The Norwegians are doing it again – reminding us that the fight to understand ME/CFS/FM is an international effort and that crucial insights are coming in from across the globe.
The Norwegian odyssey has been interesting. Fluge and Mella began with an immunological treatment (Rituximab), and are assessing another one, cyclophosphamide, but have had their eyes on energy production for quite some time. Their 2016 metabolic profiling paper suggested that something had gone wrong with a key enzyme called pyruvate dehydrogenase (PDH), and in 2019 Katrina Lien’s 2-day exercise study found high accumulations of lactate.
A Metabolic Map
Now comes a big, complex, and undoubtedly quite expensive Norwegian metabolic study. In fact, one of the authors called the study “A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome“, “the most comprehensive overview of the metabolism” in ME/CFS to date.
Given how important metabolomic studies have been to this field that was big news. Led by two researchers new to the field (Fredrick and August Hoel), and overseen by several old hands (Karl J Tronstad, Øystein Fluge, Olav Mella), the 118-person study incorporated metabolomics (metabolites), lipidomics (cellular lipids; i.e. fat) and hormonal assessments together to produce a ” metabolic map” of ME/CFS.
About 1,700 substances in the blood were assessed. The ME/CFS patients in the study met the Canadian Consensus Criteria.
A Core Energy Problem?
Particularly in an area that potentially cuts so close to home – energy production – it’s important to see consistent results – and this study had them in spades. The fact that the levels of 67 metabolites were either significantly higher or lower in ME/CFS suggested a core energy production energy problem was present. In a Science Norway report, Tronstadt noted the widespread changes found in two groups of compounds closely tied to energy production – amino acids and lipids.
“When you find such extensive changes in the pattern of amino acids and lipids, it indicates that something is going on with the energy metabolism,”
Starving Cells?
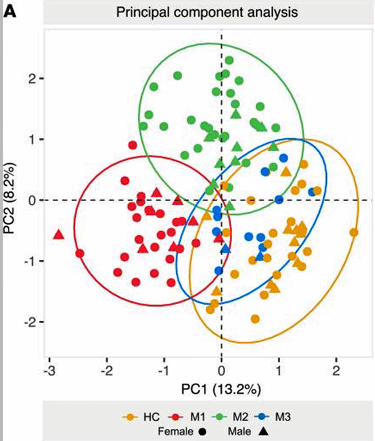
The principal components analysis showed a quite clean separation between the groups.
In a recent interview, Chris Armstrong, the leader of the OMF funded Australia said the dirty fuel scenario- the cells of ME/CFS patients have turned to a dirty fuel (amino acids) to power them – was holding up.
It certainly did in this study which suggested that ME/CFS patients’ cells are simply doing what any cell (or person) would do when starving or put under extreme metabolic stress: they’re basically consuming everything and anything they can to survive.
The Gist
The most comprehensive metabolomics study to date came up with a plus – a new kind of “comic: analysis – lipidomics – the study of fats
Widespread changes in amino acid and lipid metabolites pointed to a possible dysfunction in energy production.
This study suggested as past studies have, that the cells of people with ME/FS are trying to get a hold of energy in any way they can. Instead of relying on carbohydrate metabolism for a clean source of energy, they’re turning, for some reason, to “dirty fuels” such as fatty acids and amino acids.
Despite the fact that people with ME/CFS are neither starving nor are engaging in intensive exercise, their metabolic and lipid results are similar to those found in starvation and after intensive exercise. The authors proposed that the metabolic systems in ME/CFS are stressed even while they are at rest.
While a core metabolic issue seemed to be present in all the ME/CFS patients, three subsets of patients could also be identified. The authors proposed that the three subsets represented different ways the cells of people with ME/FS are attempting to compensate for their metabolic deficiencies.
One group – the M1 group (about 40%) was characterized by high degrees of fatty acid and amino acid breakdown. High levels of ketone derivatives also suggested this group had a ketogenic slant. Little evidence of mitochondrial problems was found, and the authors characterized this as the “lipolytic” group. Their metabolic profile was similar to that seen in starvation and after high-intensity exercise.
The M2 group (about 45%) displayed increased fatty acid breakdown but was characterized more by increased amino acid breakdown. Evidence of mitochondrial dysfunction (high pyruvate levels) was also found. This group, which had the most severe symptoms, had similar metabolomic/lipidomic profiles to diseases characterized by inflammation.
