“I had thought, it’s only about nine minutes of exercise, how much is going to change? A lot, as it turns out.” Dr. Mike Snyder – senior author of the study
How much could nine minutes of an exercise bout change in your body? Probably not that much, Dr. Snyder apparently thought. He, after all, can hop on his bike and pedal away without a second thought. At most, he might feel achy and tired for a day or two.
But people with ME/CFS have a different perspective. Too much “exercise” – make that activity – can produce a dazzling array of painful symptoms that can last days or more. In other words, it’s crystal clear to them that whether healthy people realize it or not, whatever exercise does, it must profoundly affect the body.
So, while the researchers at Stanford were surprised that a single bout of exercise to exhaustion tweaked over half the 18,000 molecules they tested in healthy middle-aged people, people with ME/CFS probably wouldn’t be surprised at all.
Plus, it intuitively makes sense. It’s only recently that almost everybody engaged in some sort of exercise regularly. Two hundred years ago, ninety percent of Americans lived on farms. Exercise is a fundamental process for humans, and the most in-depth study yet of exercise’s impacts on the human body showed just how fundamental it is.
Molecular Choreography of Acute Exercise. Kevin Contrepois,1,2,12 Si Wu,1,12 Kegan J. Moneghetti,2,3,4,5,12 Daniel Hornburg,1,12 Sara Ahadi,1,13 Ming-Shian Tsai,1,13. Ahmed A. Metwally,1 Eric Wei,1 Brittany Lee-McMullen,1 Jeniffer V. Quijada,1 Songjie Chen,1 Jeffrey W. Christle,2,3,5 Mathew Ellenberger,1 Brunilda Balliu,6 Shalina Taylor,7 Matthew G. Durrant,1 David A. Knowles,1,8 Hani Choudhry,9 Melanie Ashland,1 Amir Bahmani,1 Brooke Enslen,1 Myriam Amsallem,2,3 Yukari Kobayashi,2,3 Monika Avina,1 Dalia Perelman,1 Sophia Miryam Schu¨ ssler-Fiorenza Rose,1 Wenyu Zhou,1 Euan A. Ashley,1,3,10 Stephen B. Montgomery,1,6 Hassan Chaib,1 Francois Haddad,2,3,11,* and Michael P. Snyder1,2,11,14,* Cell. Volume 181, Issue 5, 28 May 2020, Pages 1112-1130.e16
“Everybody knows exercise is good for you, but we really don’t know what drives that at a molecular level, Our goal at the outset was to conduct a highly comprehensive analysis of what’s happening in the body just after exercising.” Michael Snyder
If you want to know what’s wrong – you have to know what right looks like. This and the studies coming out of the NIH’s $170 million Molecular Transducers of Physical Activity Consortium (MoTrPAC) are aiming to learn what healthy exercise looks like in the body. Their goal is to “develop a comprehensive map of the molecular changes that arise with physical activity” (Note – physical activity, not necessarily exercise).
This study sure looked like it was part of the Consortium – and Mike Snyder’s Genomic Center at Stanford is part of the Consortium – but it wasn’t. All the funding for this particular study came from elsewhere. It’s no surprise, though, that it came out of the Snyder lab which, back in 2012, in what was hailed as a landmark paper, carried out the first deep longitudinal profiling ever done using multi-‘omics technologies (genomics, transcriptomics, proteomics, metabolomics, etc.) that was able to predict disease risk and onset.
That big NIH exercise project that recently got underway is going to include 2,700 people. This study had 38 people – but even so it produced some fascinating, and if they are validated, potentially novel insights that could benefit ME/CFS/FM.
Michael Snyder PhD and what looked to be a good proportion of the Stanford faculty, put the 38 middle-aged healthy controls on a bike, exercised them to exhaustion and examined their blood before, and 2 min, 15 min, 30 min, and 1 h after exercise. They assessed seemingly every “ome” they could: their metabolome, lipidome, immunome, proteome, and transcriptome.
Results
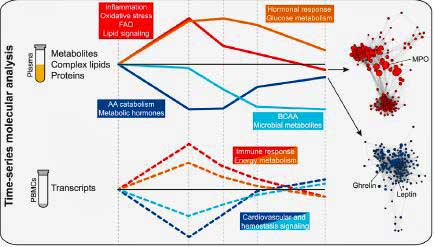
The study identified four different clusters of molecules that occurred over the hour blood was taken. Note that each of these clusters are involved in different themes. They present a kind of molecular “choreography” that needs to take place in order for exercise to work. Throw one or more of these clusters off and maybe you have ME/CFS. For the present, this study and others (Dr. Klimas has found clusters of activity as well), are identifying components of the exercise process that could conceivably be checked out in ME/CFS:
- A cluster of inflammatory/oxidative stress molecules which ramped up immediately during exercise and then settled down. One of these involved a “fitness inflammatory signature”; i.e. a burst of inflammatory cytokines fifteen minutes after exercise which proved beneficial. The signature was centered on the IL-1b cytokine and potentially regulated by IL-5 and TGF-b.
