


Prusty’s novel work on HHV-6 has been supported by the Solve ME/CFS Initiative and the HHV-6 Foundation.
Unexpected synchronies are always a good sign. Many, of course, are familiar with Bob Naviaux, MD, PhD from the University of California, San Diego (UCSD). Naviaux’s metabolomic work and his Cell Danger Response (CDR) hypothesis have opened up new possible ways of understanding ME/CFS, autism and other diseases.
Naviaux is best known for his metabolomic work, but what most people don’t know is that Naviaux is also a Salk-trained virologist, who invented some early retroviral gene transfer vectors, and was trained, to boot, in natural killer cell biology – a key topic in ME/CFS.
Bhupesh Prusty, PhD of the University of Würzburg in Germany, is newer on the scene but has been raising eyebrows with his proposal that herpesviruses like HHV-6 (and other viruses as well) may be knocking the mitochondria in ME/CFS patients for a loop.
The two authors – Prusty and Naviaux were the co-senior authors who conceived the project – teamed together to attempt to answer a question that’s been plaguing patients, doctors and researchers for years: how to tie together the energy problems in ME/CFS with the infectious onset that so many patients experience. Coming from two separate fields, Bhupesh Prusty and Bob Naviaux may have come up with a way.
They chose, what else, herpesviruses (HHV-6, HHV-7) to test their hypothesis.
Herpes viruses form a large and diverse group. Epstein-Barr virus (EBV), cytomegalovirus (CMV), and Herpes simplex viruses (HSV-1 and 2) have the ability to remain latent in the body and then explode into activity during times of stress or immunodeficiency. That has always made them a clear target in a disease largely defined by symptoms associated with infections.
The Human herpes viruses (HHV-6 and HHV-7) are a little different. Over 90% of people are infected by 3 years of age, usually through their mother’s saliva. The virus then leaves a copy of its DNA in a chromosome of a few cells, then becomes dormant. For most people, we never know if HHV-6 is reactivated or not.
Prusty and Naviaux believe this is because when HHV-6 is reactivated, it triggers cells to produce a protective factor that helps prevent other cells from getting infected (superinfected) with other viruses. This protective mechanism comes at a cost, though: mitochondrial fragmentation and a decrease in cellular energy production.
In people who don’t have ME/CFS, this phenomenon is normal and only lasts a few days at the beginning of a new infection or after exposure to certain environmental chemicals, or after physical injury. However, in ME/CFS, they believe HHV-6 infected cells continue to secrete a substance which inhibits cellular energy production, leading to fatigue, and all the other symptoms of the disease.
The very low viral loads of HHV-6 found in past ME/CFS studies have suggested that active reinfection with the virus is not an issue. A 2019 HHV-6 antibody study that turned up mostly subtle issues didn’t inspire further interest, either. (That study, it should be noted, focused mostly on late antibodies which would miss the smoldering infection that some believe may be happening.) Plus all HHV-6 serological studies to date suffer from the inability to differentiate between the more difficult to assess, and possibly more dangerous, HHV-6A and HHV-6B.
In 2018, though, Prusty, produced a controversial paper that roiled the HHV-6 research world. His cell line study suggested he’d identified very small non-coding RNA’s (sncRNA) produced by the virus in the earliest stages of reactivation, but before any virus replication occurred. The production of this sncRNA produced a signal which altered mitochondrial activity in the infected cells and caused the mitochondria to fragment. The study suggested that HHV-6 might be powering down the energy motors of the cells even as it was sitting mostly quietly in the cell. It was as if the virus was putting the cells in stasis.
If Prusty was right, you could throw the viral load data in ME/CFS right out the window: HHV-6 didn’t need to be replicating to cause something like ME/CFS – it simply needed to be a little active.
Nobody in the HHV-6 field had come up with that idea before, but Bob Naviaux in San Diego had developed a similar paradigm which proposed that the cells of ME/CFS patients had responded to infections and other stressors by getting stuck in a hypometabolic state (aka a state of hibernation or dauer, the German word for persistence).
Naviaux proposed that the stricken cells used what he called a “cell danger response” to power down their motors and redirect all energy toward cellular defense and survival, at the cost, though, of not having enough energy left over for normal cellular activity and function.
The metabolic system, in particular the mitochondria, he believed, were working hand in hand to repel invaders. In fact, in Naviaux’s paradigm, it was the metabolic or energy producing system that alerted the immune system to trouble, not the other way around.
A 2015 Nature article, which has been cited over 500 times, agreed. The study found that it only took moderate mitochondrial stress to send the cell’s antiviral defenses skyrocketing. It suggested the first goal of a pathogen was to damage, knock off, or disrupt the mitochondria of the cell it infected. Once the signs of mitochondrial damage presented themselves, however, the cell – now knowing that a pathogen was present – turned on its antiviral batteries.
Just last year, a French team showed that bacteria attempt to quickly knock out the engines powering immune cells as well. The cellular immune defense, it seems, starts with the mitochondria.
The Study
Human Herpesvirus-6 Reactivation, Mitochondrial Fragmentation, and the Coordination of Antiviral and Metabolic Phenotypes in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Immunohorizons. 2020 Apr 23;4(4):201-215. doi: 10.4049/immunohorizons.2000006.
In order to ensure that replication was not a factor, the authors chose a cell-line (U2-OS) that has the CD46 receptor that enables HHV-6 to enter the cell and integrate its DNA, but which does not allow the virus to carry out its life-cycle. This enabled the researchers to focus on the very early stages of reactivation.
These cells with a latent chromosomally integrated copy of HHV-6 DNA are called ciHHV-6 U2-OS cells. Earlier evidence suggested that in an early process called transactivation (in contrast to replication), HHV-6 began to prepare the ground for its subsequent attack by releasing small RNAs that were intent on disrupting the cells’ mitochondria.