The M3 group (@15% of the study) appeared to be intermediate between the healthy controls and other ME/CFS groups but was too small to be assessed statistically.
Calling ME/CFS an “immunometabolic” disease, the authors posited that an autoimmune reaction is impairing blood flows to the tissues and pointed to a finding suggesting that the oxygen in the blood is not getting to the mitochondria.
The idea that blood flow problems are playing a key, even central role in ME/CFS has gained traction lately with these authors, Wirth and Scheibenbogen, Systrom, Shungu, and others proposing it.
” These observations may suggest that metabolism is stressed in ME/CFS patients even in absence of activity.”
It’ll be fascinating to see Chris Armstrong’s results as he dives deep into these cells and finds out how the cells are behaving and what they’re using to power themselves.
Metabolic Subsets Identified
While a core problem seemed to exist, there was nevertheless lots of spread in the other metabolic data – suggesting that different people were compensating for the “energy strain” present in different ways. In the Science Norway interview, Tronstad explained how this might be happening.
“If one part of the system fails, you can initiate a reserve programme to save the situation. We think these reserves might be more individual, which could explain the different subgroups we’ve seen here”.
However, each person compensates for their core energy problem, a core energy problem that was found ME/CFS-wide would presumably be great news as solving one problem would be much easier than trying to solve several.
This appears to be the first study to clearly identify 3 metabolic and “lipodomic” (lipid) subsets. The principal components analysis (PCA) showed a surprisingly clear separation between the three groups.
Some people (MI) are breaking down more fatty acids, while others (M2) are breaking down their muscles to get energy. None of the groups is doing very well at breaking down the best source of energy – carbohydrates.
MI Group – a Lipolytic State
The M1 group made up about 40% of the ME/CFS participants. It featured high concentrations of ketone bodies (3-hydroxybutyrate, acetoacetate) and lots of lipid metabolites which the authors believed might indicate a shift toward a more ketogenic metabolic state and a lot of fat burning.
Although the authors didn’t say so, one wonders if this group might benefit from a more ketogenic diet which provided plenty of fat stores for their cells to utilize. Low levels of amino acid metabolites also suggested that this group was breaking down their muscles to provide energy. Low tryptophan levels suggested this group was missing this vital compound.
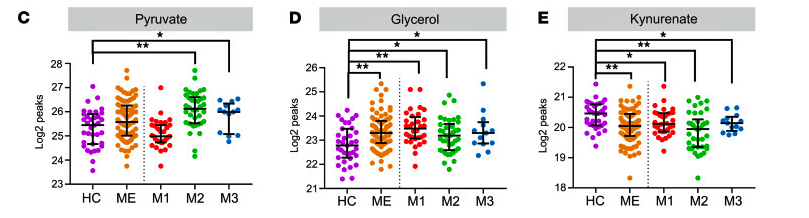
Different groups had different metabolite signatures. Check out the increased level of pyruvate in the M2 group and the decreased level of the same in the M1 group.
Pyruvate and Krebs cycle (aerobic energy production) metabolites, interestingly, displayed only minor alterations suggesting, perhaps, that pyruvate was being broken down mostly properly and the Krebs cycle was not broken.
The lipidome analysis (thankfully) largely matched the metabolomic results – as it found indications of lipid breakdowns (high levels of free fatty acids, glycerol, and ketone
The authors proposed that the people in the M1 group were caught in a “lipolytic state” due to problems utilizing carbohydrates for fuel. No mention was made of problems with aerobic metabolism.
They noted that this pattern is found is similar to those found in starvation or after exercise.
M2 Group
The M2 group was found in about 45% of the ME/CFS participants. In contrast to the M1 group, the M2 group had lower levels of fatty acid derivatives – suggesting that they weren’t breaking down their fatty acids as extensively. Higher levels of amino acid derivatives, on the other hand, suggested that this group was breaking down amino acids, instead.
The M2 group may also have mitochondrial problems.
An increase in serum pyruvate levels suggested it wasn’t being broken down properly and that the mitochondria perhaps weren’t getting the acetyl-CoA and NADH they needed to power up. Increased tryptophan levels suggested this vital compound was readily available but the lack of tryptophan derivatives suggested it wasn’t being broken down properly.