- A cluster of molecules centered on metabolic hormones (leptin, ghrelin) which decreased immediately and then returned to baseline within an hour.
- A cluster of molecules involving carbohydrate metabolism (cortisol/glucose) which showed up later and was deemed essential in replenishing energy stores. At 2 minutes, the body was metabolizing amino acids for energy, but then it switched to metabolizing glucose, a type of sugar, at around 15 minutes.
- A cluster of molecules which involved amino acids which were metabolized to assist in muscle repair. This cluster was not replenished by the end of the testing period I hour after exercise.
Interestingly, the general sequence of molecular changes – an immediate explosion of inflammation and oxidative stress, followed later by a hormonal response (steroid hormones, corticosteroids) – was similar to that reported by Dr. Klimas in ME/CFS. (Dr. Klimas also reported autonomic nervous system changes).
Other interesting findings included the following:
- A substance called myeloperoxidase (MPO). Secreted by neutrophils and a marker of oxidative stress, it turned out to be an important bridge to other pro-inflammatory and growth and protective factors.
- Other processes identified included muscle repair, nitric oxide signaling in blood vessels, a decrease in mTOR signaling.
- The authors suggested that an increase in ketone bodies later may have provided an additional energy substrate.
- The production of long-chain polyunsaturated fatty acids that increased immediately post-exercise drew interest: did they reflect inflammation?
- The two most significant pathways associated with peak VO2 (calpain and integrin pathways) are both involved in muscle regulation.
Somewhere in these key factors identified in healthy controls may lie the answer to ME/CFS. Something, after all, has gone wrong. So long as this and future studies identify what must go right for healthful exercise, we should be able to figure out what’s gone wrong.
Do the metabolic hormones that replenish energy stores not kick in? Is cortisol not elevated enough? Or do nitric oxide levels not increase enough to open the blood vessels? Or are the muscles not being repaired? Is our “fitness inflammatory signature” missing? Is the liver not producing enough ketone bodies?
Predicting Fitness er … VO2 Max with a Blood Test?
“It gave us the idea that we could develop a test to predict someone’s level of fitness,” Kévin Contrepois (1st author of study).
The most immediately important finding from the study, though, may be that the study found molecules in the pre-exercise period which predicted levels of VO2 max and ventilatory efficiency. Finding factors during rest which track VO2 max levels (a measure of energy production) could be groundbreaking for ME/CFS.
Think of the possibilities. A blood test which validated an inability to exercise? A diagnostic test for ME/CFS? Time will tell.
The study found a kind of poor VO2 max signature in the blood. While 1,000’s of molecules were involved, the main factor that popped out – leptin – has been associated with ME/CFS before. Younger’s longitudinal study pinpointed leptin as a possible factor driving the immune changes in ME/CFS, and higher leptin levels have been associated with pain in fibromyalgia. Snyder’s study found that higher leptin levels were associated with lower VO2 max in the healthy controls.
Two other fatty acids – triglycerides (TAG) and BCAA were also associated with lower peak VO2. Reduced levels of BCAA, have been found in ME/CFS before. BCAA’s have been used in athletes to increase endurance but can also inhibit tryptophan entry into the brain.
On the other hand, other factors such as the transporter of thyroxine and retinol transthyretin (thyroid, hydroxy-fatty acids, corticosterone, hippuric acid, and bile pigments (i.e., biliverdin and bilirubin) were all positively associated with peak VO2; i.e. higher levels of each of these were associated with higher VO2 max. (Are some of these low in ME/CFS?).
One of them, hippuric acid, interestingly, is associated with microbial or gut diversity – which has found to be low in ME/CFS. The thyroxine transporter could fit in with the thyroid issues in ME/CFS.
The Gist
- This is the first of what will presumably be many studies to come which will provide many new insights into how exercise affects the body – a key concern of people with ME/CFS, in particular – given their post-exertional malaise problems.
- The small, but intense, study involving 38 middle-aged healthy controls, came out of Michael Snyder’s lab at Stanford. Snyder is also part of the $120 million NIH study seeking to understand how exercise affects the physiology of the body at the deepest levels.
- The study had the healthy controls exercise to exhaustion and then tracked how their different “omes” (genome, proteome, lipome, transcriptome) responded over the next hour.
- To the researchers’ surprise, a single bout of exercise affected the levels of over half of the 18,000 molecules tracked.