Prusty and Naviaux first treated the cells containing a latent copy of HHV-6 with a chemical called TSA that causes cellular stress and then examined changes in the cells’ mitochondria and their protein production. They found that as the cells with HHV-6 began to produce the small RNAs, the mitochondria in the cells started to fragment. Altered levels of several key mitochondrial proteins involved in cellular metabolism (glycolysis, folic acid metabolism, fatty acid oxidation, etc.) suggested the RNAs were impacting the metabolism not just of the mitochondria but of the cells as well.
The Gist
- When the low viral loads of HHV-6 have suggested it may not be involved in ME/CFS, some researchers have suggested that a smoldering infection may be present.
- Bhupesh Prusty has provided evidence that a low, smoldering infection may indeed be present in a subset of ME/CFS patients.
- Prusty believes that very early in HHV-6’s reactivation phase, HHV-6 attempts to cripple a cell’s mitochondrial output by producing small non-coding RNAs that cause mitochondrial fragmentation and metabolic decline.
- In response to the mitochondrial damage the infected cell senses, it amps up its antiviral defenses, putting it, Bob Naviaux believes, into a hypometabolic state – in which most of the energy of the cell goes to antiviral defense – leaving little energy left over for anything else.
- The infected cells also appear to secrete something which puts other cells around them in a similar “cell danger response”. This is an important step as few cells appear to be directly infected.
- The kicker came when Prusty and Naviaux showed that same process occurred when serum from ME/CFS patients’ cells was added to healthy, uninfected cells; mitochondria began to fragment and the formerly healthy cells developed a strong antiviral response.
- Prusty’s and Naviaux’s study could help explain 2016 findings suggesting that putting ME/CFS patients’ cells together with healthy cells causes the energy production or other factors in the healthy cells to drop.
- They also believe the cell danger stance ME/CFS patients cells are in could explain why some ME/CFS patients rarely get colds.
- Attempts to determine the nature of the mysterious substance that may be putting other cells on high alert and sapping their energy production at the same time are ongoing.
- Naviaux and Prusty are also examining ways to block the signal that they believe sends uninfected cells into “cell danger mode”.
- Given the novelty of their research, it was no surprise to see that it was supported by private foundations including the Solve ME/CFS Initiative and the HHV-6 Foundation. Private foundations play a pivotal role in supporting researchers with creative approaches to ME/CFS.
The downregulation of a protein involved called pyruvate dehydrogenase – a core enzyme in regulating glycolysis – was of particular importance, as infected immune cells need to get their energy from glycolysis (ATP production that does not use oxygen), instead of mitochondrial oxidative phosphorylation (ATP production that uses oxygen). (Several studies suggest pyruvate dehydrogenase problems could be at the core of the energy dysfunction problems in ME/CFS). The downregulation of an antioxidant – superoxide dismutase – similarly checked the box of increased oxidative stress in ME/CFS – something we also know is occurring.
(In a 2012 paper, “Oxidative Shielding or Oxidative Stress?“, Bob Naviaux turned the oxidative biology research world on its head when he asserted that high levels of oxidative stress were not the result of a breakdown in the antioxidant system, but were an intentional and protective response that was evolutionarily conserved in all plants and animals. In 2016, Naviaux proposed that cells under threat produce “danger signals” such as ATP and ADP, Krebs cycle intermediates, oxygen and reactive oxygen species (ROS) that alert other cells to danger. )
Noting that mitochondrial fragmentation, not surprisingly, lowers the ATP production of a cell, they next measured the ATP production and mitochondrial fragmentation of cells with and without HHV-6 infections.
Finding reduced levels of ATP and increased levels of mitochondrial fragmentation in the HHV-6 reactivated cells, they concluded that HHV-6 does not need to be replicating to inhibit a cell’s energy production.
Then it got REALLY interesting. Next they transferred the culture media from cells with early HHV-6 reactivation to separate cultures of naive cells to see if something secreted by the infected cells could ramp up the immune defenses in uninfected or naive cells.
The naive cells were then tested for their ability to ward off an infection with RNA and DNA viruses like Influenza A virus, and Herpes Simplex Virus 1 (HSV1). The researchers found that once the naive cells had been treated with the culture media from the infected cells, they too were able to fight off the infections thrown at them. Something interesting had clearly been dumped into the blood.
The ME/CFS Connection
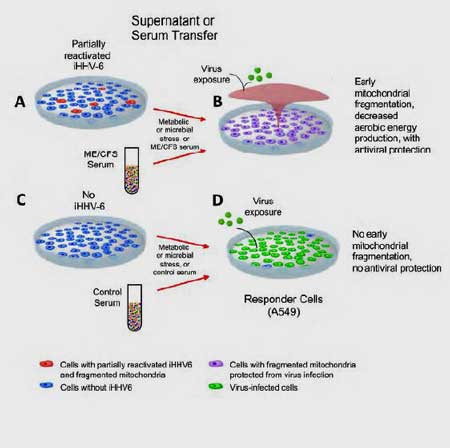
They repeated the same experiment, but instead of using culture medium, they exposed the naive cells to serum from 10 ME/CFS patients.
The serum from ME/CFS patients protected naive cells in the lab dish, but the serum from healthy controls did not. Something in the ME/CFS patients’ serum appeared to have taken on the flu virus and HSV1 and wiped it out before it could infect any of the cells that were exposed.
When the naïve cells were treated with the serum from healthy controls, on the other hand, the cells were not protected, and they died.
That’s a fascinating finding given Ron Davis’s reports, and Oystein Fluge’s 2016 finding, suggesting that putting healthy cells in ME/CFS patients’ serum knocks out their energy production. Could a lightly smoldering HHV-6 infection be the culprit?
