


Cortene has one product – CT38 – that is focused entirely on ME/CFS.
Cortene’s appearance in the chronic fatigue syndrome (ME/CFS) world a couple of years ago was like a breath of fresh air. The small drug company wanted to do something unusual in the annals of this neglected disease – run a clinical trial – and not with a drug anyone was familiar with. They weren’t trying to repurpose a drug used for other diseases: instead, they proposed testing a novel drug not being offered in any other disease.
We last heard from Cortene almost two years ago when they announced the results of the 14-person InTime trial which took place at the Bateman Horne Center between July 2018 and April 2019.
Since then, one change has affected me personally. In 2019, I was a blogger reporting on Cortene’s efforts – now I’m a member of Cortene’s advisory board. That means that should Cortene make it through the long and treacherous drug approval road and become an FDA-approved drug for ME/CFS, I could financially benefit. My commitment is to be as objective as possible and support Cortene’s efforts, as I have supported other efforts that have the potential to help people with ME/CFS.
In 2019, Health Rising reported that Cortene had concluded that its drug, CT38, was safe, that its hypothesis – that the CRFR2 receptor was involved in ME/CFS – was validated, and that the limited CT38 doses given in the trial could bring about a lasting improvement in symptoms.
Now we have the full deal – a peer-reviewed paper detailing exactly what happened. Strap yourself in – you’re in for a kind of wild ride.
The Study
The title, “Acute corticotropin easing factor receptor type 2 agonism results in sustained symptom improvement in myalgic encephalomyelitis / chronic fatigue syndrome”, doesn’t indicate how novel Cortene’s approach is, but it does present something rather unusual: it states that the treatment delivered “sustained symptom improvement” in ME/CFS, and you don’t see that every day.
The CT38 trial is specifically about myalgic encephalomyelitis/ chronic fatigue syndrome (ME/CFS) but it’s also potentially about other chronic disease states. It’s all about “homeostasis” – the ability of the body to respond to a threat and then return to a normal resting state. Cortene believes the pathway they’ve targeted – the CRFR1/CRFR2 pathway – plays a special, even fundamental role in homeostasis.
Cortene became particularly interested in ME/CFS because it presented the most extreme case of “dyshomeostasis” they could find. Where else, after all, do you find dramatic declines in functionality, such difficulties responding to stressors such as exertion, mental activity, even such seemingly innocuous things like lights or sounds?
Cortene believes a neuronal “switch” exists in the limbic system which turns the threat response on and off. The switch is found in the corticotropin-releasing factor (or hormone) system which regulates serotonin. Two receptors – the CRFR1 and CRFR2 – are involved.
Under minor stress or at baseline, CRFR1 dominates; sitting on the surface of GABA-producing neurons, the CRFR1 receptors trigger the release of GABA – a neurotransmitter that inhibits serotonin. During these low-stress periods, CRFR2 is inactive and remains embedded inside serotonin neurons.
Under high (or prolonged) stress levels, the two receptors switch places. CRFR1 drops inside the GABA neurons and goes dormant. CRFR2, on the other hand, pops up onto the surface of serotonin-producing neurons and triggers the release of serotonin.
Once the stress/threat is resolved, the CRFR2 receptors should drop back into the neurons and the CRFR1 receptors should return to the surface – thus restoring the calm state one usually associates with health.
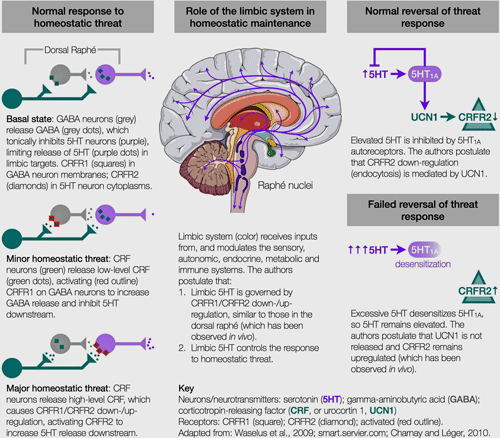
Cortene’s hypothesis.
Cortene doesn’t believe that happens in ME/CFS. They believe the CRFR2 receptor remains upregulated, leaving the stress response system in a hypersensitive, easily-provoked state – which can have major consequences. From the metabolic to the autonomic, to the immune, to the endocrine systems, the authors cite papers demonstrating the wide reach of the CRFR1/CRFR2 system. They believe everything from movement problems, to energy issues, to stimuli sensitivities, to the cognitive and emotional ramifications of ME/CFS, could potentially be caused by an upregulation of this system. Because the upregulation exists on the neurons, the type of symptoms any one person with ME/CFS experiences would depend on which neurons are affected.
The fact that we can’t actually measure what’s happening with the CRFR1/CRFR2 switch Cortene is attempting to turn off means problems with it will not show up in the blood tests – which, potentially fits ME/CFS – the disease that perpetually seems to fall between the cracks – quite well. The only way to assess if CRFR2 has been turned on or off is to hit it with a drug. Fortunately, CT38 is a good drug to do that as, so far as we know, it only interacts with CRFR2. If an effect with this drug is seen then, we can assume that it was because CT38 impacted CRFR2.
Cortene’s Approach
Cortene chose an interesting way to damp down what they believe is an overactive CRFR2 pathway. Instead of using an antagonist to block the pathway – as is usually done – their objective was to use an agonist to overstimulate the pathway.
It might seem strange to attempt to overstimulate an already hyped-up pathway, but that approach simply takes advantage of how neurons function. If they get overstimulated, they basically fold; i.e. they turn themselves off. Faced with the CT38 agonist, the neurons should move the CRFR2 receptors back inside the cell – returning it to its normal resting state – and turning off a multitude of problems.
Cortene’s approach is different, but it potentially has some real advantages. Pathway blockers (antagonists) may stop the final expression of the pathway, but because they don’t turn it off, they often need to be taken for life. An agonist which overstimulates the pathway, on the other hand, might allow it to reset itself and return to a healthy state.
The Trial
The 14-person open-label trial took place at the Bateman Horne Center under the direction of Dr. Lucinda Bateman and Suzanne Vernon Ph.D. With some animal trials and just one human trial done, Cortene didn’t have a lot to go on regarding dosing, and several dosing regimens were tried. The total exposure to the drug each person would receive was estimated from animal studies. Four dose levels were used in the trial. In general, treatments consisted of three, 3.5-hour infusions.
Different infusion rates and times were tried.
The primary endpoint compared the mean 28-day total daily symptom score (TDSS) before and after treatment. The TDSS consisted of 13 individual, patient-reported symptoms, each assessed daily throughout the trial. The 36-Item Short Form Survey was also done. Metrics taken from a Fitbit (activity, heart rate, sleep) were taken (but the trial ended up being too small to use them).
Of the 14 people in the trial, one discontinued the drug after the first treatment produced headaches, dizziness, flushing feelings, shortness of breath, and some other symptoms, but remained in the trial until the planned exit. Another had a problem giving blood samples, and received only 2 treatments (instead of 3), but remained in the trial until the planned exit.
This was an exploratory study in several ways. The first goal was to determine that the drug was safe. Next, the investigators wanted to learn if it had the potential to be effective. They also wanted to get some idea about dosing. Determining optimal dosing was out of the question – the trial was too small for that – but observing how people with ME/CFS reacted to the various doses given turned out to be illuminating.
Results
The twist came learning how to properly administer the dose – a key objective of the trial. Providing more of the drug achieved better results – so long as it was done in the right way.
Providing higher doses of the drug (concentrations>0.25 ng/ml) over shorter periods of time didn’t work. Providing lower doses of the drug (<0.25 ng/ml) over longer periods of time did. When giving the drug in smaller amounts, Cortene was able to provide the drug for longer and get better results (26% improvement -mean TDSS). The same was true with regard to the SF-36 functional status assessments. (It should be noted that only ten people in the study received what turned out to be the correct dose; i.e. Cortene attempted to get statistically significant results – and did get them – from a quite small sample size – not an easy thing to do. )
Imagine a beaker (the neuron), filled with a liquid, whose level represents the degree of CRFR2 activation present. Overstimulation is achieved by filling the beaker. The almost full beaker in ME/CFS overall, and in the more severely ill ME/CFS patients, in particular, should only require a small amount of liquid to fill the beaker; i.e. the worse off you are – the less of the drug you need.

The more severely patients need less of the drug to overstimulate the neurons and turn them off.

The less severely ill need more of the drug to overstimulate the neurons and turn them off.
When the drug was given in this manner, the more severely ill patients saw – as expected – more symptom improvement with less of the drug. This also explains why people with ME/CFS reacted more strongly to lower levels of the drug than did the healthy controls in a prior study. The ME/CFS patients’ CRFR2 receptors were already primed, so to speak, to respond to the drug.
This unusual pattern suggests that something is indeed up with the CRFR2 pathway in people with ME/CFS. Hunter Gillies, MD, InTiME’s medical monitor, asserted that, “These data support there being a pathological hypersensitivity in the CRFR2 pathway.”
Note that this runs opposite to what happens with most drugs. More severely ill patients usually need more of a drug – not less. This is a counterintuitive result that obviously messes with some of our medical norms. Given that ME/CFS seems to mess with just about all our medical norms, perhaps it’s fitting that a drug that does the same had a positive effect in this trial.
Side Effects
The authors believed that the side-effects initially seen – almost all of which reflected ME/CFS symptoms – resulted from the CRFR2 problems that were already present in ME/CFS. Once the investigators were able to determine the proper dosing over time that worked for patients, the side effects virtually disappeared.