- The study uncovered four clusters of molecular events involving inflammation/oxidative stress, hormonal signaling, and carbohydrate and amino acid metabolism.
- A signature in the blood that occurred prior to the exercise predicted how aerobically fit the participants were. Such a test, if developed for ME/CFS, could conceivably be used as a diagnostic test.
- Many other findings involving inflammation, hormones, oxidative stress, nitric oxide, muscle repair, etc. could provide a kind of roadmap for future investigations involving ME/CFS.
- Next will come larger studies out of the 2,300-person NIH initiative focused at getting at the molecular roots of exercise in healthy people. Those studies should provide us with plenty of new insights into exercise that we can use to better understand ME/CFS and FM.
The Stanford team is developing an algorithm to select a small number of molecules that are highly correlated with peak VO2; i.e. aerobic fitness. Expect to see that test in the future.
If an aerobic fitness signature can be found in the blood of healthy people my guess is that it should be much easier to find in the blood of exertion challenged people.
It’s possible that because this study was done in healthy controls, the results do not reflect what’s happening in people with ME/CFS. Our reason for reduced VO2 max may be different from theirs, but the fact that these researchers were able to uncover factors present at baseline that predict how much energy one can produce during exertion provides a lot of hope that the same could be done in ME/CFS.
Validation?
Maybe findings like this will also will help more people get it about “chronic fatigue syndrome”. If a single bout of exercise affects that much of the body, then breaking that system should cause some real damage. Overloading a broken system like that by exercising should result some pretty stunning biological changes.
Dr. Klimas – who has already done similar gene expression and immune testing in ME/CFS and GWI – is, no doubt, lying in wait, waiting for this data to come out. Her studies haven’t been as comprehensive, but they do indicate that exercise causes higher amounts of inflammation, oxidative stress, autonomic nervous system and hormonal shifts in ME/CFS patients vs healthy controls.
A big question facing these huge omics studies is when they will produce tangible, actionable results. They’re very good at spitting out interesting masses of data. Translating that data into concrete treatment approaches hasn’t been so forthcoming.
Because Dr. Klimas has embedded her exercise/omics results into a computer model, her approach has resulted in concrete results: clinical trials in GWI and ME/CFS. We don’t know if the very, very complex process she’s engaged in has worked – time will tell with that – but her work does indicate that big data studies can produce on-the-ground, tangible results.
The Future
As more studies like Snyder’s get done – and we get a better understanding of what exercise in a healthy person looks like – we should be able to show just how messed up the process is in ME/CFS. That should help researchers like Nancy Klimas, David Systrom, the folks at Workwell, Betsy Keller and others focused on exercise get funding for more intensive ME/CFS exercise studies. If a single bout of exercise affected the levels of over half the molecules tested in this study, one wonders how many molecules a two-day exercise test would alter in ME/CFS.
This study is just the beginning of the in-depth molecular explorations of exercise we’re going to see happen over the next couple of years. If this is what we get out of one 38-person deep dive into exercise, the future may be bright.







This study is very good. WHen I recovered from ME/CFS I could run on a treadmill for 45 minutes, I was completely exhausted and it took a while to recover but at least I did recover from it which showed I had recovered from me/CFS. Now since a small relapse, I would not dare try it. I just wish I had got the molecular markers when I was recovered so I could compare them now and see what went wrong. finding out why we can’t exercise properly if at all will surely help treating the condition and make life easier for us all even if it does not cure us.
No kidding Martin…How great it would have been to have you in one of these studies. Hopefully they will provide a bunch of markers that can be tested in people ME.CFS – without having to study every molecule in the book.
That’s my hope. I think we will have to do big intensive studies like this – but hopefully once they’ve been shown to work in these studies they’ll be available for us. Then, we’ll tease out the factors that result in the exertion problems in ME/CFS.
How did you recover Martin?
Martin, if you don’t mind me asking, what do you attribute for your ME/CFS recovery?
Dakota and Freda. I don’t really know. I had PVFS for around 16 years with a bunch of mild or moderate symptoms. I tried various things which made no real difference (abx, b12, thyroxine). Then I just realised one day my symptoms had gone. No explanation really. I had some sort of relapse last month though out of the blue and am wondering if I have the CV asymptomatic which caused it so I am waiting for the test result. It is prob negative I guess. One thing I found out. ME is supposed to be a slight exertion which causes muscle fatigue which takes a few days to recover. Mine never took that long. In fact I think my muscles always functioned even though they twitched after exercise in those days.
Martin, vaccines triggered my ME/CFS. I wonder if you were getting flu shots or other shots during the time that you relapsed? And that you weren’t getting shots when you had recovered?