That suggested that ME/CFS antiviral defenses were on high alert indeed – and could explain why some people with ME/CFS rarely get colds. Naviaux, in a press release, wrote:
“This provides an explanation for the common observation that ME/CFS patients often report a sharp decrease in the number of colds and other viral infections they experience after they developed the disease. Our work also helps us understand the long-known, but poorly understood link of ME/CFS to past infections with Human Herpes Virus-6 (HHV-6) or HHV-7.”
But what kind of antiviral kryptonite were the ME/CFS cells producing? The most likely guess was that the cells were pumping out their main intracellular virus fighter, interferon. Tests found that interferon activity, surprisingly, was reduced not increased. The same was true for a key pro-inflammatory virus fighter tumor necrosis factor alpha (TNF-a).
The identity of the immune enhancing/energy depleting mystery substance in the ME/CFS patients’ serum will remain a mystery for now, but the authors suggested that other cytokines, or the NLRP3 inflammasome, or even autoantibodies could be inducing a strong pro-inflammatory state that made it difficult for intracellular viruses to flourish. (Recently, one of the co-authors of this paper, Carmen Scheibenbogen, published a separate study showing that a significant subset of ME/CFS patients have increased levels of adrenergic autoantibodies.)
Naviaux proposed that a two-edged sword is to blame for the symptoms of ME/CFS. On the one hand, many of their cells secrete a signal that protects other cells from superinfection with many (RNA and DNA) viruses. On the other hand, this signal comes at the cost of disrupting normal mitochondrial form and function, leading to depleted energy reserves, and susceptibility to crashes with either physical or mental effort. With most of the energy of the cell going to intracellular defense, not much is left over for normal cell functioning – hence the fatigue and widespread problems in ME/CFS.
Conclusion
Could a very lightly smoldering HHV-6 or HHV-7 infection be whacking the ability of the uninfected cells in ME/CFS to produce energy? And turn them into antiviral energizer bunnies at the same time?
The sheer novelty of those ideas may be why the Schriener/Prusty paper ended up being published in an immunology journal instead of a virological one. For now, the HHV-6 community appears to be taking a wait and see attitude to the idea that a herpesvirus, sitting mostly quietly in a cell – and not in that many cells either – could be having such an effect.
The findings jive with Naviaux’s “Cell Danger Response” (CDR) hypothesis, which proposes that mitochondrial disruptions maintain a process which causes the cell to buckle down and enter into a hypometabolic state as a protective response to stress. This study is also the first to potentially explain Ron Davis’s and Oystein Fluge’s observations of four years ago that something in ME/CFS patients’ serum is sending healthy cells into a state of seeming hibernation.
Given the small study size, much larger studies need to be done to validate these results and many questions remain. If reactivation of a dormant copy of HHV-6 DNA in just a few cells is enough to secrete a signal that can put other cells to sleep, why is that happening in people with ME/CFS and not others? If HHV-6B is usually acquired when a child why does ME/CFS mostly show up in adolescence and adulthood? Could the difficult to diagnose HHV-6A be to blame? Or mighjt this strange immune metabolic derailment happen with any virus (as the authors suggest).
Naviaux suggests that an altered cellular or genetic factor may leave ME/CFS patients highly vulnerable to entering into a more or less permanent cell danger response (CDR).
Naviaux and Prusty summarized the highlights of their paper with two take-home messages. The paper proposes the existence of:
- a possible universal mechanism that can also be exploited by HHV-6, HHV-7 and other viruses.
- the discovery of a host cell signal and factors that may cause the CDR to become persistent in ME/CFS patients but is normally turned off after the danger has passed in the general population.
- Check out the Naviaux-Prusty Press Release
Naviaux and Prusty reported that they are “hot on the trail” of the mysterious substance they believe is causing a chronic cell danger response in ME/CFS. They’re also testing suramin and several other potential treatments in the lab to see if they can turn off or block the signal. Successfully doing that in the lab could pave the way to possible clinical trials in the future.
Viral research is changing. Prusty and Naviaux are not alone in suggesting that viruses may be injuring the body in surprising ways. Marshall Williams at Ohio State University has been steadily chasing down a hypothesis that smoldering and non-replicating Epstein-Barr virus infections are also producing proteins that are causing fatigue and other symptoms in a subset of ME/CFS patients.
It should be noted that funding for this novel study came not from the NIH but from private foundations such as the Solve ME/CFS Initiative, the HHV-6 Foundation, the Khosla Foundation and others. That’s not surprising for this field.
From Ron Davis’s nanoneedle to Robert Phair’s Metabolic Trap Hypothesis, to Cortene’s novel drug, to Workwell’s Two-Day Exercise studies, Gordon Broderick’s and Travis Craddock’s modeling efforts, to Marshall Williams’s EBV work, and Naviaux’s cell danger hypothesis and Bhupesh Prusty’s HHV-6 work, the ME/CFS field is full of creative researchers who are bumping against long-standing norms.
That can make it difficult at times to get funding from institutions like the NIH. In fact, NINDS director Walter Koroshetz has suggested that the answer to ME/CFS will not come from within the NIH. (Once it appears, the NIH will support it, but he does not believe the answer will originate from within the NIH.) That makes it all the more important that we keep these creative and vital research efforts alive.



 Health Rising’s Quickie Summer Donation Drive is On!
Health Rising’s Quickie Summer Donation Drive is On!




Most Excellent Article, Cort!
Exactly why they are important and special to all of us! 100 %??
In my current state, I don’t know where else I could be finding the type of information shared here, and certainly in such a digestible form. Thank you for what you’re doing Cort. That such research is taking place inspires hope. It also converts dejection into curiosity and hints at the possibility that some sense of meaning could eventually come from all this suffering.