Longer-Term Results
CT38’s half-life in the body is only 1.5 hours, and is essentially gone from the body after six or so hours. Dr. Bateman’s follow-up data (from 1 to 2-years) from 9/14 study participants suggested that some long-term shifts in the CRF2 pathway may have occurred. They reported subtle but recognizable long-term improvements in a wide range of symptoms (sleep, brain fog, appetite, activity, and PEM (crashed less often, recovered more rapidly).
While the participants are clearly not over ME/CFS, that’s not a bad result for a couple of first-time treatments done over a year ago in a small exploratory study of a novel drug that’s never been tested in ME/CFS before. Dr. Bateman MD, one of the co-authors of the paper, and the principal investigator in the clinical trial, reported:
“The persistent improvement in symptoms over weeks using a limited exposure is encouraging. Many patients are still showing signs of improvement almost 2 years after treatment. In fact, a few patients expressed a desire for ‘just a little bit more drug’.”
The Gist
- Several years ago, Cortene presented the ME/CFS community with a surprising commitment – to produce a clinical trial using a novel drug.
- They proposed that a key stress response pathway called CRFR1/CRFR2 had become dysregulated in ME/CFS and was producing a hypersensitive, easily provoked stress response.
- They believe that the surfaces of serotonin-producing neurons in ME/CFS had become overloaded with CRFR2 receptors – causing them to fire at the slightest stimuli. These twitchy neurons could be affecting systems across the body. Cortene proposed that the kind of ME/CFS one has might depend on which neurons are affected.
- Cortene’s unusual approach was to take advantage of an interesting neuronal property: they would attempt to overstimulate the neurons with CT38 – which should cause them to stand down and allow a healthy state to resume.
- This is strikingly different from how many drugs work, which try to block the pathway in question. That course, however, leads to a chronic drug regimen that essentially never stops. Cortene, on the other hand, hoped to reset the pathway in hopes that it would remain in a healthy state.
- Since Cortene presumed that the neurons of the most severely ill patients were the most activated of all, it stood to reason that the more severely ill patients would need less of the drug, while the less severely ill needed more.
- The 14-person, open-label trial done at the Bateman-Horne Center was done to determine if the drug was safe, if it might have efficacy, and to learn about dosing regimens. Several different dosing regimens were tried. In general, the trial consisted of three several hour-long infusions.
- The study found that more of the drug was better – but only if the drug was administered in a certain way. Providing higher amounts of the drug over shorter periods of time produced side effects. Providing lower amounts of the drug over longer periods of time did not produce side effects and resulted in symptomatic improvement.
- When the drug was given properly (a smaller concentration of the drug over longer periods of time), patients reported about a 25% improvement of symptoms. Dr. Bateman indicated that many of the participants reported subtle but noticeable improvements of their symptoms over the 1-2 years following the trial. Dr. Bateman called the study results “encouraging”
- As Cortene suspected, more severely ill patients needed less of the drug to receive improvement than did less severely ill patients.
- The trial provided validation for Cortene’s hypothesis that the CRFR1/CRFR2 pathway was, as Dr. Hunter Gilles put it, “pathologically dysregulated” in ME/CFS.
- In an interview, Cortene proposed that partial downregulation of the CRFR2 receptor had indeed occurred, and that further trials would determine if they could fully return it and the stress response system in ME/CFS to a healthy state.
- Cortene hopes to embark on a 60-person $4 million trial next.
- Please note that my situation with Cortene has changed. Two years ago, I was a blogger reporting on their efforts. Now I am a member of the Cortene Board. That means if Cortene were to get FDA approval for their drug, I could benefit financially. My goal is to be as objective as possible.
“Cortene worked long and hard to raise the money to fund InTiME. I hope this paper piques the interest of many and makes it easier to raise money for a larger trial. I also hope the results of this trial get the attention of pharmaceutical companies to show them that treatment trials for ME/CFS are possible, feasible, and urgently needed.”
There’s also the broader issue of whether Cortene’s approach could ultimately be applied to a broad swath of chronic diseases that feature “disturbed homeostasis” including fibromyalgia, long COVID, and many others. The potential reach of the serotonergic system is so broad that a dysregulation could impact many systems. This is the first time, I believe, that anyone has directly attempted to affect this system in this way.
The trial demonstrated that CT38 is safe, suggested that the CRFR2 pathway is indeed impacted in ME/CFS and that the drug has promise. Larger studies with more extended dosing regimens are needed to determine how effective this unique approach to ME/CFS may be.
_______________________________________
Cortene Interview
How did you latch onto CT38 in the first place? Were you thinking in the broad terms that you are now – of a drug that might have the potential of restoring homeostasis in stress-response disorders?
Not at all. CT38 had been shown to prevent muscle wasting, but by an unknown mechanism. The search for the mechanism, led to fundamental biology involving control of calcium, and a vast body of literature on the CRF system, and its control of serotonin (misunderstood to be the ‘happy hormone’) in the limbic system (historically relegated to an emotional role). Further studies showing that various body systems input into, and receive output from, the limbic system led to the ideas expressed in our paper, and partially ratified by the trial (and supporting animal and Phase 1 data).
Can you explain how CT38 is different from other drugs you’ve worked on and what challenges it presents?
The last 40Ys of drug development have focused primarily on the development of drugs for chronic conditions. The paradigm involves identifying an abnormality in a bodily fluid (e.g., cytokines in the joint of a patient with rheumatoid arthritis), and then masking this with a drug (an antibody in the case of the wayward cytokines).
Three caveats: (i) the reason for the joint cytokines remains unresolved, so the drug has to be taken chronically; (iii) the chronic use of the drug results in drug concentrations in the body that optimize between efficacy and side-effects, so greater efficacy is not possible (e.g., 35-39% of patients achieve at least 50% improvement in the number of tender/swollen joints, versus 8-10% with placebo), and (iii) the patients exhibit other symptoms unrelated to joint cytokines that are not resolved by the cytokine-masking drug, and may require other partially effective and side-effect-limited drugs.
This is critically different with CT38. It attempts to remove the cause of symptom and is not side-effect limited. The treatment was limited by the planned number and duration of the infusions, which had been estimated without an animal model.
The small trial resulted in improvements – some potentially long-term – but didn’t produce earthshaking results. Are you satisfied with it?
The results were dose-dependent, which, with caveats for the small trial, suggests biology over chance. Of course, we would have preferred larger improvements, but it must be remembered that a therapeutic based on removing a receptor from a neuron (or cell) has never been done, and so we were delighted to see an effect, more so because the results shed light on the dosing paradigm. The next step is a larger trial (60 patients), with a now known dosing paradigm.
The goal was to reset the CRF system, turning off the CRFR2 receptors and allowing the CRFRI receptors to return. The hope was that a permanent system reset could result. If that’s possible how would that occur?
With caveats for small numbers, the trial showed that a peptide which only binds to CRFR2 and only lasts hours in the body, was still having effect 2 years from treatment, suggesting partial, but not full, CRFR2 downregulation. We project that at the right dose, the treatment will achieve full CRFR2 downregulation.
What’s the status of CT38 now? Do you have funding for the next study and what would it look like?
We are seeking $4 million to conduct a 60-patient trial in possibly 2 indications.
More on Cortene
The Cortene Series on Health Rising
- Cortene I: The Cortene Way – New Drug to Be Trialed in Chronic Fatigue Syndrome (ME/CFS) Soon
- Cortene II: A New Drug & A New Hypothesis For Chronic Fatigue Syndrome (ME/CFS)
- Cortene III: A New Drug for Chronic Fatigue Syndrome (ME/CFS): The Clinical Trial
- The Cortene Chronic Fatigue Syndrome (ME/CFS) Drug Trial Begins
- Cortene to Move Forward on New Drug for Chronic Fatigue Syndrome (ME/CFS)
- The Cortene Drug Trial Results for ME/CFS Are In






Sounds promising. What sort of time frame do you’re thinking for a drug like this coming to market Cort
That’s a toughie. I don’t know where exactly they are with regard to funding the next trial. If that was successful I wonder, if they could, with the interest in long COVID and its connections to ME/CFS, raise money pretty quickly for the big phase III trial. They should be able to get fast-track status so that would help. I’m just guessing but several years I would think.
Thanks Cort.
Hi cort,
thanks! i wonder if europe could help for the next trial for money so that it go’s fast. Hopefully no 4 years… I thought Europe wanted to work international on ME/cfs. I forget so much, maybe you know, was it euromene, horizon 2000 or follow up, but to ill to search, i thought it was something else for money but something with euro in it. I also do not know if they would give a compagny money but maybe bateman horn center. Or a piece in the US and a piece somewhere in Europe like some study’s do. A fundraising like mentioned would also be great but would it be available in europe if it works in a larger group of patients placebo controlled study? in the us it is the fda that go’s over aproval i thouh-ght (i can be wrong)? here it is something else. would it be available worldwide or only in the US?
UK is no longer europe, they split
As a ME/CFS person with severe hyperadrenegic POTS, i find it quite scary to try something that initially increases sympathetic anything until the drug was MUCH FURTHER ALONG and fully tested on people with POTS and Hyperadrenegic POTS. Fingers crossed this will work out but only 9/14 or 26 people may not pan out in the big phase III trials. Not to be negative, but look at interferon, which was tested for 30 years and it still not an FDA approved drug.
THANK YOU CORT for all the info you share with us and all the time you spend.