Sandy, no I have never had a flu shot in my life. I also got my corona virus test result today and it is negative which is what I thought. Actually, I am better than I was, there are less myoclonal jerks and tremors at night. The mental fatigue seems to have disappeared too. I have been taking b12 pills so whether that is the reason I don’t know. I have had some other vaccines since getting ME/CFS but they did not make my ME/CFS worse or relapse that I can remember. I am sorry you got ME/CFS after a vaccine. I know this does happen to people. Stay Safe.
Martin
Interesting to hear of your recovery. I have also sort of recovered. I was never as badly affected by ME/CFS as some unfortunate people. But from been a keen mountaineer, I was unable to undertake any exercise without it wiping me out for days.
I am now able to walk all day in the hills, and to jog and cycle too. But I still have to be very careful. I can take part in long duration activities at a sensible pace, but the symptoms can come back if the exercise intensity is too high. So I am clearly not fully recovered recovered, but recovered enough to get out in the hills again provided I am sensible. I wonder if you would comment on if have recovered enough to take part in high intensity exercise? I would be very interested to compare your experience with mine.
Ben
Yes, I was able to exercise quite strongly during my almost complete recovery. I went to the gym in London for a week and went on the treadmill and although I was 48ish then and not in shape much, I was able to run for 45 minutes about 3 times in that week. I was exhausted at the end of each but I recovered without any ME symptoms then. I could probably still jog now for a while but I would not want to risk 45 min on a treadmill. I really want an exercise test and heart function test but my GP never suggested anything like this. I also went mountain trekking with a heavy backpack when I felt recovered. No problem whatsoever. I have no idea why I used to have OI and cognitive problems when exercising or walking about. They have all gone now except soetimes I feel some cognition mental fatigue for a short while sometimes. It is good to know people can recover from ME/CFS.
I too recovered what I thought was 100% after 25 years of getting better and relapsing. Activity brought me to bed and house bound with periods of feeling ok, slowly improving. So after 3 years of normality, this Feb I walked everyday long distances, rather than have a rest day and ME\cfs has come back with a vengeance. I suddenly hit the wall. I’m doing the keeping tabs on my pulse rate, keeping it between 80 and 75. But still after 5 months of lying around, I have been slowly getting worse. Perhaps I am on a plateau but can’t look after myself again. I’m still taking imunovir, glutathione, vitB12, zinc, magnesium, low dose naltrexone, NAC. All things I thought had made me better for ever. Sadly not.
SubQ IgG has changed my life. So how is this explained? I am progressing through the Levine protocol month 4 now after a year if SubQ. Why? I have a skewed autoimmune profile and am using AI doses.
My heavy legs and exercise intolerance is almost gone. I have some. What gets in the way is the EDS pain and I get stuck in hyper pots attacks still at times. These do seem mast cell driven. Mast cells improved moving to arid desert because environmental mold was constantly triggering me to the point of bedridden by the time we moved.
My brain was mush and I could not put one thought in front of the other to even pack my bags. The mold season just extended from 6 months to about 11 months a year where I lived on east coast. No breaks for my brain. That mostly stopped here.
Then the IgG miracle (for me). Doctor and I rated me about 70-80% now and I don’t’ know how much EDS pain impacts that rather than CFS-ME. I went back to recumbent last doing 50 minutes before this last POTS/MCAS attack. Now waiting for BP to stabilize.
Nervous system can be fragile but I also am in process of divorce and my mother just died most likely due to COVID and horrible death. So don’t know how much of this is normal or CFS-ME remnants that can flame back up. Doesn’t seem to impact exertion level I have reached.
So I am wondering why more are not trying this and how it is working. I guess I have CFS-ME still and maybe always did by a certain point in my life though highly functional. Now I am getting back to more highly functional with CFS-ME. Maybe this is it. I don’t know.
That’s great to hear, Sue. Subcutaneous IgG is the kind that appears to be most recommended by ME/CFS doctors. The problem has been getting it approved by insurance or paying for it out of pocket.
Do you remember which of your immune factors were off. (Low IgG subclasses?) Did you get tested for peripheral neuropathy? and if so did that help?