Agreed, Winston.
And so well said: “ It also converts dejection into curiosity and hints at the possibility that some sense of meaning could eventually come from all this suffering.”
Yes it’s much better to be in a state of curiosity and wonder than dejection! Thanks for mentioning that.
I’ve noticed something quite interesting. Every time I get a influenza my ME symptoms decrease in intensity and my PEM get less.
It became even more obvious during my Covid-19 infection because the infection took much longer to clear.
There must be some sort of connection between the immune system fighting a virus and some sort of switch that temporary gets turned back to more normal energy production.
My ME history date five years. The first three years I couldn’t get a cold/influenza but for the last two years I get influenza again but temporary less ME symptoms
I wonder if CDR persistence, which seems to paradoxically confer protection against other viruses, might confer a measure of protection vs. Covid-19. Also curious about any reports of treatment outcomes using Suramin outside the US.
I wondered that, too, as I was reading this article.
All I know of suramin is the small Naviaux autism trial which had good results.
As to COVID-19 I would think it would help and I imagine it would not be sufficient against a really strong virus. In this small study 40% of the participants appeared to have this process going. We need much larger numbers to really know how many people have this issue.
Thankfully in the last blog we saw people who appear to have gotten really nasty cases of it and who are recovering.
Note also that that work was done in the lab and we don’t know how it translates to bodies. Some people with ME/CFS have, of course, noted that they don’t seem to get colds very often. Others do! I know someone when she gets a cold – it can linger and linger and linger. She may not be part of the 40%. The immune system is a complex thing.
It’s a fascinating study and I fervently hope the researchers can get the funding they need to really ramp things up. I imagine that funding will have to come from us.
Feeling better during the flu??? 1st I hear of this. Glad for those of you. My experience is totally different. One of my first signs was extreme sensibility to cold and constant flair up, and fever blisters, eye infection,….the brain fog and fatigue that has little by little increased has turned me 80% bedridden, I reached this point while seeing one doctor after the other. We tend to be a good fat fish for many doctors who supposedly are so good but do not accept insurance. Especially in Switzerland. I am reaching a point that keeping track of my medications and doctor appointments are hard. My daughter of 15 wants a mother to do things with her. I can not. Being very reasonable, maybe my family will be better off without me. So they can go on living.
Hi Jade,
I just read your comment and I feel sad that you’re having such a horrible time. I can see how you may believe that you are not the kind of mother, you would like to be, for your daughter. However it’s my belief that your family would not be better off without you… You are undoubtedly a very important part of their life, even if you don’t currently feel that way
Tracey ??
Bottom line is, no one know for sure. See Prusty carify here: https://twitter.com/MBVanElzakker/status/1253510009771692034
But a lot of ME/CFSers have already had Covid-19, including some who don’t normally ever get flu or other illnesses.
Exactly what I was thinking as I read this excellent article.
It is very interesting, and exciting how it all ties in. Thanks for your articles Cort.
Ive had m.e 5 years now and no colds but a couple of fever viruses in the last couple of years. Like Tove Jensen above, my m.e seems ro go into remission when I get viruses, I actually have more energy than usual when sick and brain fog goes.
This paper raises some interesting ideas but as you pointed out, we need other mitochondrial researchers to replicate Dr Prusty’s findings.
Also does Dr Prusty’s findings affirm what Dr Maureen Hansen and the intramural study at NIH found studying metabolism perturbations using Seahorse analyzer and metabolic chamber? When Seahorse used to study mitochondrial function of PBMCs, are “serum factors” included or are cells washed before analysis?
Lastly, is it possible that the “protective factor” produced by cells that are reactivated not only cause mitochondrial fragmentation but also serves as a signal to the brain/CNS. (Which cell lines are chronically infected with HHV-6? ie central nervous system tissue and/or peripheral. Nervous system of the gut? Vagus nerve? Skeletal or smooth muscle?)
Does exercise or stress trigger increased production of “protective factors”?
Dear Jade,
Please don’t kill yourself!
Watch this. https://plandemicmovie.com/ Info on Surinam!
“Suramin” 😉
I think this is the most interesting research I have ever seen in our field. It sounds quite compelling too. Fingers crossed!
Thanks for the article Cort.
Nobody has put infection and energy production together the way these two have – and then to tie it to the mystery substance in the serum….Wow.
Let’s hope they get the funding they need to really dig into this and validate it.
Read Anthony Williams book Medical Medium, as this man has already done this in great detail.
Agree with you, Cort! Crossing my fingers and toes! Ouch..lol!
I think we need to suggest that researching ME/CFS/FM will provide invaluable deeper insight into complete immune function and diseases. For aging politicians and bureaucrats, the connections to old age functionality, immunosenescence, and quality of life could be compelling.
It’s an opportunity because it provides many differentials between patients and healthy people. The ME/CFS/FM medical community’s recognition of the subsets and immune system complexity has them at the cutting edge. They’re simply among the most ready to make some major medical breakthroughs that will demystify many immune-mediated pathologies.
Feels like the biggest paper since Naviaux first came to my attention! I’ve been reading up on it since Prusty announced its release; I’m still digesting and formulating questions…
Good write up! 🙂 Did you correspond with Prusty or Naviaux for it? And Have you got a link, please, for the “Naviaux, in a press release, wrote:”? I couldn’t search it up, or any other direct comment from him on the paper. Thanks.
I added the Press Release to the text in the conclusion. Thankfully, was able to communicate with Naviaux regarding the blog.
Cheers! Oh, looks like that release was published here: https://health.ucsd.edu/news/releases/Pages/2020-04-27-for-me-cfs-patients-viral-immunities-come-at-lifelong-cost.aspx
I have reactivations of EBV since 2006.