Sounds encouraging. Hopefully they meet the funding target sooner than later.
And congrats on having a drug named in your honour 🙂
Agreed. So far as medical funding goes it’s not that much money. To test a drug in a disease that needs it as badly as ME/CFS and which presents a novel way to treat the stress response system which is so badly messed up in some many diseases- I would hope the funding would be available.
The name – a nice coincidence (lol)
Love the name … no coincidences 😉 … how do we help raise funds …
This does sound encouraging, well done. Let’s hope it fulfills its promise, and is available within 3-4 years.
Fingers and toes crossed.
Are the researchers connecting with long covid researchers Cort? If it might also be effective for long covid then surely that will help it’s case for funding and urgency.
They mentioned long COVID in the paper but I think they’re really focused on ME/CFS. Anything proven to work in ME/CFS, would, I would think, rouse interest in the long COVID crowd.
Cort my point is in reverse to your answer- given the size and cost to society of long covid, and given the theorized associations, could not funding from long covid help towards funding of a trial for a potential CFS treatment?
I must admit I was quite disappointed with the level of spin and misleading claims in the manuscript, given the amount of respect I have for two of the authors (Vernon and Bateman).
They dropped most of the objective outcomes and the one objective outcome they did report on showed a negative result (decline in activity). This creates a great deal of uncertainty about whether the TDSS results were genuine or just the typical changes due to response bias that we frequently see in unblinded trials.
Given all the criticism of the PACE trial, other researchers necessarily should be criticised when they make similarly exaggerated claims with similar methodological problems.
“so far as we know, it only interacts with CRFR2”
Where did you get that idea?
First of all, such claims are meaningless if no one bothers to do a selectivity/binding affinity study.
Here is an example for kinase inhibitors https://www.nature.com/articles/nbt.1990 (check the supplementary data if interested)
Secondly, we know that cortene is a Urocortin II analog, so it is likely to have related binding pattens. In fact that was stated in the “InTiME” protocol – similar binding affinity to CRF2 as Urocortin II, and while it was more selective for CRF2, there was still some (albeit a claimed 50 fold lower selectivity in rats) to CRF1.
Lastly, it is quite possible that any observed effects have nothing do to with the proposed model or CRF2 dysregulation in the limbic system/Raphe nuclei. We know that Urocortin II has vasoactive effects in the rest of the body https://pubmed.ncbi.nlm.nih.gov/17258092/ and it is possible the observed effects have nothing to do with the authors’ proposed model of disease resolution.
Secondly, the claims that patients responded more strongly to the drug is invalid as the comparison group was completely different – they were 100% males (and significantly younger) – should always call out misleading claims when there are sex biases like this.
“They dropped most of the objective outcomes”
The study did not report that it “dropped the objective outcomes”. It stated the trial was too small to statistically assess them (HR, activity, sleep). I don’t know why that was – I’m not a statistician – but that statement passed muster with the reviewers, the editor of the journal, and the authors. (Peter Rowe and James Baraniuk reviewed by paper, by the way, and it was edited by someone from outside the ME/CFS field.)
This was a small 14-person (12 of whom completed it) and only ten of whom got what turned out to be the best dose. Given that the finding were pretty good. Finding statistical significance of any kind in that small of a study is not an easy thing. it’s not that uncommon to come across much larger studies which report that if they had just been a bit larger they would have expected to get a significant result.
My impression is that it’s exceedingly rare for any clinical trials attempt to measure these kinds of things at all. Honestly, I would give Cortene a thumbs-up for attempting to do what few others have. Hopefully, we’ll see them get more data with a larger trial.
“This creates a great deal of uncertainty about whether the TDSS results were genuine or just the typical changes due to response bias that we frequently see in unblinded trials.”
While it certainly would have been nice to have statistically relevant (and positive) results regarding the objective assessments, the trial was too small to allay uncertainty with regard to any of the assessments that came out of it. All it could possibly do was point to the possibility of a larger trial. The early Rituximab trials, for instance, were larger than this trial (including a placebo-controlled trial that was over twice its size) and turned out to be misleading apparently because of bias in patient selection (although see an upcoming blog on Scheibenbogen for another slant on that).
The fact that patients continued to report positive benefits for such a long time (1-2yrs) after the trial, while anecdotal, perhaps suggested that a response bias was not responsible. Still, while this small trial had some positive results we won’t know how effective CT38 is, as you note, blinded, placebo-controlled trials are done.
I will try and find out about the basis for this statement- “so far as we know, it only interacts with CRFR2”. That statement has been made from the beginning with regard to CT38.
I will try and find out about Urocortin II having these effects, but I don’t see any problem with the idea that the drug might work in other ways than hypothesized. It seems like we’re constantly learning about the different effects drugs have on the body. The authors focused on the mode of action they considered most likely. I imagine that’s pretty common.
This statement – that patients responded more strongly to the drug than the healthy controls – is correct and thanks for pointing out that they were a quite different group. Thinking retrospectively, it was, of course, pretty much a given any healthy control outside of an ME/CFS study – was not likely to be a good match for ME/CFS; i.e. it was not likely to be a sedentary, middle-aged, female dominant group, but I had not thought of this before.
Your point demonstrates how much more we need to learn about this drug and how much we need a proper control group to assess it correctly. The next study, if it occurs, will provide that. That will tell us much.
Cortene replied to this question:
“‘so far as we know, it only interacts with CRFR2’ Where did you get that idea? First of all, such claims are meaningless if no one bothers to do a selectivity/binding affinity study.
CT38 was subjected to selective binding and affinity studies, performed independently, and tested with 85 different receptors transporters and ion channels. It showed no binding, except weakly at CRFR1 but at very high concentrations (well beyond what was used). This is all in the Supplementary Material (published with the Article).
With regard to this:
“Lastly, it is quite possible that any observed effects have nothing do to with the proposed model or CRF2 dysregulation in the limbic system/Raphe nuclei. “
It is true that CRFR2 agonists have vasoactive effects, but they also have numerous other effects that go well beyond vascular action. Moreover, our proposed model is exactly that—a proposed model. It just so happens to fit with all the observed data in animals and humans, with CT38 and with other CRFR2 agonists, but as we do not have the technological capability to probe individual neurons in the brain, it remains a hypothesis.
With regard to the objective measures Cortene reported that only 7 people attempted the CPET test and the results trended in the direction of the other study finding; ie; improvement. The Fitbit results were all over the map – making them difficult to interpret. Cortene pointed out that improvements in capability may not always translate into increased activity and that the long-term improvements many of the patients reported may not have shown up in the Fitbit data.
Cort, I’m a complete layman, but if there is a real problem with the Hpa then wouldn’t drugs like fentanyl help.
I know that’s a radical step. But the disease is radical and surely any drug that dampens down that adrenal axis could be used as a bridging drug.
Obviously it’s dangerous , with potential overdosing, addiction etc but surely the damage this adrenal overstimulation is probably worse.
I say this as I had the same response to Ativan as Whitney.
Initially, I could walk some distance again, on relatively small doses.
Surely we could repurpose drugs that effect the Hpa axis whilst we wait for cortene.
I’d be quite willing to potentially go through cold turkey as my current state feels awful.
I’ve heard some CFS patients have remission from anaesthesia.
Just thinking out loud. Another 5 years, even though I’m grateful, makes me think have any docs tried to repurpose strong pain killers etc
Yes! I too have heard of and personally experienced remission from anaesthesia. Excellent point!
If it was just a small pilot trial about learning more about the drug (and dosing), why such bold claims about how it confirms the “hypothesis that CRFR2 is upregulated” and then write a really misleading press-release entitled “Clinical trial provides preliminary evidence of a cure for myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) and Long Covid”?
It is the hype and misleading claims that I find most frustrating – claiming it is “preliminary evidence of a cure” is quite irresponsible.
We hold other researchers up to very high standard, so why not this group?
Snow leopard I replied to your comment using a new comment below. (This thread was getting too long and too narrow to read well.)
Oliver – the anesthesia stuff is fascinating and I actually do respond well to dental anesthesia. It gives me a little boost for several hours. It jives the idea (and my experience) that if we could just calm things down in our body we would all be better off.
a ha ha! dental anesthesia doesn’t work much for me. I need multiple injections, multiple times throughout a procedure.
It’s somewhat common to some in hEDS.
I do have benefit from a common cold medicine that has a stimulant as well. It’s supposed to increase heart rate/blood pressure, but it actually calms down mine
I happen to notice it had this effect on me after taking it for a cold.
It is not something I take daily, because I am unsure of the long term effects of daily ingestion.
I am also unsure of stimulating my adrenals further.
I think helping my thyroid better route.
Unless for some weird reason, i’m down on adrenaline. Test could help me figure out what is going on. Tests that my health services don’t approve.. harr harr.
I also learned that this substance was used in the 1920s/30s by a doctor that came down with something that sounds like ME/CFS and later became myasthenia gravis.
on the anesthesia and misophonia connection:
(which I also had. Comes back when under stress)
https://www.researchgate.net/publication/5674624_Hypokalemic_Sensory_Overstimulation
With only 14 persons in the study, results sn side effects couldn’t possibly be clinically significant. Is the data they have from this trial sufficient to start a larger trial at $4 million cost? Is funding prudent fo investors, NIH, etc?
Time will tell and, of course, you can’t extrapolate from 14 individuals to the ME/CFS population. The side-effect data was good with the side-effects basically disappearing when the correct concentration of the drug was found. The NIH is certainly not interested in Cortene, but it’s historically not been interested in clinical trials for ME/CFS, and from what I’ve heard its program announcement for ME/CFS actually precludes them.
