-2 use to replicate. STAT News reports that the first studies on effectiveness of Remdesivir will finish up not in May or June, but in April.
Its maker, Gilead, has been working with researchers and governments around the world to get clinical trials up and running. Six large studies are in progress, with the first, in severely ill patients in China, due to finish as early as April 3, according to a government website. A study in patients with milder disease will also finish in April, with two more due in May.
Hydroxychloroquine (Plaquenil)
Doing in 3 or 4 days what usually takes 6-9 months, New York announced that it’s testing three medications — hydroxychloroquine and chloroquine in combination with the antibiotic azithromycin. In addition to mortality and overall recovery, the study will measure patients’ overall viral load, duration on a ventilator, and number of days in the hospital.
The National Institute of Allergy and Infectious Diseases (NIAID) does not appear to be particularly interested in Donald Trump’s favorite drug. Fauci, the head of NIAID, reported that trials on “remdesivir, other drugs, immune sera, convalescent serum, monoclonal antibodies” were all in the pipeline and should soon go into clinical trials.
The failure of NIAID to support hydroxychloroquine trials appears to highlight a failing in our drug approval system with which people with ME/CFS can probably relate – the importance of the profit motive. Large drug companies appear to be expending lots of money to get their proprietary drugs into trials while hydroxychloroquine,an inexpensive, generic drug that no company stands to make much money off on, sits by the wayside. Besides the New York trial, one hydroxychloroquine trial is partly funded, another is unfunded, and the Gates Foundation is expected to fund another.
It should be noted that hydroxychloroquinine has never shown any antiviral effects in the lab. A a small French study, though, seemed to indicate that combining hydroxychloroquine with azithromycin decreased coronavirus levels. STAT points out, though, that the French study had such significant methodological issues (not randomized, inappropriate control group) that a London statistician asserted that “gave very little useful information about whether hydroxychloroquine might help”.
STAT News reported that a Chinese study was better but the data was still “compatible with a wide range of possible effects,”; i.e. “Nobody knows whether the drug helps or not.”
The ME/CFS and FM communities have been here before: hoping that preliminary study results are more than that; i.e. more than preliminary.
With runs on Plaquenil limiting access to the drug for people with lupus and other conditions, Andrew Cuomo in New York and Nevada are limiting new prescriptions of the anti-malarial drugs to patients with previously approved FDA conditions and to coronavirus patients participating in state-sponsored experiments.
Lopinavir-Ritonavir Trial bombs
The first failure. A study published last week in China indicated this hoped-for combo provided no benefit compared to placebo.
Monoclonal Antibody Drugs
At least three companies – Vir Biotechnology, Regeneron, and Eli Lily – are working on drugs that produce antibodies to the virus, thereby preventing it from entering new cells and replicating.
Monoclonal antibody drugs could be particularly effective for people who are really sick and having trouble fighting off the virus. They could even prevent infections from taking hold. Vir Biotechnology says it’s tweaking its antibodies to trigger the long-term production of white blood cells designed to fight off the virus. If they can do that, their antibody drug could work like a vaccine – rendering a person immune for many years.
Both Vir and Eli Lilly expect to begin testing their drugs in 3-5 months.
Antibody-rich Plasma
One recent study from China reported that of 10 patients given convalescent plasma, seven saw their viral loads become undetectable; it also noted other improvements in their condition. Ian Lipkin noted that a colleague in China wanted to get him shipped plasma in a diplomatic pouch. Providing antibody-rich plasma from those who have recovered has been used successfully in other epidemics.
On Tuesday, the Food and Drug Administration approved the use of plasma from recovered patients to treat some severe cases and New York will begin testing serum from people who have recovered from the virus to treat those who are seriously ill.
Arthritis Drugs
Drugs against autoimmune diseases like rheumatoid arthritis work by tamping down the immune system. This could, ironically, be useful against COVID-19, because the SARS-CoV-2 virus can make the body overreact, causing what’s called a “cytokine storm,” damaging the body. Cytokine storms are more likely with viruses like SARS-CoV-2, which can cause lower as well as upper respiratory infections. Uncontrolled cytokine storms are believed to be responsible for some of the most serious illnesses seen with the virus.