Each time I feel something is wrong, I tell my doctor to check IgG & IgM viruses + PCR or VSG ( Globular sediment speed)
Always the same:
EBV: IgG & IgM+
Citomegalovirus:
IgG + (exceeds maximum levels) but IgM most of times –
VSG sometimes above maximuns, others not.
PCR same ( some times exceeds max, sometimes not.
Always ourine infection too.
When I was diagnosed, both were + and VSG & PCR were really high, at the point doctor was scared.
He did a Phd about fibromyalgia and my diagnostic inform says is Tipe II fibromyalgia and CFS
Thanks Magda, dp you know what Type II Fibro is?
Big typo Cort? Missing the word “not”?
“the authors chose a cell-line (U2-OS) that has the CD46 receptor that enables HHV-6 to enter the cell and integrate its DNA, but which does [[[NOT]]] allow the virus to carry out its life-cycle.”
That was a big miss! Thanks junkcrap50 for pointing that out.
Once again Cort, many thanks for taking the time and effort to be such an understandable conduit for the research out there that may one day change our lives! It is extremely encouraging to know that progress is being made, bit by bit.
Thanks Cort indeed for making this work so understandable. I tried to read the original paper and can say you did an incredible job to “translate” it!!!
Also PLENTY AND PLENTY OF THANKS to all the authors being “Philipp Schreiner, Thomas Harrer, Carmen Scheibenbogen, Stephanie Lamer, Andreas Schlosser, Robert K. Naviaux and Bhupesh K. Prusty”. Their work might become a reference work!
Let me first state that this does remind me A LOT of this previous blog you made: http://simmaronresearch.com/2018/04/autoimmune-virus-groundbreaking-ebv-finding-help-explain-mecfs/
This one deals with herpes virus EBV:
“EBV consists of several proteins of which EBNA-2 is one. EBNA-2 is EBV’s main viral transactivator; i.e. it’s a transcription factor that turns on genes in an infected cell that help EBV to survive. Essentially EBNA-2 allows EBV to hijack a cell’s genetics and put them to its own use.”
=> Note it uses the word “transactivator”. Some viral factor produced by the virus when not active and “dumped” out of the cell into the bloodstream to “do something other then infecting cells”.
And from this paper (HHV-6 and HHV-7) https://www.immunohorizons.org/content/4/4/201 :
“we have recently shown that HHV-6A transactivation induces mitochondrial fission, which is associated with altered host microRNA (miRNA) as well as the mRNA transcriptome (15). HHV-6 reactivation in U2-OS cells is nonproductive (15, 25) and is marked by initiation of transcription of several viral small noncoding RNAs in the absence of substantial viral DNA replication and viral protein synthesis (15).”
=> Another herpes virus that shows the ability to do this “transactivation” stuff without viral spreading or replication, and this “transactivation” stuff can cause mitochondrial fragmentation (helping to make us so depleted of energy).
=> Combine both papers and it is likely that EBV and maybe many other herpes viruses too can do this “transactivation” trick and cause the mitochondria to go into the “Dauer state” without having a full reactivation nor detectable active infection.
=> Now let’s go a bit further: only 40% of the patients in the last paper showed this effect due to HHV-6 or HHV-7, but what if ALL herpes family viruses could do a similar trick by shedding somewhat different partial “non active” “transactivation factors”? Then maybe 90+ % of patients have at least one of these herpes viral transactivation factors active and kicking their mitochondria down. Could this be a near universal road to ME diagnosis, understanding (and treatement?)???
Great catch on the possible EBV transactivation as well 🙂 and good point that this study covers only two of the many viruses that Prusty and Naviaux believe could be involved. It was nice to see Carmen Scheibenbogen – another German connection – involved as well. 🙂
What diagnostic criteria us was used to select the survey participants?
I’ve noticed something quite interesting. Every time I get a influenza my ME symptoms decrease in intensity and my PEM get less.
It became even more obvious during my Covid-19 infection because the infection took much longer to clear.
There must be some sort of connection between the immune system fighting a virus and some sort of switch that temporary gets turned back to more normal energy production.
My ME history date five years. The first three years I couldn’t get a cold/influenza but for the last two years I get influenza again but temporary less ME symptoms
That is very interesting because I have read a couple of other patients post similar comments about amazingly feeling better during the flu. At least one thought it was due to taking Tamiflu but that was probably a red herring. I wonder if there is a benign virus that could turn off the underlying pathology.
I’ve heard of some patients doing better on Tamiflu. Just a few but it’s interesting. One thing I hope we get out of COVID-19 is better broad spectrum antivirals. (I was just told that Suramin qualifies, by the way.)
Thanks for this & for everything you do Cort! Glad to have those minds working for us! I agree with you Tove, the same thing happens w me. I’ve been sick for 20+ years. And for the most part I will not usually catch colds/flus. When I’m doing better overall, even slightly better than baseline I will begin to catch things. While I’m in the midst of it my energy def ticks up. I always feel better energy wise if I have an infection. It’s the only time I have the energy to cook! Always thought it was especially cruel that when I’m feeling most like socializing energy wise I can’t bc I’m contagious ? I don’t think I feel better than the average person w a cold or flu but I feel better than the average person w ME. The fatigue that comes w colds & flus really doesn’t begin to touch the crushing exhaustion that is ME. But after the infection the ME rages on ramped up past full throttle, requiring months to get back up to baseline. All of this fits w this discovery to me.
Yes, I’ve noticed that if I’m unwell with a cold or something mild, I feel normally ill, but with more energy.
This has happened repeatedly. I wouldn’t take any medication.
From the paper https://www.immunohorizons.org/content/4/4/201
“The virus reactivation experiments described in this study show that an antiviral state is produced both in cells with unreactivated and reactivated HHV-6. This seems to be against the viral growth and hence fails to explain the short-term benefits of viral reactivation from the pathogen point of view, unless passive transmission of viral genome to daughter cells after mitosis plays a major role in HHV-6 genome propagation.”