Oliver.
I have had a significant stable improvement form a lower dose of CLoazepam (Rivitol) .75 mg a night an dmagnesium Glycinate ~400 mg elemental
This was Dr. Cheney’s protocol> I read his report that he had partial and even complete remission with these 2 . He hypothesized that ME/CFS patents he saw were wired but tired and that these 2 particular substances slowed down the brain which he felt was going to fast.
Cloazepam has a bad rap now and ther eis a huge push to get people off it etc. etc. A:ways check with a health professional BUT it has been a game changer for me. Best of luck Namaste
Cort, thanks again. Do you think this could be fast tracked, after all ME has the highest suicide rate, and the level of suffering is really acute–bedbound life. Surely, the FDA would consider moving this along. The coronavirus was moved along which Pfizer prepared, yes, they had worked on this sort of virus before, so there was knowledge, but we had it before FDA approval. Is there any way to get access to this treatment? Folks with ME need urgent help.
The next study is the major one. If another larger study has positive results I imagine that the FDA will certainly fast-track it. As to getting emergency or temporary approval or whatever the vaccines got that requires a health emergency, if I remember correctly, and while the FDA has certainly acknowledged the urgency of finding treatments for ME/CFS, I don’t think they’ve gone that far.
There’s no way to get access to this drug until it’s approved or given some sort of designation like Ampligen has….Even that would certainly require larger studies. The FDA is a very conservative organization. They need larger studies not only to show efficacy but to show that no harms are done across a considerable swath of people with the condition.
Thanks Cort for your response. Now, as you are on the Board, perhaps you can help with time lines. Why did they have to wait 2 years in this trial? The infusions were injected and results emerged pretty quickly.
When will this larger trial start? How can one be part of it? Through Bateman? Folks tormented in bed sadly do not have years. Have a look how tragically sad poor Whitney looks in his last post. It is beyond heartbreaking.
Also, Snow Leopard, on S4ME pointed out that Fluge and Mella also
understand that the HPA axis is implicated. Is there any way to get Cortene to contact them and to work together. The more brilliant minds working the better. That’s what was done for AIDS.
Thanks again Cort.
Great question. I don’t know the ins and outs of all that. What I can say is that Cortene is not a large pharmaceutical company that can dig into its coffers and plunk a lot of money on the table and get its different departments rolling on the different aspects of doing a clinical trial. Cortene is a small company with three employees.
I saw someone asking why don’t they just publish some of the background data. It takes a considerable amount of time and energy to do that, and they have so much else going on.
They’re responsible for raising funding for the trial (never an easy thing in ME/CFS) interacting with the FDA (a mammoth job in itself), getting the drug manufactured, finding a place to test it, creating the study, analyzing the results, writing the paper, getting it published. It’s simply an enormous task.
Even though this was a small trial check out the number of analyses that went into it on the website, plus the supplemental materials. The paper itself was submitted to the Journal 5 1/2 months ago.
They’re doing all that on a tight budget. If they had more funding I imagine they could hire more people and get things done more quickly. I really don’t know but I imagine that might happen if they raise the money for a bigger trial. They’re eager to start the larger trial which will start when they raise the money.
Finally, Cortene has informed me that all the money raised has gone straight into the study and that no salaries have been paid and none are envisioned to be paid in the phase II trial, should it occur. The only payoff comes when and if the drug is approved.
Cortene did a lot of outreach into the ME/CFS research community to explain what they were up to prior to the study. I don’t know if they’re in touch with Fluge/Mella but they’ve been in touch with quite a few people.
Cort, with all the negative comments that you have made about the trial I am wondering why you reported on it at all. Surely it is too preliminary for sharing widely (and anything positive that you report on is shared widely)?
Most things don’t even get through phase three trials. While I think that phase three trials are worth sharing broadly, anything before that has such a high failure rate that it is actually the opposite of hopeful. Especially when people really really _need_ something (anything) that really does help.
Following phase 1 trials is more depressing than anything.
As a sufferer of FM for the past five years, I’m thankful for some hope, when there’s been little else that addresses FM and the other related disfunctions.
How can the me/cfs community and others contribute to funding the larger study and thereby expedite it taking place? Those of us who are severely ill need help yesterday.
I don’t know. I will try and find out. Given the exploratory nature of the trial Cortene had a commitment not to go to the ME/CFS community for funding for the first trial. Instead, they worked to gather up investors. I don’t know what they will do this time.
I found out. Cortene is looking for “angel investors” interested in contributing significant sums of money. If you know of anyone who might be in that category who might be interested please introduce them to Cortene (info@corteneinc.com).
Crowdfunding
I believe it would take a relatively short amount of time, if ME-patients went together and crowdfunded next trial?
I know I am in.
I think this sound both promising and wildly enough, logic in a way.
PS Great to hear You are in this group. You have been giving hope and insights to the ME-community for so long, and I think Your engagement does nothing but build trust. Is it possible to buy stockshares to support Cortene?
I don’t believe Cortene is in the stock market. I might not know though.
Can we as patients, families and caregivers help fund it? If everyone with ME/CFS put some money in knowing there’s potential to have a drug to be proven and approved, it would raise a lot more money than usual
How do we join the next trial?
If and when the trial occurs that information will certainly be available through Health Rising. In fact, we have something coming up which will make it easy to find all the relevant open studies and trials for ME/CFS, fibromyalgia, POTS and long COVID 🙂
Hi, Cort
Thank you for this info and ALL that you do… I have reached out to Cortene to see 1. What the enrollment/selection process would be for the next trial and 2. How I could support this effort financially.
I also reached out to Dr. Patterson’s group to ask to be included in his database of CFS patients, since, along with Long Covid, he has expressed an interest in looking at ME/CFS..
Like most here, I have done multiple deep dives into treatment and lifestyle modalities… Hope springs eternal…
I have ZERO issues regarding you being appointed to Cortene’s board—- Who better???
Regards,
Nicole Martin
Lots of action Nicole! Good luck in getting in the trials and if you hear anything more about Patterson and ME/CFS please let us know:) Thanks for your trust as well 🙂
I think Cortene’s novel approach is very interesting but my stress levels rose significantly when I read it was a small, unblinded trial, with no objective outcomes, relying on individual patients reported symptoms.
There’s a massive conflict currently playing out in the UK because NICE decided to ‘pause’ the publication of the revised NICE guidelines because some of the Royal Colleges are refusing to implement them because GET and CBT (as a curative treatment) have been removed. This all goes back to the infamous PACE trial. So if Cortene is to go on to be tested in a larger trial, it will need to be done in a scrupulously scientific manner!
With myself, I came to the view that my sympathetic nervous system was stuck on and in an endless vicious cycle. I’ve made sustained improvements, over the last few years, though I am still a bit weird. I can still trigger my sns, if I eat the wrong food. I don’t seem to be able to process something in the food and my HR & BP will rise significantly and I sometimes stay awake all night, completely wired. It’s not a normal stress response, this is some other sort of trigger. So I just have to avoid setting it off. If I wasn’t so intolerant to so much food, I’d be doing so much better. I have more understanding of how to manage my brain and my energy levels but this whole NICE fiasco, is wearing me out (in addition to having no support, being poor etc!) So if Cortene can produce a drug that, at the correct dosing level, can safely switch off the hyped up sns then I think that may be very helpful for pwME.
I’m glad you brought this up. The subjective measure problem is not unique to Cortene. It’s something that occurs across the spectrum of ME/CFS clinical trials. I just looked up a bunch of clinical trials in ME/CFS and found that most rely on subjective measures.
Those that don’t have ways to assess the parts of the body that they’re attempting to enhance. The NAC trial (https://www.clinicaltrials.gov/ct2/show/NCT04542161?term=NAC&recrs=abdf&cond=chronic+fatigue+syndrome&draw=2&rank=1), for instance, (which is funded amply by the NIH) had a built-in objective measure – the trial came out of brain scans which showed low glutathione levels in the brain. The oral rehydration solution study was able to do tilt table tests.
Even when an objective measure seems to be present the trial may not use it. The big Florinef trial published in JAMA on hypotension in ME/CFS (https://pubmed.ncbi.nlm.nih.gov/11150109/) used a “global wellness scale” as its primary outcome measure.
The double-blinded, placebo-controlled Rituximab trial (https://pubmed.ncbi.nlm.nih.gov/30934066/) which took years to get going used fatigue scores to assess efficacy!. So does a probiotics trial underway, a solriamefetol (never heard of that before) trial, a creatinine trial, etc.
The same is true in fibromyalgia by the way. The clinical trial that helped win Lyrica approval (https://pubmed.ncbi.nlm.nih.gov/15818684/) used subjective pain scores as its primary outcome. Other subjective scores were used for secondary outcomes.
I know that Cortene devoted quite a bit of effort to find good ways to measure progress. It’s an essential, even a crucial part of a study. Using these subjective measures doesn’t mean they are not valid scientifically by the way. They have been “validated”. They are not what we want but they are the best we have in most circumstances.
Cortene did try to do cardiopulmonary exercise tests but most patients, understandably, didn’t want to do them. (Fitbit scores, by the way, are not “validated” – so in some important ways (which I don’t know) they apparently don’t “count”).
A final issue is that since it’s not possible to measure what’s happening with CRFR2 there is no biomarker that can be tested.
How to accurately assess efficacy is a big problem in ME/CFS – and is not at all exclusive to this study.