The authors concluded that this group may have had problems with lipid metabolism, mitochondrial oxidation, and “lipid trafficking and storage”.
The lipidome analysis found high levels of triglyceride fats (TAGS) and low levels of non-esterified (“free” or unsaturated) fatty acids (NEFAs). They noted that high TAGS are often associated with metabolic imbalanced and cellular and mitochondrial stress responses. Similar types of findings have shown up in chronic diseases characterized by inflammation.
This group, perhaps not surprisingly, given the emphasis on lipid and amino acid metabolism and possibly mitochondrial problems – were the severely affected.
M3 Group
The smallest group (15%) appeared to be intermediate between the healthy controls and the other groups. Too few people were in this group, though, to assess them properly.
Immunometabolic Disease?
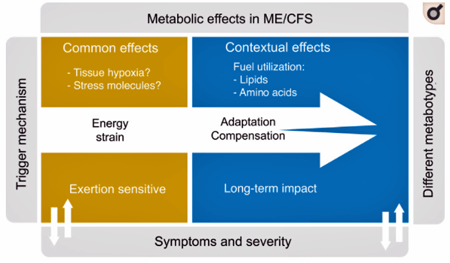
The authors proposed that different compensatory responses to the “energy strain” in ME/CFS are producing different metabolic subsets.
The authors don’t believe that metabolism is simply about energy production – not in diseases like ME/CFS, diabetes, multiple sclerosis and rheumatoid arthritis. Calling the problems in ME/CFS “immunometabolic”, Fluge, Mella, and Tronstad proposed in this paper, as well as their recent hypothesis paper, that the energy problems in ME/CFS have an immune origin.
They believe an autoimmune reaction impairing blood flows to the tissues is keeping oxygen from getting to the mitochondria and turning on the powerful aerobic energy production pathways. They pointed to the group-wide elevation in purine nucleotide metabolites found as evidence that oxygen utilization problems are present.
Impaired Blood Flows Highlighted
Running low on reserves the authors believe ME/CFS patients cells are “straining for energy”; i.e. they’re attempting to get it however the heck they can.
They’re certainly not alone in their focus on impaired blood flows. Autoimmune processes that interfere with blood flows play a central role in Wirth and Scheibenbogen’s now three-part ME/CFS hypothesis.
Systrom has actually found evidence of poor oxygen extraction in ME/CFS.
His invasive exercise studies also suggest that microcirculatory problems are impairing blood flows. Shungu’s findings suggest that blood flow problems are present in the brain. Using a novel technique Van Campen/Visser/Rowe have found reduced brain blood flows exist in virtually everyone with ME/CFS doing a tilt table test. Ron Davis is continuing to work on red blood cell deformability.
Several fibromyalgia studies have also highlighted possible endothelial and microcirculatory issues. Blood flow and red blood cell issues are also being seen in long COVID and could be the tie that binds all these diseases.
Time will tell if an autoimmune or immune reaction (Bruce Patterson believes it’s an immune reaction) is whacking the blood vessels and/red blood cells in these diseases. It’s good to see the research findings and hypotheses in this often rather rdiscombobulated field cohere around problems with energy production and blood flows.
BIG (little) Donation Drive Update

Keep the information flowing – support Health Rising
If there’s anything Health Rising’s been has focused on it’s been energy production. Over the past nine years, HR has devoted over 60 blogs to the energy production issues in ME/CFS, FM, and now long COVID. That’s because our goal is to keep you up to date on the potentially most far-reaching findings in these diseases. Regarding that, nothing beats energy production.
Ask yourself where else you’d be getting this information and please support Health Rising.






Hope these researchers are able to make a diagnosis test based on groupings. Glad they used the Canadian Consensus criteria for patient selection.
(hope taking bigger doses of vitamins wouldnt mess up their testing)
Starving for amino acids/protein, can identify with that……until that seemed broken too…..and energy made gets stored as fat? cause no energy/O2 to move??….,,
Does anyone else have thalassemia minor with their ME/CFS? I wonder if the red blood cells’ decreased ability to transport oxygen in my thalassemia interacts with my ME/CFS.