I would add another explanation for this “fails to explain the short-term benefits of viral reactivation from the pathogen point of view”:
Maybe the whole point is EXACTLY to increase antiviral behavior by stopping the spreading of the virus ITSELF to other uninfected cells AND at the same time cripple the cells defenses against the viruses that are already “fully integrated” into the cells.
From the paper:
“Additionally, mitochondrial fragmentation often allows virus to acquire persistent or latent state under a hypometabolic state (57).”
And when reading that reference 57 https://www.pnas.org/content/111/17/6413?ijkey=69f9cfc513b6dd737054f1337e26c02861888610&keytype2=tf_ipsecsha :
“This study provides a new insight into how HCV disrupts mitochondrial dynamics and evades apoptosis and innate immunity to sustain persistent viral infection.”
=> So this says that mitochondrial fragmentation sort of “cripples” the innate immunity and allows the infected cells to live longer then they would when not being infected AND at the same time “safeguard” the infection inside the cell.
On the other hand the paper and Cort explained very well that this transactivation also has potent anti viral properties. That seems to be contradictory, but it is not. Remember the immune system is not one single thing!
So these herpes viruses seem to be able to exploit two parts of the immune system: safeguarding infected (by themselves!) cells and keep those cells living and infected on one hand AND to prevent non infected cells to become infected by other viruses AND themselves.
Why is that latter so important?
I wrote on that one before. The incredible trick that EBV and other herpes viruses can do is to infect the body, be “uneradicable” but not kill it. It may seem normal but it is not.
If we look for example at Covid, then we see some people feeling fine today, going tomorrow with complaints to the hospital and be dead the day after. So fast has the virus spread in two days and so strong was the immune response to the virus that it killed the body. So, when left unchecked chances are big that a virus will replicate that fast that either the virus or the immune reaction against the virus will kill the host.
That is very bad for the host but in the longer term eradicates the virus itself. So it’s bad for the virus itself in the long run. These herpes viruses however “remain with us” for the rest of our lives AND they spread successfully to other people (so they MUST keep spreading themselves a lot of the time). Many of these herpes viruses are so contagious that the large majority of people are infected.
Quoting what Cort wrote: “Over 90% of people are infected by 3 years of age, usually through their mother’s saliva.”
So the virus must at least partially reactivate in near all infected mothers from time to time. But (assuming a too high % of infected cells would be very bad for the mother) the mother is safeguarded from being overrun by these many succeeding reactivations, meaning that the virus largely stops infecting new cells of the host.
That’s quite the trick: only very easily infect others but not reinfect the host so that the host does not get very sick and die.
One could say “but the adaptive immune system knows the virus so it protects against reinfection”. But IMO that logic is faulty. The immune system will never prevent ALL viral particles from increasing the infection of the self. And with infected cells staying that long infected that SHOULD increase infection rates a lot each time the virus gets into a contagious state and that seems to be very often.
IMO only if the adaptive immune system can kill off enough infected cells or the infection inside it, it can prevent the virus from flooding the host during when the virus gets contagious many times.
So IMO for herpes viruses to live that long in each cell they infected and be contagious so many times during life it has to prevent further spreading or “self limiting” in the body itself. And it does this “wonderful”:
it does trigger the immune system to not attack itself when it infected a cell and established itself in it very cozy (weakening that *part of* the innate immune system) AND at the same time it prevents new infection of other cells of the host by ANY viral infection including ITSELF (strengthening that *part of* the innate immune system).
IF these viral “transactivation particles” would prove to have potent antiviral properties possibly including to Covid, Ebola, HIV… then our disease might become the most studied disease overnight.
Imagine research being able to just extract and copy these particles and administering them to patients who are in strong actuate danger. Imagine it might provoke a strong antiviral working whereby the innate immune system breaks down these particles so that they disappear after that they are needed as the particles themselves would not be able to self replicate.
It would be tricky to get it working and safe and likely require the mitochondria to unfracture / refuse again by resting enough after treatment, but if it worked then one could say “they have healing blood” rather then “they are no good fainters”. And studying each treated patient would offer an opportunity to study part of ME onset (and healing in the absence of transactivation particles self replicating) time and again.
We might open up the age of strong, broad working and safe antivirals. Where is that research funding now??? If it worked we are talking a hundred + billion dollar a year market.
I definitely believe Naviaux’s CDR framework will (should) overhaul medical practice and understanding.
I’d thought about using the fatigue factor for viral defence, too. Problems are, it would presumably only be useful a prophylactic (acute infection would already cause this) and would give the recipient a chronic fatigue phenotype (hopefully only temporarily, but maybe sticks also).
So interesting and hopeful. I also rarely get colds, and felt better for three weeks when i did get one.
I wonder why mostly female, and also why it seems to affect athletes so much?
Cort,
I have both CFS and POIS. Both simultaneously onset same day
In 1993.
Post Orgasmic Syndrome is a NORD validated rare disease. Where men crash with severe CFS like symptoms for 2 to 5 days after every ejaculation takes place (does not matter how (sex, nocturnal emissions etc.)).
The symptoms are amazingly similar as as a bad CFS crash. But most men go back to higher plateau of functioning than people with CFS (unless they have both) in 2 to 5 days. For me 3.
Imagine the study gem this entails. The whole cycle ALWAYS happens. So it could show volumes if tracked daily monitoring multiple biomarkers.
Dr Maria Vera who works in Dr. Klimas office said to me “EBV has an affinity for sexual organs like the testis”.