Fair point but I think the legacy of the PACE ‘trial’ will live on in the field of ME/CFS for a long while. So how to conduct a trial, without biomarkers (as there currently aren’t any), that will indicate accurately enough if a particular drug/treatment works or not?
The main problem with the PACE trial was actually not the use of subjective markers. Those are used all the time in these diseases. The big problem with the PACE trial is that they changed the criteria for success and made it so low that even people entering the trial could be already counted as recovered.
There were other problems – enough problems that, as I remember, a course was taught using the PACE trial as an example of how not to do a trial (lol). Imagine that!
Certainly, it would be better and would be a big breakthrough for this disease if we had a validated objective measure which could assess reductions in fatigue properly. That’s probably way up there on the list of things to get accomplished in this disease.
Thank you for summarizing this and working toward being able to summarize on-going relevant trials. I used to periodically search the NIH clinicaltrials.gov site and have been unable to do this so it’s appreciated.
I hope you will include trials that address symptoms – for example relevant sleep studies. Although given our other symptoms we tend to get excluded or age out.
Also if the results showed stronger outcome measures results then the sample size would not be as much of an issue.
Honestly I think we need to find ways to complement the clinical trial stop/start methodology and rely more on longer term self-quantification. Did the subjects have long term prior measurement data, clinical trial data, and are they continuing to collect on their own the same a posteori measurement data after the trial? For example, fit bit data can be helpful for self-management.
That really is true. We have way too many studies that stop at their six or eight-week mark and that’s it. I don’t know they collected the longer term data. I only know that it was Dr. Bateman who did that.
The topic allows me to put in a plug for the Solve ME Patient Registry and using their app to follow symptoms over time. The nice thing about that is that your stats get infolded into the group stats where patterns can show up.
Given that only 10 patients got the proper concentration of the drug – and then received it only three times – while no one recovered I think the outcome measures give reason for hope.
True. So hopefully, NICE will publish the revised guidelines and we can all move on…
Big sigh of relief that Cortene was able to complete this study.
A novel company, novel idea, and glad that Cort did not hide the parts of this study that people objected to.
This IS what we want, imo. We want people with cfs/me and like diseases who are represented here ti be able to have their say. For this is what is needed. People who have it “get it.”
Voices of dissension are as important as voices of agreement. For novel thoughts and ideas that inform the next steps CAN come from patients ( and NEED to, imo).
So many patients HAVE done so much reading, researching, and learning. Add this to patients’ FIRST-HAND experiences, and patients are a FORCE to be reckoned with……….. of priceless worth to companies engaged in research.
Kudos to Cortene for ‘gambling’ on cfs/me when so many won’t.
Hi Sunie, I totally agree with you that pwME, who have the lived experience and may have researched and thought extensively about ME/CFS may have a vital inputs into future developments. What I would be fearful of – is a valid idea being dismissed because there appears to be no evidence of improvement, lack of improvement or even worse, harm experienced by the study participant. I think there’s a great opportunity for researchers in the field of ME/CFS (Thank you for trying – we know it’s very difficult!) to learn from the glaring mistakes made by the GET/CBT crowd. David Tuller, Brian Hughes, Keith Geraghty and Tom Kindlon have been shredding their basic errors over the last few weeks on social media and there are many in depth studies they’ve produced over years. NICE’s ‘pause’ of the revised guidelines has, in my view, laid bare the very worst in poor quality studies and their supporters. It seems the biopsychosocial crowd had decided what was wrong with pwME and then they went on to prove their idea was correct, changing the criteria when their findings in the trial contradicted their biased thinking. So study, after study replicated what they were expecting to happen.
So what is the best that we can do, until some sort of biomarker is found? Like, I know I have improved my own functioning because I can generally make it through my day (I work p/t) with a bit more ease than I could before. I can think more, remember more, I’m not so emotionally all over the place etc. And this is an ongoing situation I still react very badly to things I eat and recently I’d figured out a diet that was suiting me but I was losing too much weight really fast. So, I have to eat things that are a problem (chocolate & cheese) in terms of intolerance not in terms of how tasty they are! But the darker the choc, the more problems I have with processing it (I think I’ve turned into a dog) and it sets off my sns. Milk choc has milk in it (!) and more sugar and I have an issue with fructose – sugar is 50% glucose and 50% fructose. Welcome to my life…
I’m totally in favor of your financially benefiting, Cort, if Cortene succeeds, given all the work you do for the ME/CFS community!
Thanks. Given my paltry retirement savings and my pretty dismal social security social security payments – all of which I attribute to having ME/CFS…it would certainly be helpful.
Cort: If you profit so do we, and that would be very good. Do I hear an Amen.
Nice point, Ron.
AMEN!
I would really like to join the next study. I’ve been mostly bedridden for almost 12 years. I was diagnosed with CFS circa 1990.
New Jersey, but more mon lives on the CT/NY border.
Thank you.
I must say I was appalled by both Bateman and Vernon being in attendance at the FDA meetings which clearly spelled out the need for 1) large and 2) Controlled studies being done to be meaningful in the study of CFS. This was at best an exploratory IND study done incorrectly. As such it took too much time and spent too much of Cortene’s
money that should have been reserved for a proper phase 1 study. Another example of a doctor not a researcher becoming involved with CFS research- what a shame.
We would all have loved for a big double-blinded, placebo-controlled study to be the first study done on Cortene (or any other treatment for that matter) but this is where we are in ME/CFS in general. This field is not bursting with funds. It’s a poorly funded field that kind of scratches along. We’re going to see long COVID studies the size of which is going to blow our minds.
Cortene raised enough money to do an exploratory trial. That trial gave them the data which they can use to try to raise more money. If the next trial occurs and is successful, they’ll use data from that trial to raise the boatload of money that’s needed to do the big phase III trials.
I don’t see that they had any other options.
Suzanne Vernon, one of the co-authors by the way, was the research director for Solve ME (then the CAA) and before that worked for many years with the CDC. She’s been a co-author of over 100 research studies. (https://pubmed.ncbi.nlm.nih.gov/?sort=date&term=Vernon+SD&cauthor_id=33168001). I don’t know if you need a researcher to do a clinical trial so much as you need people from the pharmaceutical industry – which Cortene certainly has.
Get someone to write about this who doesn’t have a financial conflict of interest. It’s pure BS to say your job is to promote the interests of the company and also expect people to trust you as “objective.” This is really basic stuff, and your bold refusal to abide by basic scientific and journalistic ethics undermines your credibility and that of the research itself.
Really. Silly me for thinking that declaring my potential conflict of interest – at the top of the paper no less – was how this was ethically done. That is actually how it is done. You openly declare a potential conflict of interest is present.
https://www.rasayely.com/what-is-conflict-of-interest-how-to-declare-it-and-why/
“Declaring conflicts of interest is critical for maintaining the integrity of unbiased professional editorial assessment of the publications.”
The document does not say you cannot write an article on a topic you might potentially benefit from. It states you must declare a potential conflict of interest. In contrast to your assertion regarding my “bold refusal to abide by basic scientific and journalistic ethics” I am actually following standard procedures in this area, and I encourage you to investigate this further so as to relieve your mind.
As to getting someone else to write about Cortene….HR has occasional guest bloggers who are interested in writing on topics they have a special interest in but it does not have a pool of guest bloggers ready and willing to write on technical topics that HR chooses.
We basically have a pool of one – me. In the vast majority of cases either I do it or it’s not going to get done. It’s up to you to decide how objective any blog is.
Ellie, I would hope that you would kindly take back your words. Cort has done an amount of work in the ME/CFS (and beyond) field that I don’t believe could have been done by another. He’s not selling something here, he’s trying to help us … every day.
It was such a bummer to read your post. Maybe you’re new to this blog and don’t realize what an incredible gift Cort has been to us over the years. I have spoken with various CFS researchers. Some have chosen to exit this area of research because people with CFS can be very unpleasant. Your vitriol hurts our chances of finding the answers we are so thirsty for.
I agree.the posts before the trial were like an advertisement for the company.
Posts explaining the company position on various issue s are like PR department of the company.
Also they are interested in FM lyme POTS so not just ME
So strange, then, that the company has never based the ME/CFS community for money.
Thank you for presenting this info. The goals of cortene seem like a good fit for my symptoms.
I obviously don’t know if Cortene is going to work out but personally – based on my symptoms and that weird wired and tired symptom found in ME/CFS – anything that addresses the stress response should be looked at carefully. I think it must be involved.
Wow, Cort this is exciting!!! I am impressed that the authors collected objective data and whilst this was not statistically relevant nor reported on it is heartening that the need for objective data was recognised. As an avid HR pacer and objective data collector of my own data I recognise the difficulties in obtaining statistically meaningful data. My HR data collected over 5 years helps me manage ME/CFS better as I know what I am doing when. Whilst the HR data looks similar from year to year hidden in that data is my ability to do more and more, incrementally tiny but sustainable and life changing improvements. A tough call to get the data but worth the effort. For me any improvments are tiny, so tiny that I often only recognise them when I reflect back over what I can now do or when I over exert and a symptom that has magically melted away reappears. I am delighted that you are on the Cortene board and blogging about the same. Fingers crossed you do financially benefit from the drug being effective as you have done so so much for all of us with ME/CFS, at enormous cost to yourself.