We did a 3-part IgG series a while ago:
An IVIG Chronic Fatigue Syndrome (ME/CFS) / POTS Treatment Success Story: IVIG#1 – https://www.healthrising.org/blog/2018/08/11/ivig-treatment-chronic-fatigue-syndrome-me-cfs-pots-success-story/
Are Chronic Fatigue Syndrome, POTS and Fibromyalgia Autoimmune Dysautonomias? IVIG #2 – https://www.healthrising.org/blog/2018/09/13/ivig-chronic-fatigue-syndrome-pots-fibromyalgia/
The Case for IVIG Treatment in Chronic Fatigue Syndrome (ME/CFS), Fibromyalgia, Small Fiber Neuropathy, and POTS: IVIG#3 – https://www.healthrising.org/blog/2018/09/13/ivig-chronic-fatigue-syndrome-pots-fibromyalgia/
R here, Cort. No low IgG classes. This was for autoimmune disease. I have autoimmune urticaria, SCL-70, titers not pointing specifically to an known AI disease and as my doctor sees with people who have my profile, small fiber neuropathy. I don’t have AI dysautonomia as per Mayo test. I fit the profile that is responding as per my doctor. It was approved with multiple peer reviews until insurance finally let go and continues to re-approve. I change insurance companies in December and since I have gone from 20-40% functioning to 70-80% functioning (not factoring out EDS from lifestyle quality) and for over a year now, I hope it will be deemed medical necessary by next insurance company. The IgG is addressing CFS-ME specifically for me. PEM. I don’t have it. If I did a spin class, then I would guess I would struggle because I’ve been bedridden/sofa bound 15 years prior to this last year and I am 58. IDK what would have happened if I had this treatment earlier. I might recover from the class though. I’m not at level of working out I was before illness. I worked out 5 days a week and very hard before. Now I cannot stop pushing myself because it seems to be in the DNA of my mindset. I don’t slowly bike, rather consistently fast. I don’t push my limits either like in a race. I hesitate less about risking exertion in every day activities but the mindset is also there that I will fail.
WHo is the doctor you are working with getting the IgG?
Hi! Would you be open to get in contact with me regaeding your treatment? I am very severe and want to try your approach, would appreciate your insight a lot! Here is my contact: christoph.stroeck@stroeck.at
This reminds me of the processes behind the blood passport/doping suspicion list in the Tour de France. Given the extreme demands of the sport, athletes’ blood changes through out the race. Although an outright banned substance may not be detected, an athlete’s blood may still not track as expected. The further away from an expected normal result the greater the suspicion of doping. A scale of 0 to 10 was used in the Tour de France. From what I remember (based on the occasion the list was leaked to the press) that technique proved pretty accurate. Scoring above 6 out of 10 correlated with being busted for performance enhancing drugs (at some point). I’m not sure ME/CFS is a great analogy for performance enhancing drugs though. It may be long bow to draw, but the similarity of the technique, and the presumed robustness and utility is encouraging (even when athletes and their doctors were actively trying to hide their condition). Particularly considering that ME/CFS sub-sets may be non-heterogeneous in pathology. The potential benefits for symptom sharing illnesses like GWI is hopeful too.
I think that’s a great analogy.
One question is whether people with ME/CFS are worse off in the same way as healthy people with altered molecular levels; i.e. if their levels of leptin, hippuric acid, BCAA are just much worse or if they’ll have entirely new things show up. I imagine and hope the second is true. It would be really nice if one molecule or a couple of molecules were just off the charts bad…Let’s hope that happens and a clear pathway to what’s going on emerges.
There could be nothing wrong in CFS body at molecular level. Instead, it could be just that CFS brain is overly sensitive to the molecular changes.
All exercises, regardless of intensity, brings about molecular changes. And CFS brain could be perceiving small changes (from walking from the bedroom to the bathroom for example) as a catastrophic one and put the brake on in order to prevent you from doing more damage. If that’s the case, then you may not learn much just by looking at the molecular changes because they’ll look no different than those in non-CFS bodies.
Teasing out where this is coming from is going to be interesting. We know it shows up in the body in the form of problems with aerobic energy production – so they are going to find things in the body. But could it start in the brain? Could the brain not be sending the signals to the muscles that it should? Is an over-reaction in the brain sending pro-inflammatory cytokines soaring causing pain and fatigue? It’s been proposed that the fatigue in ME/CFSD is “central” i.e. that its being produced by the brain Or is it a blood vessel problem or a mitochondrial or ? problem? There are so many options….
I have mild ME and nitric oxide for sure plays a role in my case. I get less PEM when I get some sun.
This study is interesting, but it is the 24 hour and 48 hour differences that are of particular interest among those of us who wish to uncover the reasons for PEM. Notably, these time frames match that of various muscle repair functions (Vascular endothelial growth factor and the like), but weren’t discussed in this study.
The relationship between peak VO2 and integrin pathways is of particular interest to me, given some of my hypotheses, but the authors don’t seem to discuss it much at all.
Aside, I’d like to point out that focal adhesion dysruption has been previously discussed:
https://phoenixrising.me/research-2/the-pharmacogenomics-papers/the-pharmacogenomics-studies-on-chronic-fatigue-syndrome-mecfs-iii-the-gene-expression-studies
The most important time to look is an interesting question. Dr Klimas looks longer but also has focused most of her attention during and following exercise where shes found significant differences as have Systrom. Workwell, Keller and others. Intuitively it makes sense to focus on the inciting factor but you’re right there is that mysterious delayed symptom flare that often occurs that certainly needs to be accounted for.