Light went on. These thousands of men could be the missing men in CFS! Testosterone goes down after ejaculation and it takes a few days to come back. The things that help them are exactly the same as what helps people with CFS. The similarities are perplexing. To the extreme that I have given them lots of good material from CFS that they use now to alleviate symptoms. Many are just waiting for the CFS cure with the hope it will somehow benefit them.
I have long suspected that herpes type virus are involved in POIS. I came down with EBV then POIS + CFS simultaneously. So the link is there for me. I am not the only man who has both. Note that hormonal changes activate herpes viruses as well.
Studying this cohort could shed so much light into both CFS and POIS.
The problem I have is that few people with one label bother to look at the other. That is different they conjecture.
I have both and I believe they are one and the same. Except POIS. Is what these men call their short term CFS episodes.
More on POIS at
https://poiscenter.com/forums/index.php
Can you please consider taking a look bringing the group to researches attention? It could be the missing link in these diseases spectrum.
This condition is predominantly men but a few women also. ME CFS is majority women. I totally believe these should be studied together.
Thanks for pointing out something a very interesting disease.
Very interesting..Agree, that should be brought to their attention..
Great Thought!?
Thank you Ric for bringing this up. I don’t know you but I’m also a POIScenter member and I only came to the blog here for general research because I buy into the virus theory.
For others, here are some threads to read:
Ideas on Herpes Induced POIS https://poiscenter.com/forums/index.php?topic=2683.0
Transiently Induced Immune Deficiency and Therapy https://poiscenter.com/forums/index.php?topic=3151.0
Naviaux’s PhD was all about a class of viruses related to Polio and how they used ways to block the interferon response.
He could never have developed the CDR hypothesis without his training in virology. This is what makes his work with Prusty a natural match.
Naviaux’s most highly-cited publication is a virology paper describing the invention of a new series of retroviral gene transfer vectors—the pCL vectors.
See: https://www.ncbi.nlm.nih.gov/pubmed/?term=8764092
UCSD just published Naviaux’s SUMMARY:
https://health.ucsd.edu/news/releases/Pages/2020-04-27-for-me-cfs-patients-viral-immunities-come-at-lifelong-cost.aspx
Cort, As always a brilliant distillation of complex science. What would your intuition tell you about the etiology for those patients that partially or fully recover?
It would be interesting to try to solicit more information from those patients with ME/CFS who have developed Covid 19. How would that be possible?
Thanks for keeping the light on.
Hi,
Open Medicine and the You+ME Register is going to that when they
Launch it, so it is important to sign up for it! We can help those who are helping us!
Correction Open Medicine Foundation (OMF) which will be International..
As well as I am seeing Stanford is doing alot in that area as well!
Many heartfelt thanks to them all!
Our little poll (https://www.healthrising.org/blog/2020/04/22/coronavirus-silent-hypoxia-pulse-oximeter/) suggests that many people with ME/CFS with symptoms of COVID-19, are, thankfully, recovering. Other than not I don’t have much info. Maybe as things wind down with the bug (as hopefully they will) we can do a more complete survey.
Solve ME will also be determining how people with ME/CFS who get COVID-19 do in their Patient Registry which will hopefully be launched soon.
Living with a spouse who suffers from severe ME/CFS has been a challenge. Her suffering has come from both the fatigue for which it is know as well as the misunderstanding that people have of her condition.
I work hard to get people to understand her, and have had some small success, but it remains a mystery to most. It is encouraging that clues have been found as to what is taking place within the patient’s body. If we could only get the folks at the NIH to support the research with serious funding.
I pray a cure be found soon. Not just for my wife, but also for everyone of the millions who are similarly afflicted.
I am always happy to see funding in this field. But we all know mitochondrial plays a big role in this disease, we should not be funding who are coming back to say what has already been pretty much established? but how to tame the ill mitochondrial, very simply said.
Remember XMRV? A retrovirus.
New book just out.
Plague of Corruption: Restoring Faith in the Promise of Science
by Kent Heckenlively (Author), Judy Mikovits (Author)
So great to see Naviaux’ work continuing and the collaborations Cort! I’m intrigued by the mention in the article that the reactivation process seems to be triggering a hypometabolic response – the freeze-like state of dauer he identified in his 2016 metabolic study.
“This effect of viral reactivation on mitochondrial morphology of nearby cells provided an interesting scenario that could be used to study ME/CFS pathophysiology. A transferrable hypometabolic phenotype in responder cells was further supported by observations of decreased intracellular ATP content (Fig. 2D).”
In other words – the process they are encountering with viruses is consistent with past studies about this intelligent, persistent stuck CDR being strengthened in the direction of hibernation / freeze / dauer for those of us with ME/CFS.
It makes me wonder if infections act like triggers that INTENSIFY and strengthen freeze responses in the subset Naviaux et al are talking about. Perhaps this group is more sensitive to infections because of (more or more serious) infections earlier in life (such as in childhood?).
I hadn’t heard about the autoimmune studies and this seems so hopeful for continued work towards finding a biomarker. In that vein, I wonder if our bodies are actively trying to decrease sympathetic activity in ME/CFS to keep strengthening the freeze based CDR – such as through autoimmune attacks on adrenergic functions (which I see is some of what Carmen Scheibenbogen et al found) ie: attacks on sympathetic function that – in health – creates vasoconstriction in most parts of the body while causing vasodilation in skeletal muscles, brain and heart to support mobilizing for fight/flight. An attack that could be trying to reverse that function in support of more freeze, leading to vasoconstriction in the brain heart and muscles when we get stressed and when we most need that function.
You’ve elicited much curiosity and intrigue – as usual! Thanks Cort!
quote from earlier HealthRising comments section:
“Catherine on September 5, 2016 at 2:15 am
How about a name change from ME/CFS/SEID to Naviaux’s Disease?! Very exciting research”
IMO, there are so many who have contributed significantly over the years, including having info ‘lost’ and ‘re borne,’
that there is difficulty in choosing one name to stand for this disease.