Hi, a group of researchers in France are getting ready for phase one clinical trial based on amino acids. The reason the clinical study has been delayed is “ there don’t use medication” 😳
I find this very interesting, especially that ‘the energy problems in ME/CFS have an immune origin.’ ‘Immunometabolic.’
Also that ‘an autoimmune reaction impairing blood flows to the tissues is keeping oxygen from getting to the mitochondria and turning on the powerful aerobic energy production pathways.’
Years ago, I remember saying to my doctor, I’m just not getting energy from the food I eat. I described myself as like a vintage car that ran on some obscure fuel, that I didn’t have access to. Back then I was thin, pale and at times, frequently felt I was on the point of collapse. I have extensive food intolerances and immune issues.
What made a difference for me I think, was that my reaction to some food – chocolate and chocolate chip cookies actually fired up my system, probably because I’m sensitive to them. What happened, when I ate them was that it put my blood pressure up (which had been lowish) and I then could feel more energy and the colour in my cheeks came back etc. Very low tech and controversial!
I have really had to focus on all the well known and boring lifestyle changes, like pacing, diet, supplements, lessening sympathetic nervous system response where possible etc., over years to make headway. But I have improved my energy levels. I can still exceed them, if I’m not careful but I can do much more now. If I didn’t have the food intolerances, I’d be doing better – food is a constant challenge.
So, it’s not just that the chocolate is giving me energy, it seems to trigger a reaction in me (for whatever reason) that then fires up my whole system.
“a vintage car that ran on some obscure fuel” – nice. I was trying to think of a car analogy – a car trying to run on water or something like that. Yours is much better.
“lessening sympathetic nervous system response where possible etc” when people recover using mindfulness based practices my guess is that one thing that happens is they stoip the blood vessels from clamping down, stop the gut from doing the same and who knows what else – letting things flow freely.
I’ve often described this illness like a car running on empty or dirty fuel. I never realised just how accurate this analogy was. The time I collapsed as I walked back to my car reminded me of that time my car conked out due to dirty fuel in my tank. When people with ME run out of energy there’s absolutely nothing left in reserve. We just simply stop working and drop to the ground. We need our own broken down service to come and collect us😂
I agree Amanda but it’s very difficult to explain that to anyone, isn’t it? I lived on the edge of collapse for a long time. A few years ago, I felt that getting through each day was like attempting to walk across a frozen lake, never knowing where the thin ice was located. So each day I lived with the threat of dropping through the ice, into the cold, dark water below. Not exactly the same as being a bit ‘tired’!
You’re not the only one finding chocolate helpful
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3001690/
High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome
I had a look at that article, thanks E. It might seem silly but for whatever reason, eating the chocolate makes a significant difference to me. If I decide to leave it out, or I run out of it, I really don’t function very well and I seem to be more prone to feeling faint, with a racing heart and nausea. It definitely helps me with my p/t work.
I don’t know exactly what it’s doing but I know it’s more than would be considered a normal reaction to eating chocolate. However, I can’t eat the chocolate with the very high cocoa content, as it raises my blood pressure too much, my heart races and I don’t seem to be able to process it.
hi cort, i am not able to read whole the norwegian study.
did they (and others) also looked at sex differences? ME/cfs is much more common in women than in man. not saying that there are no man with it but there is a hormonal difference like in many deseases. thanks, a bit of hope again. But it is such a complex desease that go’s from head to too. And we are all so hetrogenous.
i am verry brainfogged, would the typical supplements help? Long time ago i was on a diet from someone who got miss bodybuilder worldwide. at that time, all the products (shakes) with everything in did not help. also the diet not. but creatine “helped”, i could do more. it was as if my brain was leteraaly half working/ one side awake :-). i could walk again for some time. but my periods stopped immediately and i found myself agressive with it. als with using my legs (the most wait) i got verry thick painfull nodes in my muscles. and i do not know if it is true, but an internist said that it was bad for my kidneys.
Yes, they did – it was quite a thorough study, Interestingly, they did not find that gender made a difference as I remember. While the hormone tests they did do had some abnormalities they were pretty minor. I don’t believe they assessed estrogen/testosterone.
I’m sure some supplements help some people. B3 – which is a vasodilator and mitochondria enhancer – definitely helps me for short periods of time. So does CoQ10.