I imagine we will see longer study times in the MoTRPAC studies which seek to explain the molecular changes that occur as a result of exercise – which extend several days even in healthy people, showing up in achy muscles, fatigue, etc.
24 hour delay is typical of DOMS. There has been a paper that investigated and concluded that there is no micro trauma in PEM muscles, but I think it only means that the investigator did not see it. It could be so minute in CFS that you may not be able to easily see, and maybe a few strands are enough for CFS to react violently.
As for 48 hour delay, I just had one after a bike ride. It seems to me that the happy chemical (dopamine?) from exercise suppresses PEM. And when that happens chemical dissipates PEM resurfaces. 48 hour delay is typical for me whenever I do a strenuous exercise. Well, strenuous for a CFS patient, that is.
Sorry typo: “when that happens chemical” -> “when that happy chemical”.
TK, I believe I am very similar to what you are describing, passionate cyclist for close to 20 years diagnosed with possible Fibromyalgia about 10 years ago because after tons of tests nothing else is “wrong with me” (other than history of sinusitis which is mostly under control) and almost 100% of the time when I ride I have a delayed (48-72 hour) flare up of my fibromyalgia pain and as I like to describe it to my doc, “
flu like symptoms after I exercise. I can string together a few good months of consistent riding, even rides up to 50-60 miles, but then something triggers, something Not sure what, and I fall back and can’t do much of anything exercise wise, for days/weeks/months. I come back every time, starting over with short walks building into slow short rides but inevitably it happens again and again. I’ve tried everything, medications, supplements, read everything and been to many many doctors. I have many high level cycling friends, and even am friends with a few former pros that I’ve talked to that had access to the worlds top doctors that understand exercise physiology, still nothing. I have been searching for years for an answer to that what may be happenIng to the body when I exercises..with no luck. Heck, I know I’m luckier than most because I can exercise at all, but I seem to be getting worse as I get older (47 now), and there are so many days that I have made excuse after excuse to for years to my friends why I can’t make the ride today, and days I feel like selling my bikes, but I am determined to not give up, yet!
This study is one of the first that speaks to what I’ve been saying and the questions I’ve been asking for years. I’m hoping there are some more answers soon!
This study is fascinating to me because I have noticed that using supplements that high performance endurance athletes use, seemed to help my sister and I the most . That makes me laugh, because my idea of high performance endurance exercise is walking down the hall! Using things like BCAAs and L-Arginine + L-Citrulline (to boost nitric oxide) helps me, but they irritate my dysbiotic gut. Right now I’m trying epicatechin, because it increases nitric oxide production, increases mitochondrial biogenesis, increases angiogenesis, decreases myostatin, increases follistatin, increases oxygen extraction, increases exercise performance in rodents, and is thought to preserve lean muscle mass. I have had ME/CFS my whole life, so I’ve never really been able to build muscle despite trying many different methods. I haven’t noticed much change yet, but I will give it a full four weeks. I’ve also been using a chlorogenic acid supplement (green coffee bean), because it is supposed to keep you in aerobic metabolism. I hoped it would keep me from producing so much lactic acid, when I have to be active. I think it has resulted in slightly less PEM after activity, and my weight is more what I would like it to be, so I think I’m burning more fat or sugar, and less muscle. However, the treatment that always helped me (and my sister) the most was hydrocortisol. With doses of hydrocortisol (1-4mg), I was functioning almost normally, and not suffering the PEM punishment for normal activity. I always thought it was strange from a biochemical standpoint to add a “stress hormone” to a body that’s stuck in it’s sympathetic nervous response, especially since symptoms of high levels of cortisol are:
Fatigue
Irritability
Headaches
Intestinal problems, such as constipation, bloating or diarrhea
Anxiety or depression
Weight gain
Increased blood pressure
Low libido, erectile dysfunction or problems with regular ovulation or menstrual periods
Difficulty recovering from exercise
Poor sleep
Sound familiar?! It’s strange to me that the symptoms for both too much cortisol and too little cortisol would be the same. To me, it seemed like my body was saying “I need cortisol, but I’m too tired to produce it! Help me!” So, I would take the hydrocortisol and my body would say, “Ahh that’s just what I needed! Thanks!” The symptoms listed above would actually go away with the addition of more cortisol to the system. Go figure!
Great work as always Cort! I look forward to delving further into the study.
There seems to be a connection for me, between amino acids and energy. I feel I have to eat meat, fish and eggs otherwise I’d grind to a halt.