Stat News reports that a small Chinese study found that Actemra reduced fevers and the need for oxygen has prodded Roche to begin a study in the U.S. Trial results from a similar drug called Kevzara could be ready as early as April. Both drugs target the IL-6 cytokine.
Check out two excellent, but probably underutilized, coronavirus news sources:
The Coronavirus Series From Health Rising
- Coronavirus #I: Dark Sun: Reflections on the Coronavirus as it Heads For Town
- Coronavirus #2: Scary Models, 8 Reasons People with ME/CFS and Fibromyalgia Should Be Careful, How to Stop an Epidemic, Why You Should Trust No One and More
- Coronavirus #3: Is the U.S. Becoming Italy?, A Singapore Success Story, More Scary Models, Remdesivir to the Rescue?
- Coronavirus #4: Lipkin Gets Hit, Testing Woes, Could the Models Be Wrong, Ikea Ventilators?, and What’s Next (???)
- Coronavirus #5: Lipkin, Bateman and Klimas Talk Plus Treatment Updates
- Coronavirus #6: Will COVID-19 Leave An Explosion of ME/CFS Cases in its Wake?
- Coronavirus #7: Records Broken, An ICU Doctor Talks, The Peak is Coming, Hot Spots, Is it in the Air? Dr. Hyams on COVID-19
- Coronavirus #8: The Grand Experiment, Starting Up? Social Distancing – For 2 Years? WHO Did It?
Now May Not Be the Time for a Big Donation
But Guess What?
Those Small Donations…
They Add Up!
Please Support Health Rising in a Manner That Works for You






TWiV Special: Conversation with a COVID-19 patient, Ian Lipkin
http://www.microbe.tv/twiv/twiv-special-lipkin/
Vincent Racaniello – yes! Thanks.
Cort,
Thank for your stellar coverage of the pandemic!
Since Covid 19 can cascade into sepsis, I thought readers may be interested in a sepsis protocol that has been shown to really lower mortality and organ failure.
“…giving patients 200 mg of thiamine every 12 hours, 1,500 mg of ascorbic acid every six hours, and 50 mg of hydrocortisone every six hours for two days reduced mortality from 40% to 8.5%…” Mercola.com
“…Our results suggest that the early use of intravenous vitamin C together with corticosteroids and thiamine is effective in preventing progressive organ dysfunction, as well as in reducing the mortality of patients with severe sepsis and septic shock,” says Dr. Marik. …”
https://www.physiciansweekly.com/iv-vitamin-c-hydrocortisone-thiamine-for-sepsis/
I’m so glad you mentioned Dr. Marik and his protocol. So many people have poo-pooed his three-pronged drug combination yet he’s saving lives! An ME researcher said a couple years back that ME looks similar to Sepsis. Why doesn’t even one ME doc try this combo on patients? What if it works?
How did Fauci get to be Mr NIH? What happened to his boss NIH Director Collins? Is Collins too busy handing out guitar picks? Both of them should’ve been put out to pasture years ago.
Fauci wants to dismiss chloroquine. Well then, fund a study and show us the data that it doesn’t work. But what if he funds a study that shows success instead? Oh how embarrassing! That might throw a wrench in the mad drive to find an expensive and profitable drug.
It sure would be handy to have a journalist to pick apart NIH and CDC managers’ connections to drug companies, patent royalties, “public-private partnerships”, etc. There is plenty of muck to rake, if anyone will bother.
Since Fauci botched the NIH response to the AIDS epidemic decades ago (the documentary “How to Survive a Plague” shows him being burned in effigy), CDC and NIH have worked overtime to improve their public image. CDC and NIH together spend about $45 Billion per year, but they can’t even plan for an epidemic. It’s time to strip away the shiny covers and examine the rot underneath.