Thanks Ellie, the only thing I would be careful about is that while we can’t in any manner extrapolate the results to the broader community – the study was too small that – within the bounds of the study the outcome was statistically meaningful. It is not, as you pointed out, statistically meaningful for the entire community and there was no way it could be. Even the expanded phase II trial wouldn’t fit that bill. It would take the large Phase III trials before we could say that.
Kudo’s to you for watching your data so closely. I’ve found a new “toy” which can assess functionality I guess you would call it with much more precision than I’ve seen before that and will report on that hopefully soon. It’s kind of exciting! I’m ever so slowly emerging from the dark ages and may even become a data junkie at some point. 🙂
“I’ve found a new toy…..”. Can’t wait to learn about this Cort. Like Ellie I use heart rate to manage my pacing but brain fog has prevented me from anything but very basic analysis to detect improvements. I was a maths teacher in a former life.
I’m also surprised (and delighted) to read about developments in ME that fit with my experience of the disease. It developed after an extended period of stress and not in response to a viral infection as the majority of sufferers have experienced. Maybe we stressed individuals get to be at the front of the queue. (Joke btw).
It’s that something – a math teacher in your former life – and even then those well-worn circuits in your brain are not working. Darn!
Have you tried thyroid?
You’ve mentioned your ME/CFS was not precipitated by an event/infection. More of a slow decline over time?
That sounds like hypothyroidism to me.
Measured your temperarure and heart date through out the day?
B1? Niacinamide (instead of niacin)?
Concerted effortof low doses multiple times a day.
Couldn’t the NIH pay for the next study? Wouldn’t they find interest in it??
Clonazepam and Magnesium Glycinate should be tested. It will help a lot of people imo.
Hey Dan, really appreciate your recommendations. Gonna look into it. Thanks man
Certainly help the drug company who make clonazepam.
I hope this drug works and you get rich and we all get healthy again.
🙂 Wouldn’t that be nice!
Thanks for sharing this great article Cort-we’d be lost without your excellent summaries!
This really interests me-as I became ill after a number of insults to my system in a row. A virus (EBV) which i slowly recovered from-90% of baseline. Then I foolishly took the combined contraceptive pill-which I think flared mast cell issues/autoimmunity.
But then the final blow was that I was given a trial of high dose steroids-which were also tapered very quickly. Since then, I have had things like POTs, etc., develop along with diagnoses of fibromyalgia, erythromelalgia, and a host of inflammatory skin issues. I have often wondered since if the final insult of the high dose steroids-which I guess is equivalent to being put in a very high stress situation- sort of messed up my crf/crh receptors. I am a lay person who is sick, but I keep saying to myself, have I basically induced cortisol resistance of the cortisol receptors, akin to a high sugar diet inducing insulin resistance, with a resulting downstream plethora of issues. I have also wondered if this is why IVIg is helpful to some-I have read an article that stated IVig improved cortisol sensitivity, albeit in the context of steroid resistant asthma, but still, makes you think.
Keep up the great work you do! And thanks again!
Thank you so much for your work on this Cort. I was very interested when the initial study came out about Cortene as it did seem a fresh new approach. I am not a scientist so do not understand a lot of what I read but I am severely ill now and most definitely come into the wired category. To be able to have something that switched off that response would be a game changer even if it didn’t ‘cure’. Just wish that these studies didn’t take sooo long. I will be 80 soon and would just love to think that there was still some life in the old dog yet.
I have found that the low frequent dosing principle works very well for me with all substances, including food. Diluting in water and sipping throughout the day, or a drop transdermally, I open a cap and dump just a little bit of the contents into my tongue. I’m talking about 10mg of a B vitamin as opposed to 500, etc.
I first got the idea to try this approach after learning of Norman Cousin’s experience with IV ascorbic acid.
– – – – – – – – – – – –
I wonder if one way to assess the effects of the drug (cortene) would be to measure CO2, glucose, temperature and heart rate. Cortisol. Perhaps even magnesium (if the metabolic rate increases, ATP comes back into the cell, then we won’t be wasting it away and hence, levels should go up)
Both before and after activity. Just one measure in the morning seems useless and incomplete of a picture.
Remember the graph from McGregor’s study?
Glucose staying the same before/after a meal for some
Rising after a meal but coming down quicker than in the controls
Or the rise in cortisol with stress (ACTH test perhaps)
There are also a few studies on the movement of cortisol throughout the day that you have reviews on the site.
These would be objective measurements, to assess improvements.
I bet there are specific substances that indicate protein breakdown and catabolism, that theoretically when there is a switch to anabolism, that should also be reflected in measurements.
There is a specific pattern of raised methionine and homocysteine, and lowering of all the other amino acids in a simple qualitative AA blood test (it measures about 40 of them). Raised 1,3-methylhistidine for muscle tissue breakdown. Hydroxyproline/lysine, though there is a division here between those with hEDS and those without from what I remember. You know who know these patterns well inside and out? I think McGregor.
You would need to test every participant before the trial, be aware that there will be different responses. The interesting things would be that even thought there are different presentations, you would also be tracking how they change before and after. And even those that get worse, maybe it can give an idea of why.
This probably requires even more funding.
I think that simply measuring metabolic rate could be enough. and less cost.
And YES to the high serotonin = good and healthy myth!
! ! !
for example :
https://davidhealy.org/wp-content/uploads/2015/07/2015-Serotonin-and-Depression-bmj.h1771.pdf
Thanks Meirav – always interesting stuff you provide 🙂
Oh wait: what about using the nanoneedle?
Testing before and after?
Or testing the blood for if it changes the behaviour of cells? That X mysterious factor..
Then this could apply to the other chronic conditions where it has been found.
Did you know that an infection (viral or bacterial) can precipiate diabetes onset too?
Thank you for all your gems. There are soooo many scattered through out the site. We forget them when new findings come along.
You have one on CO2 as treatment for hyperPOTS (you know the type that no doctor can agree on for treatment because they can’t find one)…
I’d love to know what are the treatments that Nancy Klimas is setting up in experiements. They also involve resetting HPA axis?
I think you missed your calling Meirav – should have been a researcher. 🙂
Am glad the early trials have shown some positive signs. Fingers crossed that a larger trial can commence soon.
The Cortene theory really resonates with me because in the many years before I got diagnosed, I always thought of my illness as a form of ‘stress diabetes’. My body can’t process stress properly, similar to how a diabetic can’t process sugar. Although my illness was triggered by a virus as a teenager, it was preceded by a period of intense life stress and trauma and I am certain that this, along with genetics, played a role in me developing ME/CFS.
I know not all patients have a broken limbic system like I seem to, but I don’t think I am alone either given the number of people who report recovery after making life changes that reduce stress: setting strong boundaries, leaving toxic relationships and jobs, removing dietary or environmental allergens, or undertaking neural retraining programs based on meditation or visualisation. I too have achieved partial remissions and improvements a number of times over the past 30 years following positive life or dietary changes, but the improvement never sticks. Life just doesn’t provide enough clear water from stress and trauma and soon enough I find myself back stuck in my four walls in a small life and a body that feels like its unravelling faster than a loose piece of wool on a knitted jersey.
What I like about the Cortene hypothesis is that it marries up my intuition about what is wrong with me with a concrete scientific mechanism that is far less vague and unscientific than descriptions used in neural retraining programs like “maladapted stress response”. The Cortene theory might be wrong but at least the hypothesis is feasible and it offers some hope.
Since life doesn’t appear to be getting any less stressful, and there are no magic bullet cures on the horizon, I may need to renew my previous failed efforts to rewire my brain to react less to stress. If only it didn’t take so much effort though. A magic bullet would be so much easier.
I think I get what you’re saying debsw. I have completely overhauled most of my life, tried to resolve or come to some sort of understanding and acceptance of major difficulties – all fairly draining but I’ve tried to have a clear out. But there’s still ongoing issues, like I do manage to work p/t but I can’t work full time and the work I do is on a zero hours contract, so it’s inherently unstable. I have no diagnosis and no help in managing my health issues and no support from family, friends or neighbours. But people on Health Rising and Twitter are mostly really lovely and supportive and that makes a huge difference.
So hard Tracey Anne. They are some tough hurdles to conquer. Take care of yourself.
Thanks Debsw, you take care too 💕
Very interesting Cort. Could it be autoimmunity against CRF(R2) receptors? Or are ME-patiënts born with a hypersensitivity for stress? What could be the cause that we’re stuck in this loop?
I always say that ME is a disease where the stress system reacts abnormally to any kind of stimuli. I do think that 2 groups can be distinguished 1. hypersensitive group 2. undersensitive group
Just ask patients if they feel (completely) relaxed or if they feel rushed that you are on the run. Especially when you are going to rest. Do you have the feeling that your body is calming down or does it seem as if you are running or over-stressed?
These questions are missing from most questionnaires.
Good luck with the investigation even now that you’re involved. I think you’re on the right track. Too bad we can’t measure anything. Can’t we measure substances in the blood that have to act on those receptors?
I trust your objectivity on this topic in your blogs. I look forward to more information.
“Just ask patients if they feel (completely) relaxed or if they feel rushed that you are on the run. Especially when you are going to rest. Do you have the feeling that your body is calming down or does it seem as if you are running or over-stressed?”
You are right sir! Those questions just encapsulate so much and I think we are missing them.
I wonder about measuring the substances that interact with these receptors or which they effect. I imagine there are many ways to assess general homeostatic mechanisms (cortisol, tilt table, exercise stress tests) . For Cortene’s purposes in these clinical trials, they need validated ways to assess reductions in fatigue, etc. It’s a tough problem.