I used to have to eat nuts, like peanuts and almonds, otherwise I’d start to shut down. However I became intolerant to nuts and that caused huge problems for me. Dairy would also give me energy before I became intolerant to that.
In my previous life, as a vaguely normal person, I didn’t eat very much protein and was fine. In fact eating meat, eggs etc was too much and I was largely vegetarian. In fact the less I ate, the more energy I had.
I have a feeling the whole cortisol issue is very relevant to me now. I wonder if I’m boosting my cortisol with the dark chocolate I’m eating at the moment. I’m no longer wired, I’ve sorted that at the moment – so I am able/need to eat chocolate to function.
I can’t eat milk chocolate, as the dairy appears to cause me to faint (drop in blood pressure?) and does terrible things to my mental health.
But I do have to provide things – protein and dark chocolate, amongst other things, to just function – which luckily, I’m managing to do fairly well.
K: Thanks for an interesting post. Would you kindly provide the dosages and when & how taken for the supplements that helped you, including BCAA, L-Arginine, L-Citrillune? Also, how did you come to take hydrocortisol, which I gather is a prescription, and why did you stop? (I’m still looking for things that help after 28 years of CFS, which hit me at age 42.)
Thanks, too, to Cort for another superb post.
“A substance called myeloperoxidase (MPO). Secreted by neutrophils and a marker of oxidative stress, it turned out to be an important bridge to other pro-inflammatory and growth and protective factors.”
MPO? That points to neutrophils going into NETosis, the process where neutrophils immune cells “burst” and throw out their DNA that forms nets around all things sticky or thin and creates plenty of reactive oxygen and HClO. That later one is a very strong inhibitor of the mitochondria and all respiratory things.
If that would happen in a “dirty” environment with plenty of things to clean up (like I suspect is the case in the finest capillaries in ME) then the patient is in for a nasty ride IMO!
With constricted veins, unflexible RBC and plenty of pro-inflammatory debris this would give IMO a very sharp rise in oxidative stress that would be far far higher then what is seen in a healthy person. All once again would be worst in the smallest capillaries.
I came up with one thing that could make it worse, a lot worse probably: if MPO were to bind with the already heavily strained RBC. And guess what?
A paper titled “Red Blood Cells Serve as Intravascular Carriers of Myeloperoxidase” with first author “Matti Adam” (I learned to refference papers like that to get through WordPress’ spam filter):
“Red blood cells serve as intravascular carriers of myeloperoxidase. … In summary, we find that MPO binds to RBC membranes in vitro and in vivo, is transported by RBCs to remote sites in mice, and affects endothelial function as well as systemic vascular resistance.”
A paper titled “The Effect of Myeloperoxidase Isoforms on Biophysical Properties of Red Blood Cells”:
“Myeloperoxidase (MPO), an oxidant-producing enzyme, stored in azurophilic granules of neutrophils has been recently shown to influence red blood cell (RBC) deformability leading to abnormalities in blood microcirculation.”
A paper titled “Binding of human myeloperoxidase to red blood cells: Molecular targets and biophysical consequences at the plasma membrane level”:
“Myeloperoxidase (MPO) is an oxidant-producing enzyme that can also bind to cellular surface proteins. We found that band 3 protein and glycophorins A and B were the key MPO-binding targets of human red blood cells (RBCs). ”
“These findings suggest that MPO functions as a mediator of novel regulatory mechanism in microcirculation, indicating the influence of MPO-induced abnormalities on RBC deformability under pathological stress conditions.”
=> IF the last quote could be confirmed we would have a potential VICIOUS CIRCLE FOR POOR DEFORMABILITY OF RBC!!!!!
A paper titled “The effect of myeloperoxidase isoforms on biophysical properties of red blood cells”
“Myeloperoxidase (MPO), an oxidant-producing enzyme, stored in azurophilic granules of neutrophils has been recently shown to influence red blood cell (RBC) deformability leading to abnormalities in blood microcirculation. Native MPO is a homodimer, consisting of two identical protomers (monomeric MPO) connected by a single disulfide bond but in inflammatory foci as a result of disulfide…”
=> Yay, a potential mechanism to rip through glutathione stores:
A paper with title “Oxidation of neutrophil glutathione and protein thiols by myeloperoxidase-derived hypochlorous acid.”:
“Neutrophils, when stimulated, generate reactive oxygen species including myeloperoxidase-derived HOCl. There is an associated decrease in reduced glutathione (GSH) concentration. We have shown that neutrophil GSH levels decrease on exposure to reagent HOCl, whereas the equivalent concentration of H2O2 had no effect.”
And a paper with title “Production of glutathione sulfonamide and dehydroglutathione from GSH by myeloperoxidase-derived oxidants and detection using a novel LC–MS/MS method” telling enough.