Patterson has a different take – he believes an immune reaction is whacking the blood vessels and he’s found that statins and other drugs help. A blog on the very interesting Solve ME Webinar is coming up.
thanks cort!
may i ask how many milligram of B3 you take and of coQ10 and how often on a day from both ?
i ask this because i read from someone who takes 3 times a day 30 milligram of B3 and i think that is way to much but i can be wrong… I am allways afraid of overdosing for any “price”, do not want to harm my boddy even more.
thanks!
Enough to produce a “burn” which is generally about 100 mg. at a time. The results last from 10 to 30 minutes or so – and include increased calmness and mental clarity. It doesn’t always happen but when it does its really nice.
The first time I tried it I inadvertently took 500 mg – which made it seem like my skin was going to burn off (lol) but it also left me with an extended period of deep calm!
To be honest I never understood these kinds of studies. We have a disease marked by severe misery perfusion – who would expect normal metabolisms and energy production? And yes, at times your misery perfusion is severe (e.g. in phases of PEM), at times it´s better, in severely affected patients it´s more severe than in the less affected – so of course you have different kinds of surrogate pathways being active. I am sure, if somebody took a single person with ME/CFS and did metabolomics at several time points you´d see the whole gammut of metabolic derangements. I´d volunteer. For me, all this is just coffeeground reading (expensive coffee though).
The KEY is the ACiD Erythrozytes which made them unflexible !!!
Missing oxygen
more than 256 Symptoms under acid-base bloodcirculation
The omics studies are certainly indicating problems are there- which is good – and they are pointing in a general direction – which is good as well. The question I have about them is if they will actually ever end up with a specific target? They have done that in other diseases but it’s quite a journey.
What kind of studies would really spark your interest?
See, I also have the following problem with these studies: we do not know anything about intraindividual variance. Just look at the scatter plot in the article. Possibly a given patient would be M1 on one day, M2 on another and M3 on yet another (or have normal results). We just don´t know. I have never seen such longitudinal studies published. Yet every single ME/CFS patient knows that you are a different person with a different physiology on a good day than on a bad day. And of course these fluctuations in blood flow/perfusion have something to do with the status of our immune system, for those who live with this hell this, too, is a no brainer.
The sad part is I believe we have a whole collection of data to prove some of this and can’t get anyone to take it seriously. I have repeated data to prove most of this with my Whoop data (almost 3 years worth). Who knows how many others there are in the system.
Taking a shower registers as a full blown workout and I’ve typically used up my energy reserves after that. I never get sufficient sleep. The list goes on. And who knows what else the data is showing that we just don’t understand yet.
It’s so frustrating to see it every day and not get anyone to take it seriously.
It is beyond frustrating that such a debilitating disease can be so slighted by the research field for so long. While I think of it as a fascinating problem – what could cause so many people to become debilitated – think what you could learn about the body by studying it – obviously, is not how most view this disease.
ME/CFS is a real outlier – it’s different (exercise hurts instead of helps, for instance), fatigue has never been taken seriously by the research field, most researchers have never heard of PEM, and it’s invisible – no obvious structural problems. It’s different.
The growth of the Norwegian research effort and the two new lead researchers – and the positive results is a good sign. Yes, that needs to be duplicated 50 times over but the arc, as Carol Head puts it, is in the right direction.
Does anyone know any solutions to all this? Loaded question I know sorry x
It depends, in part, on what’s driving this. Klaus Wirth and Carmen Scheibenbogen have ideas about drugs that may help the blood vessel problem but I don’t know what they are. If autoimmunity is driving this – that opens one door. If Bruce Patterson is right (and he’s quite confident that he is) and an immune reaction involving macrophages is driving it – that opens another door. Mitochondrial enhancers could help the group with the mitochondrial problems. Blood volume enhancers can help some people a lot – Health Rising actually has some recovery stories involving something as simple as that. Mindfulness practices may, I would think, tamp down sympathetic nervous system activity – helping the blood flows. I’m sure there are quite a few other possibilities that I haven’t mentioned.
I’m encouraged because while the treatment isn’t obvious at least the field seems to be heading in a general direction.
Cort, well said. Thank you for expressing the frustration we all feel .