Thanks for your support 🙂
Reply to Snow Leopard regarding this comment
“If it was just a small pilot trial about learning more about the drug (and dosing), why such bold claims about how it confirms the “hypothesis that CRFR2 is upregulated” and then write a really misleading press-release entitled “Clinical trial provides preliminary evidence of a cure for myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) and Long Covid”?
It is the hype and misleading claims that I find most frustrating – claiming it is “preliminary evidence of a cure” is quite irresponsible.
We hold other researchers up to very high standard, so why not this group?”
For the record, I think “improvement” would have been a better term to use :).
Cortene, though, is not basing their assessment of this drug’s efficacy on that small trial. They’ve been working on this drug for somewhere around five years and these people are wicked smart. They’re working without salary and will be doing for quite some time to come; ie they really believe in this drug. Some more reasons for their support of the drug are below.
(i) stimulating the CRF2 receptor with a drug in animals induces MECFS signs/symptoms – which are dose-dependent; ie the higher the dose the more the symptoms – until the drug wears off
(ii) similarly stimulating CRF2 receptor with a drug in healthy humans produces ME/CFS signs/symptoms – until the drug wears off
(iii) high-dose CRF2 stimulation in ME/CFS patients does the same except that symptoms remain. The dose does not readily wear off – suggesting that something is wrong with the CRF2 system in ME/CFS.
(iv) Stimulating CRF2 using CT38 in ME/CFS patients simply produces more ME/CFS symptoms; that suggests this pathway plays a role in ME/CFS.
(v) the response to CT38 in the MEcfs patients was dose-dependent: when given at the correct concentration the more of the drug the patients got the better they did. When given at too high of a concentration, the more of the drug they got the worse they did.
I don’t know if the results of the trial are enough to validate their hypothesis but when too much of the drug was given and when the right amount was given – both tracked with what Cortene hypothesized.
You know you’re quite the gadfly, Snow Leopard – virtually all of your comments on HR have poked holes in something or the others. That’s not necessarily a bad thing. If everyone did it I think we’d be all depressed and go hide under the covers but you obviously bring quite a wealth of knowledge to the discussion and your insights are welcome.
Thanks to your “question” I found out, for instance, that the drug had been tested with many different receptors. I didn’t even know that was an issue (lol). The accusatory tones, though, they don’t help in my book. Dragging the most controversial and universally derided trial into the conversation was not helpful IMO.
We are all weary of the lack of progress in this disease but I think we need to continually remind ourselves of the tremendous constraints the small research and clinical trial efforts that permeate this field operate under. So many times, I see patients getting upset at the size of a study or something that was left out of it. It’s not the researcher’s choice to do that. I’m sure they would love to have the large, buttoned-down studies that we want. Really, we should celebrate the fact that they’re willing to work in a field with so many obstacles.
While we need to acknowledge the deficits that will necessarily be present and point any mistakes that might have been made and we need to support these efforts at the same time.
That doesn’t mean not questioning but the accusatory and ugly tones that sometimes show up – I don’t think they help.
I don’t know if it needs to be said but we are ALL struggling, the patients with their health, often their finances, etc, and the researchers, advocates, patient groups, and small drug manufacturers like Cortene with their lack of resources – we are all in different compartments of the same boat. Questions have even cropped about whether our big placebo-controlled, double-blinded effort – the one trial we wanted to make sure was done correctly – the Rituximab trial – was done properly! The issue was the same one as always – not enough funds.
Let’s have some compassion for everybody. If we could all get together and break bread and drink a little wine (or maybe water for a lot of us :)) I think we’d find we’re all actually pretty decent people.
Great job finding a novel way to address the very complicated ME/CFS Disease. Cort, you are always my go-to for information that is easy for me to understand. You, my friend, are awesome. Hopefully you do reap some benefit from this, there’s no-one who deserves it more. THANK YOU, and GOD BLESS YOU 💜
Thank you, Cort! Sign me up for the next trial! Please keep us informed of any updates when next phase and patient recruitment will begin.
We are all eager, hungry for a treatment that will restore our health and lives as they used to be.
But, the halls of the pharmaceutical industry are littered with the bones of phase I studies that never made it to phase II and phase II studies that never make it to phase III.
A study of only 14 ME/CFS patients compared to a healthy male control group is almost useless in predicting positive effects and potential side effects.
Finally, should we be tamping down or accelerating neuronal hyperactivity when we don’t know what is causing it.
Until we know the cause of ME/CFS, we won’t have a safe and effective treatment. That is where the Covid Long Haulers have an advantage; the triggering agent is known.
I was really hoping that cortene was going to help me, but alas it did not in any way. I have recently tested negative for all of the celltrend lab tests and I don’t seem to be fitting into the standard ME/CFS parameters even though I have all the same symptoms. I have EDS and SFPN so its possible that just CCI issues are at the root cause. I really do commend Bateman Horne for getting behind this and other initiatives. I’m glad to hear that the study helped others and Bateman has been more than amazing in their care.
Sorry it didn’t work out but I acknowledge you, Adam, for being willing to try a brand new drug! Good luck with your search. CCI and related issues certainly do seem to be cropping up more and more.
This is a little old, I hope you see it Adam:
Have you ruled out inherited metabolic disorders?
There are those that have been misdiagnosed with ME/CFS FMS hEDS. It does happen.
It should be the doctor’s responsibility and their job to rule out conditions/diseases that have established diagnostic tests before arriving at clinical ones.
You may have to take on that job.
If you have joint hypermobility – you could test for the homocystinuria and hypophosphatasia. The testing is easy.
I personally think it is worth ruling them out/in.
Maybe a real dedication to de-stressing could reset or modulate the CRFR2 pathway, at least for some of us. Maybe this is why there are many stories of people who have been able to make positive lifestyle changes that reduced their symptoms. I was already thinking about this the last couple of weeks that I need to implement a serious program of sleep and stress treatments bc at this point it’s just a waste of my time seeing and waiting on Doctors they don’t come up with anything for me. Most days I do very little in the way of regular, daily, habitual stress relief. I don’t think I’m particularly stressed out or under heavy stress but I’m really not doing anything I would call relaxing other than mindlessly watching youtube videos.
I wonder if this would help both my ME/CFS and my long Covid…..2 birds with one stone……
I’m so sorry you have both! It must be a tremendous struggle. If you haven’t already, you might look into going gluten-free.
Interesting. I am curious whether SSRIs (one of which I take) would influence this in any way. From what I can gather, SSRIs do target and inhibit 5TH1a receptors.
The study article (https://www.frontiersin.org/articles/10.3389/fnsys.2021.698240/full) does mention SSRIs briefly:
‘Many signs, symptoms and anomalies of ME/CFS have been linked to 5HT (and downstream mediators), but studies involving SSRIs require cautious interpretation as these drugs initially increase extracellular 5HT, before activating the 5HT1A autoreceptors to decrease 5HT and induce effect (Andrews et al., 2015)’
..but the linked study seems to indicate that SSRIs ultimately result in lower extracellular serotonin. I’m very much a layman though, so I could well be misinterpreting it..
‘The brain develops compensatory responses over chronic treatment that reverse the energy disruptions and reduce symptoms. Specifically, both the reduction in the synthesis of serotonin and the tonic activation of the 5-HT1A heteroreceptor act to reverse the elevated glutamatergic activity induced by the direct effects of SSRI treatment. If the 5-HT1A heteroreceptor is still activated as extracellular serotonin returns to baseline over chronic treatment’ – Full study here (see pages 11 & 12): http://www.jandersonthomson.com/wp-content/uploads/2009/10/Andrews_et_al_serotonin_upper2015_NBR-1.pdf
The TL/DR seems to be that SSRIs result in lower 5-HT and an increased sensitivity of the 5-HT1a receptors. If i’ve interpreted that correctly, wouldn’t taking an SSRI have some sort of similar effect to what the Cortene study is trying to achieve? I ask this as someone who’s been on an SSRI for 20+ years.
I was also going to ask regarding the effect this would have on the endocrine system. One of the treatments I have pursued in the past is hormone treatment. I have had some success but nothing spectacular or long lasting. But, I think I found the answer to my question here https://www.news-medical.net/health/Corticotropin-Releasing-Hormone.asp
According to this article it’s CRFR1 that controls ACTH and not CRFR2.
SSRIs have been a life saver for me, and I believe they have acted on my CFS more so than any depression that I have had, which has been mild-moderate. I too have been on them for more than 20 years with a few pauses.
SSRIs are known to act on several channels, including immunological ones.
Very glad to hear they’ve also helped your CFS, Matthias. They didn’t have any effect on my CFS, which makes me wonder whether this Cortene theory would be of help to me. There’s still a lot unknown though, so I can’t really make that generalisation.
Interestingly, CRF2 has been linked with IBS…
Wouldn’t the best thing to do be for the people who own the Cortene intellectual property to sell the rights to a large, well resourced pharmaceutical company in exchange for future royalties? This could potentially speed up regulatory acceptance by many years. With the potential revenues to come from any successful long Covid treatment adding to the attractions of this drug.