Too busy handing out guitar picks! 🙂 The story I learned about the NIH’s unwillingness thus far to fund hydroxychloroquinine trials cited someone stating something to the effect that the profit motive is a big factor. Somehow and I don’t know how the NIH bends towards therapies which big pharma supports.
I don’t know why that’s necessary – the NIH certainly has the capability and certainly does fund many drug trials – but I thought Jarred Younger’s inability, after trying several times (and we know he can get grants) to get the NIH to fund LDN and dextro-naltrexone trials spoke volumes.
If Younger can’t get the NIH to fund trials into something like that – a type drug – for a disease with no FDA approved treatments – I just don’t know what to say. They’re the ONLY ones with the money and one would think the interest – isn’t this about the public good – that could fund trials like that. Who else is going to fund a trial like that? No one!
It’s remarkable how utterly broken the NIH – which does wonderful things in so many areas – is in some respects.
I am seeing it widely reported that hydroxychloroquinine is being fast tracked into clinical trials and is being used on critically ill patients now even if they are not in a trial.
Screw profit. If a drug works, the governments of the world should fund its mass production and delivery.
I’m an ME/CFS pt who’s been using home Ozone therapy for 7 years and haven’t had a cold OR the flu during this entire time. And how many medical professionals on TV have mentioned this treatment even once? That’s right – ZERO. For the sickest people who are dying from this, it’s absolutely unconscionable. Do a 10 second google search on COVID-19 and ozone and you’ll see how this is being talked about. Also in Asian countries like Thailand.
Mark, that’s great that you haven’t had a cold or flu in so long!
I have a home ozone machine. (Small one.) And can do rectal, vagina, or ear insufflations vs. IV. I wonder if those methods would help.
“if you get sick bear in mind that the worst part is usually the first week.”
I’m not sure where you got this information from but it is not the experience that I or my girlfriend have had (both moderate ME/CFS patients, not home-bound but not able to work or exercise much). I do not think we know much about how the course of the illness goes in different patients given the lack of testing and the attention of the medical profession focused on only the cases with the most extreme symptoms.
I did not have a fever or much of a cough but the difficulty breathing, weakness, and chills were at least moderate and at times bordered on severe. And back to the original quote, I had days the first week I felt great and thought “this isn’t too bad” only to be floored the second week with much worse symptoms. Days 10-14 are when you have to be very careful, and are when most people end up the hospital if there symptoms become severe.
My doctor has confirmed that her patients have experienced this same roller coaster ride of much better or much worse over the course of the illness, with things changing quickly. I found overexertion of the smallest amount could bring on a huge exacerbation of symptoms. So my advice it to take this very seriously and be careful even if your symptoms seem mild (no fever, no cough – perhaps just a tickle in your chest – that’s how mine started). I also do not agree with saying “if you are sick you probably don’t have covid-19”. If you know how your body normally reacts to various viruses you will know if you have covid-19. It has been unlike anything I’ve had before and I’ve had a lot of illnesses over my life. It targets the lungs in a way I have never experienced, and hope never to experience again.
The quote came from Dr. Klimas. Notice the “usually” in there. Thanks for providing your experience.
Dr. Bateman said that if you have COVID-19 it will be different from your typical symptoms. Then again, so, come to think of it, that would probably be true for the flu.
Statistically speaking, if you have flu-like symptoms the data based on hundreds of thousands of tests (a nice data set!)- suggests that you probably have a cold or the flu.
If you have symptoms that are different from typical flu-like symptoms – I assume difficulty breathing is one – then that is, of course, a different story.
Everyone is agreed that this should taken very seriously.
Sounds like a bioweapon to me.
Hey Cort,
Thanks for your ongoing reporting! I want to reach out and offer my story. I’m dx-ed with ME/CFS, Fibromylgia, asthma, AERD/ASA, presumptive POTs, etc. I’m Day 32 since symptom onset (presumptive positive, still waiting for test results 11 days on!). I say say 32 because symptoms persist. I had been in recovery from ME, I was even working full time, but now I’m left wondering if these lingering symptoms are lingering COVID or if it triggered a flare. I don’t have the energy to write up my story but open to being interviewed if that would be helpful (good news is it feels much more manageable than I feared, I’ve been able to avoid hospital).