As far as sleep is concerned, Dr. Cheney told me to take 1 mg of Klonipin at night. It takes a bit of time for it to kick I but it works very well for sleep. Sincerely, Javen Morell. Hipjaven@gmail.com
I have that problem with showers, when I am bad. Thankfully not at my base level. Cold showers was the only way I could go.
Some evidence that the calm the nervous system as well (once I stop screaming of course)
🙂
Hi Cort, I’m a long-time follower of you but my first time commenting. I’m a 65 years old male and have suffered with chronic fatigue/ Lyme disease / mold toxicity for 16 years…been to 14 clinics in the past 6 yrs. I have of late tried a ketogenic diet and have been on it for 5 weeks now with no real benefit and possibly even more weakness,especially PEM since I am an
electrical contractor still trying to make a living. In light of this latest article and research do you think I am barking up the wrong tree by trying to get my cells to use healthy fats for energy? This was actually recommended to me by my cardiologist & he wants me to try a water fast for 3 days as of tomorrow in an attempt to “reset” my cellular metabolism…feeling too weak already but I am ready to try ANYTHING! Thanks so much. Alan B.
The keto diet can be very helpful for some and can take a while to kick in but you do have to be careful. I’ve heard of people had a negative response to it and kept on it and got in real trouble. It doesn’t sound like you’re in that category – you may be experiencing more weakness – but if things start going south then I would abandon it.
Maybe someone more familiar with the diet can be more helpful.
For me, I was sure I would benefit greatly from a keto diet as I don’t do well with sweets and starches and I do benefit somewhat from it but not a lot. (I’m still on a low carb/high fat/higher protein diet).
As to the water fast – I would be careful. It works fine for some people (my sister loves them) but I, for instance, am unable to tolerate longer fasts. I would abandon anything like that if my symptoms really ramped up while I was trying it.
Have you tried intermittent fasting first? Courtney Craig, a nutritionist with ME/CFS – knows fasting and highly recommends intermittent fasting for ME/CFS – https://www.healthrising.org/blog/2014/07/10/craig-fasting-health-fibromyalgia-chronic-fatigue-syndrome/
You might want to try that first and see how it goes and then decide if you want to try the longer fast. Good luck!
I suspect that ketogenic diets may have affects on the gut microbiome that are damaging to many PwCFS. I have tried ketogenic diets quite a bit over the years – ranging from as few carbs as possible (mostly just non-starchy veg and meat – “paleo diet”) to eating only small amounts of starches and fruit. I have not found those really ketogenic diets to be useful, but I have found limiting glycemic load (glycemic index multiplied by amount of carbs) to be very important. Since about 3 years ago I consume a moderate amount of complex carbs (since much less carbs than than in the average diet and all from good whole foods). Eating even small amounts of simple carbs quickly has a negative affect on me – anything beyond 1 bit of cake for instance can give me a headache, faintness, more lethargy, brain fog. So I avoid food intake that would involve a high glycemic load since that definitely affects me badly. I have a bowl of porridge most mornings and that is about the highest glycemic load I can handle (40g oats, 10g butter, 40g soy milk, 80g blueberries; cooked the day before, chilled in fridge to form resistant starch and reduce the GI, then slightly warmed up). When I was eating few carbs and lots of fat, my gut microbiome analysis revealed very low diversity and a complete absence of f.prausnitzii (a highly beneficial species that suffer from high intake of fat and protein). I have had chronic constipation since even before developing CFS and it got really bad – I’m taking rocks that made tears – on the paleo diet. Since I’ve been eating more carbs (but healthy ones and never in very large quantities), the constipation has improved though I haven’t repeated the microbiome analysis.
I have tried intermittent fasting – mostly for almost 24h by skipping breakfast and lunch – and it has seemed to have some benefit sometimes, but I think the biological stress of it maybe counteracts the benefits (since PwCFS are hypersensitive to all kinds of stressors). It made my sleep much worse on day when I had skipped brekkie and lunch. So I got the impression that overall the effect was neutral. Responses to fasting may well vary greatly from person to person and depending on the exact details of the fast.
To clarify a typo: more than 1 BITE of cake (not “bit”) is risky for me, even 1 bite sometimes brings some unpleasantness.