There is one thing to be said about NETosis however: it is one of the best possible immune reactions for the ratio “inflammation / immune clean up and repairing activity versus permanent damage”. That may be a key reason why we have so few “overt” permanent damage to show for.
In more plain English, note that it OVER simplifies things:
Assumption: MPO rises immediately and quickly during exercise in healthy people. Given Cort’s summary this seems likely but the full paper is locked behind a pay-wall so I can’t confirm this.
* MPO rises quickly when anybody exercises
* MPO together with inflexible RBC gives plenty of ROS, generates HOCl that inhibits mitochondria, depletes glutathione, impacts microciruclation and further worsens the flexibility of RBC
* Having constricted capillaries and stiff RBC makes it IMO a lot easier for MPO to stick to the RBC, making this effect *a lot* worse in ME patients.
* Glutathione levels are depleted when this bonding of RBC and MPO gets intense and things get even worse when blood flow is hampered a lot.
* As such, RBC are unable to defend themselves well against massive oxidative stress. Oxidative stress and strongly mitochondria inhibiting HOCl (bleach) flood the capillaries and nearby cells.
=> This IMO could easily explain the very quick onset of inflammatory feelings, vast pain surges, mental confusion and a deep air hunger within minutes after ME patients start exercising.
=> The delayed fall out of this would be massive and would be strong over the categories short term (immediate), mid term (hours to days) and long term (weeks to months). That obviously includes PEM.
“The production of long-chain polyunsaturated fatty acids that increased immediately post-exercise drew interest: did they reflect inflammation?”
The quick production of long-chain fatty acids is IMO a strong marker of the body going into deep anaerobic functioning.
When glycolysis (the anaerobic step of energy production) goes too quick, plenty of NADH piles up and NAD+ gets depleted. The first creates massive oxidative stress when piling up too high. The later makes near all energy production impossible when dipping too low.
The “classic” way of the body to handle that is to produce lactic acid (plus analine in the brain) that needs to be detoxified in the liver. That detoxifying however costs plenty of oxygen (that obviously is short when the body goes through anaerobic exercise).
An alternative route to get rid of excess NADH is to convert NADH to NADPH and create fat out of it. That also regenerates NAD+ so that energy production can continue longer.
The benefit of reducing excess NADH and replenishing too low NAD+ that way is that it does not cost oxygen. It does cost ATP however so it likely will be done outside of the zones with the worst energy needs.
That could be also the liver: detoxifying lactic acid and analine also costs energy and ATP. That can lead to non alcoholic fatty liver disease in the long term in some ME patients. The alternative however might be worse.
Important note to researchers: the reconversion of excess NADH to NAD+ occurs more efficiently (costing less scarce ATP) *in* the mitochondria. It would be *very* interesting to try and track the fatty acid production during exercise in the mitochondria if possible.
Something bizarre that happens to me but I’m not sure if it happens to others is that my ability to exercise is radically better if I exercise after 4PM. If I do even moderate walking in the morning I’ll crash by the afternoon, but if I jog in the afternoon i’m good.
Something bizarre that happens to me but I’m not sure if it happens to others is that my ability to exercise is radically better if I exercise after 4PM. If I do even moderate walking in the morning I’ll crash by the afternoon, but if I jog in the afternoon i’m good.
My energy is a lot better mid to late afternoon. That’s when I can walk my dog reasonably well but still for no longer than 15 minutes non stop. I then have a short sit in the park and eat a very small amount of carbs and fat and then recover enough for just 5 minutes more.
I would love to know what is EXACTLY happening at the molecular level in a person with ME/CFS but I am sure it would vary from person to person. As I live in the UK I just cannot imagine the NHS taking this too seriously though. The energy/muscle connection I feel is critical to this illness, well it has always been that way for me and I have had this illness for over 20 years now and as I am 72 I find that the energy/muscle problem has been a lot worse this past 2 years.
Thank you Cort so much for keeping us updated, it is really appreciated.
I’ve felt exhausted – NOT SLEEPY – for 40? plus years. I actually felt much worse in the 1980’s than now. Every day was like having a combination of the flu and a mid level hangover that diminished as the day progressed, but the exhaustion didn’t. I started using caffeine which helped some for the exhausten then 70 mg of dextroamphetamine every day since about 1990 (which never did increase my energy level nearly as much as ALL the doctors thought they did) and were less effective as time passed but we’re better than nothing. Seems that doctors think everyone has the same tolerance!! A new doctor decided that I didn’t need them so no prescription. I’m hurting. Too exhausted to search around for replacement.
I’m writing this to see if anyone has heard of anyone (me) feeling NO energy before beginning very hard exercise ie breaking up a concrete sidewalk with a sledgehamner but 1/2 to 1 hour Afterwards getting a LOT of energy.