Dmorgan505 @ icloud.com if you or researchers want to contact me.
Thank you for your excellent work covering this. I’m too ME/CFS – sick after 3 weeks with no caregiver 5 out of 7 days to check out the excellent resources listed, but so many WILL be able to and that’s just fabulous!
I kept waking up panicked, gasping for air last night, but that’s typical of my response to over-exertion with ME/CFS. I can’t breathe. Out of breath lying down, but unable to stay awake.
And being totally alone as my entire family has scattered to protect me from exposure just in case as I live in Washington and people are still packing the stores and the streets from where I live in Southwest Washington.
I expect half of this County to come down Sick in the next 2 or 3 days. People think they’re Invincible in this County but we’re not that far from Thurston County and we’re on the I-5 Corridor so with that attitude will probably have a major Breakout down here too if the president does not do something.
For the most part people have a very laissez-faire attitude still. I look at all them like a bunch of typhoid Mary’s down there on the sidewalk when I look out my balcony and during bathroom visit to try to keep my body from freezing up from fibromyalgia.
Being seriously ill with this disease is not a good position to be in when everybody needs to stay away. I am definitely not a person that should be home alone for all of these days and I wonder if I’ll come back from what’s happening with my ME/CFS, even if I manage to avoid covid-19.
Just be safe everybody and look at that picture of Ian Lipkin. that is how serious covid 19 is.
Thanks again!
Thanks for relating your experience from southwest Washington. We, as human beings, are apparently just not very good at reacting pro-actively to threats that are looming but are not visible.
We’re also not very good in the U.S. – the most technologically advanced country in the world – at trusting experts. Somehow we think we know better.
“We’re also not very good in the U.S. … at trusting experts.”
Might have something to do with the fact that over and over the “experts” turn out to be incompetent frauds whose claims are more “eminence-based” than “evidence-based”.
The “experts” abandoned us decades ago. If “experts” like Fauci tell me the sun rises in the east, I would check my compass before accepting the claim.
Hi Cort
In Australia we are told that 70% or more, alcohol strength is needed to destroy the virus. Thanks for all the info and best wishes to all.
Got it! I guess it’s best to err on the stronger side if possible. Good luck down there.
Cort, Re: the Santa Clara County numbers. I am an elected City Council member at a city in Santa Clara County, and so get to participate in special conference calls for elected officials that are held by County Health Officer Dr. Sara Cody (an amazing person!). Two factors affect the numbers you saw: 1. Until last Tuesday, March 24, negative tests were NOT REQUIRED to be reported to County Health. That has now changed by County Health Order. So, we will get better testing data going forward. 2. Nearly all testing through March 27 (Friday) has been AT hospital admissions, so the percentage of positives compared to known test results has been very, very high. As testing becomes more widespread, the data and ratios will change.The only really reliable indicator so far is number of deaths. Feel free to contact me for any info about the Silicon Valley hotspot.
In 30 days one person with this virus will cause infections in an average of 406 people as each person they infect goes on to infect others 75% social distancing will reduce that to an average of just 2.5.
An asymptomatic person has no idea they are doing this – but if everyone practices physical distancing (a better name than social distancing) you avoid the asymptomatic.
The last thing you want to do now is go near medical care. Your doctor could be in the early stage of an infection and asymptomatic.
Of the first patients in the UK to be ventilated 50% died. https://www.theguardian.com/society/2020/mar/28/coronavirus-intensive-care-uk-patients-50-per-cent-survival-rate
I suspect 70% alcohol kills the virus faster but 30% left in contact longer works – need to see the full report.
https://twitter.com/i/status/1244372773423558656
A potential treatment being tried in Australia…a century old vaccine that is an immune booster.