Thanks again Cort for another great writeup 🙂 I am pretty certain it is an immunometabolic disease too. I watched this really exciting talk be Dr. Patterson at IncellDX, where using AI techniques, they identified immune dysfunction and available drugs to treat long Covid, and the immune dysfunction was very similar to their ME/CFS patients! They plan ME studies in the future, but right now their drug regimen is really helping long-covid patients. So they are expanding as fast as they can to include more long covid patients, and I’m wondering if ME patients who got covid might be able to participate now? Not sure but it might be worth an ask to the company. Here is the video of the talk he gave to SolveME https://www.facebook.com/SolveMECFSInitiative/videos/1081944279223432/?comment_id=1082046925879834¬if_id=1638995996896716¬if_t=feedback_reaction_generic&ref=notif and the study site https://covidlonghaulers.com/ I love AI and complex systems, so completely geeked out. I SO wish my math brain didn’t break so badly with ME. I wish I could help 🙁
Yeah I have heard good things for the Long hauler treatment programme. I think from memory they used asprin a statin an antihistamine and a expensive HIV drug. I have not heard personally how M.E folk are doing on this regime but I’m keeping my ear to the ground.
So I see myself as an M2.
The data stacks up beautifully.
From the beginning of my illness – generated by a toxic exposure – I wanted hyperbaric treatment – just KNEW it would help. Ten years passes and I still don’t have the right ‘condition’ that qualifies for a an appointment. Let alone a session.
beyond frustrating.
Jensy, I see myself as an M2 and I too had a chemical exposure trigger. Early on my internist did a treadmill test and my 02 sat hit 66% during it. My 02 recovered after I got off the treadmill but I was knocked back for weeks. Much later I got home O2 after a fingertip monitor showed my sat was dropping randomly down into the 80s & sometimes the 70s. I can go a few hours without O2 in low-load/resting conditions but it still sinks without warning eventually.
I’ve done the protocol for one month and have improved, I’d say about 25% (I have long covid and ME). I take low dose aspirin, propranolol, a statin, and a short course of ivermectin.
Thanks for the link. That was a great talk. A blog is coming up.
Yes, he is accepting ME/CFS patients. I’ve never had covid or the vaccine but I signed up for his treatment in Sept. due to various events I haven’t started on the meds but I do have various abnormal cytokines, an elevated d dimer and CRP, extremely high lactate and pyruvuse (sp?) and a few other tests that show an abnormal immune and metabolic system. Hoping to start the meds in a few weeks and for some improvement! Feel free to sign up anytime, they are always looking for more volunteers and data.
Nick, your observations about keto are correct. Unfortunately I was put on it and it completely destroyed my microbiome and gave me fatty liver. I am trapped with high levels of methane now and many food intolerances which has taken me down to 3 foods. This research fits me exactly and lines up with the results of my Acid Oats tests. Your observations also fit perfectly. I desperately need complex carbs but they actually feed my methane (research from Pimental now explains this). I’m really struggling to get out of the trap I’m caught in which started with heavy mould exposure.
I’m one who gets much sicker on a ketogenic diet, and am already severe. So I’m really happy for the people it helps, but like everything with treating ME, please go slow in implementing it in case you have a bad reaction.
At the end of 2012, I was diagnosed with a cancer. After my body had time to heal from the surgeries, I started chemotherapy in the second half of 2013 and got my last dose in 2014. Cyclophosphamide was one of the four poisons included in the protocol my oncologist chose for me.
My oncologist knew I had ME and took it seriously. He was watching me closely and even warned me that he might not insist on my going through the whole length of the chemo treatment. He was surprised at how well I did recuperate between perfusions until the end of the whole treatment.
Of course, while under treatment, I can’t say that my ME improved :). But, after the side effects of the last perfusion subsided, my ME improved drastically; I would say by close to 90%. All my family, including my children, told me that I had not been in such a good shape in years.
I told my oncologist about it. He raised his – very thick – eyebrows and answered that in the last stretch of years, he had had three other patients fighting both ME and cancer, who experienced the same improvement shortly after chemo was over.
However, whatever was causing our ME, which chemo had managed to damper dramatically, found new strength while the following radiation therapy was straining our newfound energy.
My doctor referred me for a consultation in the rheumatology department of our hospital. I never heard from them and I didn’t insist, as I was afraid that they would forbid my GP to renew the prescription for stimulants that allow me to get some work done.