Sounds like a great plan
Hey there folks… Well, all this news about COVID19 aftermath symptoms just kinda reaches out and screams…we are all suffering from POST VIRAL SYNDROME.. This is if you are conditioned in your DNA to certain pathogens.. The pathways all give us one thing in common..A Pathway!. Our immune system will follow whatever it is programmed to do after a flare of any illness.. I just read a whole thing on Epstein Barr and mono -both which I have but when they clear up….PVS [post viral syndrome} comes hauling ass down the pipe and kicks whatever part of the body it’s programmed to hit with inflammation and many different symptoms that all seem to be very much alike,, For instance MS…What ever sets it off then turns it around and out comes PVS have simular symptoms.Inflammation is one of them.. Ya know I went for years, thinking my body ached before I actually knew it was Inflammation… In MS the inflammation aims at nerve endings.. In COVID it aims at the lungs and oxygen..All of them DO ATTACK THE BRAIN..We have a brain disease.. That’s why it makes us feel wacky.Whatever the science is, I’m livin it..50 years now… All person’s with PVS have oxygen problems and especially brain fog…Ask your doctor for a Spect scan of your brain..Get the book by Dr. Amen who is the new way towards healing us all… It’s called the End of Mental Illness as we know it”.. shows the oxygen and blood that is in strangle hold from inflammation in the brain… Those damn pressure headaches and neck stiffness that feels like Meningitis. I know I’m going a little loopy tonight but I hope I’m making some sense to some of you..Natural killer cells develop in the bone marrow. Mine are not right….some are very underdeveloped..He! He.!… I always knew I never grew up.!! joke.. Naltrexone helped me.very low dose many years ago..That news is just resurfishing again..!!!!..Here we go again.. Round and Round we go with all the absolutely dumb tests. If we had all the money these guys in white coats got for research we’d be doing really great.. exercise tests, tilt table tests, and on and on… I kinda decided that it’s all so very confusing that I narrow it down to one thing..”.That pathway “that allows the PVS to crawl in to the brain and mate with your own choice pathogen!… Just think about this… we are all out here trying to figure this thing out… I think COVID may open up some things that have been in Pandora’s box all along about CFIDS and Herpes Six B and CMV and Epstein Barr and on and on and on..We rarely die, some kill themselves ( which I have contemplated) so because we aren’t dying or curled up in a wheel chair all deformed, we are not seen as a real terrible illness…..HA! I’m fed up with public doctor’s in general.. They only know what wheel to jump into like a hampster and run around and around!Closed off only to what they know.. After all, they are doctors.. A few doctors over the years have been open minded ( Lapp, Nancy Klimas) and that’s why we are getting some where.. Do yourself a favor..Don’t Call this by it’s stupid name. CFS………What??? Are we gonna LAY here and take that any more? I have learned to call it Auto immunity with post viral syndrome… Hey, the doctor can handle that.. it sounds serious!! Damn Doc, it is serious!!! So forgive me.. alot of my thoughts are not meant in anger…just using a little humor…BUT I AM SERIOUS ABOUT IT ALL BEING A POST
VIRAL SYNDROME..AND I AM SERIOUS ABOUT US USING SOME TOUGH WORDS SO WE ARE HEARD.. IF YOU HEAR ANYONE SAY CFS AGAIN…..GET OUT THE DUCT TAPE.. WRITE ON THE BACK OF THEIR SHIRTS.. LISTEN— YOU ARE MAKING IT WORSE THAN IT IS!……WE DON’T HAVE CFS.!!!!! Actual truth.. everyone has CFS. /Even my dog after a long walk…WE HAVE A SERIOUS IMMUNE DYSFUNCTION.. Good night my loved and honored” Chronic Immune post viral brain injured “friends..love you all! Nila.
This hypothesis seems to explains my ME/CFS to a tee! My ME and Fibro was triggered by a severe and immediate rxn to a corticosteroid injection. Within 24-48 hours of the injection, I was in the ER with quad muscle rigidity and quad burning and all of my current ME/CFS symptoms began immediately and they have never left. My gut has always been that serotonin/tryptophan has always been a major factor for me. My experiences with SSRI and SNRIs have caused an almost serotonin syndrome-like rxn….which is basically more of my ME/CFS symptoms.
I never had a virus or fever prior to onset of my ME.
I believe it is time to recognize as a community that there are subsets of patients under the ME/CFS umbrella. For the subset of patients like me, we are “wired but tired” and can not sleep. If I exceed my energy envelope, I want to crawl out of my skin, total insomnia ensues, my leg muscles burn, my skin is on fire, my body is exhausted but my brain can’t stop…..I know it is early for this drug but….I feel this is the closest hypothesis I have ever heard to explain what is going on with me.
Wow. Chris you sound like me – except worse. Thanks for sharing what’s going on with you.
Ironically also one of the only labs that has ever come back abnormal for me was an OAT Test showed high serotonin metabolism (5-Hydroxyindolacetate) and high kynurenate. I kind of wrote the lab off but now I wonder. Anyways thank you Cort for this blog and continuing to cover all new research. It gives me a little hope for the future and most days hope is all that keeps me going.
Keep the hope up, Chris! Lots is going on. 🙂
Chris, have you tried not eating any muscle meats, fish and seafood?
Insomnia/depression feeling/muscle tightness, pain and spams abated quite some.
I also did a round of famotadine to lower serotonin.
I can eat a bit of seafood daily now, and chicken broth. Beef is still way too much. Serotonin symptoms come back.
The trick then becomes were does one get enough protein in their diet from.
There’s more to what I have been doing. Certainly serotonin is something I am very sensitive to. Stress hits my stomach right away.
Thank you Meirav. That is something I will have to try. I have trouble with restrictive diets, as I have trouble keeping weight on. I have stopped eating turkey as I noticed it activates me but had not considered other meats.
Egg yolks are a good way to get nutrition in when cachexic. I’ve heard the juice of potatoes too, cooked.
I also had serotonin syndrome like symptoms from SSRIs after taking for just a short while. Tryptophan causes extreme symptoms too. 5htp seems to be ok though. These symptoms could possibly also be consistent with the metabolic trap theory too.
I quit my meds due to side effects. My care provider Dr Miller introduced me to VineHealth (vinehealthcenter. com) ALS/MND herbal treatment. The treatment is a miracle. I recovered significantly!
WAit a bit:
What if what the drug is doing is simply increasing adrenal production?
And then in the long run, you’ll just wear the system down and lead to worsening again?
I just had a run of adrenal hormones, I oddly felt alive and full energy like my old self. But it couldn’t be – I should have been feeling dead-tired from the little amount of sleep I was getting in.
I , like many others, subsisted on stress hormones for decades, and kept going and going until my body deteriorated like that of a 70 year old, what lead down this road to my body falling apart.
(that’s what my xrays looked like, said the doctor. It’s what finally grabbed his attention that something is truly wrong here.)
The answers to what is happening with the drug may be in measurements of stress hormones and metabolic rate, etc.
I saw there is a machine that can discern O2 consumption and CO2 production PLUS what source you are using to burn energy.
(hint: should be glucose, not protein, not fat)
I did flat line during a heart cath, yrs ago at UN of Miami. They didnt ask me if i was allergic to shell fish. So i FLAT LINED ant rose above to watch Doctors revive my heart. I was in my 20″s and was the 1st patient they lost from the sodium based IV red die.. The Vaccine has 6 parts. 4 of them are sodium based,, NOT GETTING THE VACINE! Plus the reaction from flue shots , I was told never get any VACINE EVER.
does it really help?did anyone in the trial share any results regarding long term improvement?
I’m a 34 year old who’s had chronic fatigue for the majority of my life. My stamina was definitely become lesser than my peers by the time I was 11, and I’d reached my current level of dysfunction by the end of high school. Some of the comments state that, if all the clinical trials go well, this could be available to consumers within the next 3-5 years. It’s a nice thought that this could be over by the end of my 30s.
I don’t give much credence to any of this research
Remember Jonathan kerr 2 be years to cure about 20 years ago research is just a job to them
A doctor told me while you keep giving them money they will keep testing you
Cort,
I’ve been eagerly awaiting news on this.
Do you have anything new to add???
I have to disagree with the statement that ME/CFS presents with the most extreme case of “dyshomeostasis” you can find. “Where else, after all, do you find dramatic declines in functionality, such difficulties responding to stressors such as exertion, mental activity, even such seemingly innocuous things like lights or sounds?”
The answer is fibromyalgia. Fibromyalgia has that and a whole lot more.
Fibromyalgia is very close to ME/CFS in symptoms and can stand in for it many times. In general I think they’re very comparable. I’ve never seen anything to approach the really severe ME/CFS – people totally bedbound in a darkened room, kept alive by feeding tubes, unable to talk. Check out Whitney Dafoe’s story on this website for more on that.
https://ksparrowmd.com/wp-content/uploads/Cardiovascular-functions-of-central-corticotropin-PMIDP30922523.pdf
CRF dysfunction can lead to cardiovascular abnormalities. It explains orthostatic intolerance and sympathetic hyperactivity, or dysautonomia. Low blood flow to the brain causes oxidative stress and neurochemical imbalance (gaba/glutamate, or some aminoacids), which leads to neuromuscular fatigue and wired but tired symptom. I don’t understand how dysautonomia can explain PEM, but CRF system is fundamental and regulates bodily homeostasis, so this dysfunction can explain PEM too, I believe. Since CRFR1 and CRFR2 expressed in the whole brain (especially in the limbic system and brainstem) and other parts of our bodies, I think receptor upregulation can easily explain all other symptoms too.
I believe this hypothesis it true, and I don’t understand why other researchers didn’t pick it up. There is nothing that can disprove this theory, at the moment. I don’t understand why many people says that this is 100% wrong theory and it will not lead to discovering a cure/treatments for us.
Hey Cort — What’s the status on Cortene? Anything new to report?
Thank you! Amazing. I’ve had ME/CFS for years…. this gives me hope. How can I help?