Calling the disease “Prusty-Davis-Cheney-Naviaux Disease”
((order of letters: PDCN was the only order, when i googled, that did not show up immediately as already taken))
would still be missing out multiple names of great significance,
from (almost?) every letter of the alphabet,
for this alphabet soup disease.
Diseases are often named after whoever
puts the ‘final nail in the coffin’ of a disease.
Therefore the final word
may be a name that does not refer
to the many debilitating aspects,
but instead recognizes a person
or group’s achievement.
I have been considering giving Dr. Chias Equilibriant a try. In view of this research maybe that is what I need. Any thoughts?
CFS patients rarely getting colds can also be explained by the social isolation. Social distancing does wonders for preventing infection and we are stuck at home much more than healthy people: CFS a natural stay-at-home order.
The weakness of HHV6/mitochondria theory for CFS still is the lack of clear explanation for PEM, especially for moderate/mild patient who can generate enough energy, only to keel over for days after 24 hour delay. Maybe there is a way to explain, but it’s not central to the theory.
Good point about the social isolation and while my guess is that Naviaux must have an explanation for the PEM, that weird delayed impact is something that must at some point be explained.
You know that muscle soreness a day or so after exercise is normal. I wonder if it could be an exaggerated bit of that plus some pain hypersensitivity added in. Actually given the burning sensations I feel I would guess that small fiber neuropathy is involved.
I still have sf neuropathy after my my CF somehow resolved. The PEM went away with high dose B vits. I’m on DSF now for Lyme but nothing seems to touch the neuropathy. I’m starting to suspect Bartonella. Have people with ME/CFS been tested for tickborn co-infections (not that the tests are very accurate).
Actually I thought that this theory would explain my experience of PEM after physical exertion really well. I can usually walk for 20 minutes, but if I walk for 40 minutes, I will crash. I had a time when my illness was milder, but the rule was still the same: I could walk 4km on most days, but if I walked 8km just once, even on a very good day, I would crash for months.
Physical activity is stressful to the body, even for healthy people – that’s how we get all the good-for-you adaptations to exercise. If this stress was enough to reactivate a virus in CFS patients (as exposure to certain chemicals or injury does) and this would turn on the early antiviral response that they describe, this could account for my crash.
Of course in my case this response would have to lessen over the period of a few months as I did usually get back to my baseline (but never to pre-illness health).
Not all patients are socially isolated. As a school psychologist, I work in several schools. The 15 years before I got ME/CFS, I would come down with almost everything-colds, flu, etc. I also have five children that seem to be germ magnets that “share” all of their colds. There was not one year that I did not catch at least a couple of colds a year.
I got ME/CFS 5 years ago. I did not stop working after getting sick so my exposure to viruses is the same before and after. For the first two years with ME/CFS I never got sick. I remember thinking in the first year that it was truly a blessing that I didn’t have to deal with any colds like I usually did. I believe that for me I had some viral protection that can’t be explained by social isolation.
I am another Naviaux fan, and should go and re-read his last two big papers on the “First chapter of the 2nd book of medicine” and the complex process that may lead to healing–this latest with the others is a real eye opener. I can join the list of those who have never had a cold or flu during the now13 years of ME.
Many thanks for a lucid account.
Hi Cort, dies this relate to shingles/herpes zoster (VZV) as well ?
This is,very specifically, what triggered my ME in 1987. Gemma
Try the antiviral treatments for it.
Dr Chia finds treating vzv cfs can take as little as three weeks
Good research. biology isn’t my area of interest however I have a natural aptitude for all sciences and an intuition of sorts, there was something wrong with the inconclusive or cross contradictory studies we have seen over the years on this subject, which is out of line with the ‘degree of resolution’ achieved by the technological system, ( some key factor was missing / overlooked ) so I had to accept that every theory or angle may hold truth, and I think that may still be the case, we will see that everyones opinions and perspective is ultimately validated because the mechanisms facilitate a variety of outcomes within the umbrella of the same disease.
I am just getting a strong feeling that this is the right line of inquiry, since first hearing about the CDR hypothesis I found it very compelling. I didn’t really need a study to know that periods where O.I. is experienced is associated with less blood flow to the brain, or that CFS/ME patients likely have resistance to covid-19 fatality.
Respect to everyone that stuck by their beliefs in the face of denial from the establishment, though I am not expecting to see any recognition or apologies in that regard. I don’t think Dr Prustys discovery would have necessarily found the relevant application if not for the body of work surrounding the subject already so I want to say thanks to ALL researchers.
I was wondering what has been happening with the Naviaux dauer state research. Back in 2014, they stated that the oxygen and glucose needed to power a cell were not being used. It seemed so promising. Cells can’t use the primary energy source of oxygen and glucose, so our bodies must run on the back-up system – adrenaline. So, if something further happens where we need adrenaline, it’s not there and we crash. So, more cortisol kicks in to say something is still wrong, which in turn kicks in the ACTH which disregulates the response. It over-kicks in the adrenaline, which fails. The cycle checks to see if the problem has been corrected, which it hasn’t, which kicks in yet more cortisol (stress hormone). Cortisol ramps up again, adrenaline ramps up but fails again, in an unending spiral, forcing a crash. I believe most other symptoms we suffer are part of the Central Sensitivity Syndrome issues we share with West Nile/PTSD etc. But for ME/CFS funding, we need to figure out the Cell Danger Response. Everything else is secondary, but I haven’t seen much follow-through on CDR since 2014. The similarities between ME/CFS and the long-haulers is great for funding, but unless the CDR is shown to exist in the long-haulers, it’s leading in the wrong direction. Does anyone know of anything recent about CDR?