Could it help ME/CFS as well?
https://www.bloomberg.com/news/articles/2020-03-30/century-old-vaccine-investigated-as-a-weapon-against-coronavirus
Handwashing
I heard that handwashing, with soap, was supposed to be more effective than handsanitizer and was a bit surprised. However I came across a very interesting article, with great diagrams, called Why Soap Works from nytimes.com.
So they say ‘when you wash your hands with soap and water, you surround any microorganisms on your skin with soap molecules.’
‘Soap molecules have a hybrid structure, with a head that bonds to water and a tail that avoids it.’
‘Soap destroys the virus when the water-shunning tails of the soap molecules wedge themselves into the lipid membrane and pry it apart.’
‘Soap traps dirt and fragments of the destroyed virus in tiny bubbles called micelles, which wash away in water.’
‘On the whole, hand sanitizer’s are not as reliable as soap. Sanitizer’s with at least 60 percent ethanol act similarly, defeating bacteria and viruses by destabilising their lipid membranes. But they cannot easily remove microorganisms from the skin.’
I didn’t realise soap had ‘active’ ingredients and thought this was fascinating given the current need to stem the spread of the virus.
Whoa! Thanks so much Tracey Anne. I had no idea. Great to know that simple soap is so effective (particularly since I don’t have hand sanitizers)
Seriously, I had no idea either!
Cort
I am a one woman campaign in Lawrence, Ks to educate medical professionals on ME/CFS here. I need a “quick start” guide for doctors on diagnosis and treatment which is succinct, specific and comprehensive like Dr. Klimas videos. Do you know if anyone has something like this?
Have you checked out Dr. Bateman’s short video’s? You can find them in A Basic Guide to ME/CFS on Health Rising – https://www.healthrising.org/blog/2018/12/10/the-basics-a-guide-to-learning-sharing-and-making-a-difference-in-chronic-fatigue-syndrome-me-cfs/
Good luck Janet!
That’s a great description, Tracey Anne! I was wondering the exact “mechanism of action” of soap.
A kindergarten teacher in Florida has this wonderful demonstration video of soap in action against the “virus” (with pepper in water as a virus stand-in) 🙂
https://mobile.twitter.com/reuters/status/1239287755101921280
Well I can’t take credit for it, Ferris Jabr wrote it ?
He also wrote:
‘The hydrophobic tails of the free-floating soap molecules attempt to evade water; in the process, they wedge themselves into the lipid envelopes of certain microbes and viruses, prying them apart.’
“They act like crowbars and destabilize the whole system”, said Prof. Pall Thordarson, acting head of chemistry of the University of New South Wales.
‘Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses useless’.
Fantastic video from the kindergarten teacher – I could keep watching that over and over…
Thank you Cort. This is much more informative and helpful than those waste of time daily press conferences from the coronavirus task force… Unless it’s the two doctors speaking. ?
Stay well my friend!
Anyone else noticed that the death rate from the cruise ship Diamond Princess is now 11 with 15 still critical out of 712 cases? It’s probably a mainly elderly population but is a good guide to a likely death rate before medical services become overwhelmed.
13 year old boy, believed to be otherwise healthy, died in the uk.
Take this very seriously Eat as healthily as you can, wash not just hands but face and clean your filthy mobile. Get fresh air when you can, even if it’s from an open window. And listen to doctors not your president.
My cheap oximeter is useless.
The virus is transmitted by people rarely by surfaces. So keep away from people as much as possible
REMINDER, or warning to those who didn’t already know.
Pulse oximeter gives false-positive readings IF the person has methemoglobin or carboxyhemoglobin.
Because either (or both !) of those can be caused by internal, biochemistry problems — or can be from traffic pollution or household heaters, etc. etc — best to be skeptical of oximeter readings. At home and ALSO in hospital.
Have not noticed that many doctors pay attention this fact. Do they even know ? Do nurses even know ?
There is one brand of oximeter that takes account of this. If I had a lot of money, and wanted good information, I would buy one of those.