It just so happens that I have my annual follow-up with my oncologist tomorrow. I will tell him about this new Swedish investigation. He might already know about it, since he was aware of the small study on Rituximab. He reads a lot!
Thank you Cort for all the information contained in the issue.
Isn’t that something! That is really something…
does that mean that ME. is possibly a form of difficult to diagnose cancer? hope not ?
@Genevive. Thank you for your post. Even though I don’t know you I am over the moon to hear that (any!) your oncologist takes your ME seriously. I have not seen any other pwME discussing the topic of cancer. I was diagnosed with Triple Negative Breast Cancer (TNBC) of the right breast in March 2013. When severely immune compromised from the IV chemo, I contracted a virus that landed me in the hospital for 4-5 days. I had the worst headaches of my life, and wouldn’t have been surprised if I died in my sleep. They were that bad. Ever since that hospitalization, I have not been right. It took me 6 years to figure out I had ME.
As well, in 2014 I was diagnosed with cancer of my scalp. In October 2014 had MOHS surgery and a split thickness skin graft from my left thigh to approximate the wound (about the size of an adult’s palm) of the left scalp and partial forehead. I remained sick as a dog, and found more cancerous tumors in August of 2015 in my right chest wall. Had another port inserted, more chemo, surgery, and radiation. The chemo was completed in October 2016. It has been an uphill battle to defend myself in order to receive SSDI and the absolute worst of all my health-challenges, fighting for my private Long Term Disability (LTD) with the company Lincoln Financial. SSDI has been more than helpful and reasonable. Lincoln Financial has been an absolute nightmare, obstructionist, brow beating one into giving up (which I will never give up), as well as many things I shouldn’t write on a public forum.
I found that eating vegan, no dairy, gluten-free, no processed foods, little to no SOS: Salt Oil Sugar and only utilizing organic, avocado oil, for oil, and scant amounts of sugar has optimized my health. At least one green tea per day.
For drugs and supplements, there are two medications that have made it possible for me to live. The first is IV ketamine, the second LDN. I have rambled on much too much already, but wanted to add my personal findings.
My utmost gratitude and respect to Mr. Cort Johnson for your unbelievable work and contributions f to the ME community. Thank you Sir.
Thanks again Cort for your great work!
What I am always curious about is how different I can feel from one day to the next .
Why do I feel really sick and some days feel “pretty” good – no difference in energy out put or diet. Sometimes within the same day!!
Great question. All I can think is that our systems are pretty tippy! Even small stressors can impact them. That’s certainly been my general experience.
Thanks. There certainly much about this disease we don’t understand!!
Hi Pat, my day is spent trying to figure out what’s going on and what I need to do about it. I’m very sensutive to food etc., but also I don’t think my system regulates itself very well anymore, or to the correct setting, so I have to try and regulate my system myself!
@Pat. I am 100% on board with your experience of wonder re: the lability of our “feeling well’ vs. horrendously sick…..as you’ve stated, sometimes within the same day. ME is literally an hour by hour challenge.
I am interested in hearing more about others’ experiences with hyperbaric oxygen. I’ve heard a couple of positive anecdotes, but nothing more. Any research? Any experiences? Any adverse effects? Thanks!
I have found HBOT very helpful and my experience with it would fit in well with this theory.
The big problem with HBOT is that it is very expensive for the benefits that it provides.
I do know a lot about the science of HBOT. I think that the initial intensive daily protocol helps cells to repair any anaerobic damage that has been done (this phase can feel quite awful for many) and then maintenance helps to reduce the damage of anaerobic periods.
I do wonder what an extended protocol period would be like (going everyday months instead of weeks) but that would be incredibly expensive!
Is there a correlation between weight fir M1’s or M2’s, i.e. wasting or weight gain?
It would be interesting to see if the M1/M2/M3 groups related to severity of symptoms at all – mainly whether those in M3 were perhaps more mild and then developed more metabolic derangement alongside more severe disease
also would be interesting to see poll here of how many people feel they fit into which group most, and which group secondly in the M1 M2 M3
and rate severity —( but add one more group between each ratings eg severe and very severe have another in between……..etc
Of the recent research studies you’ve written about lately, the gut bacteria one, the small fiber neuropathy, and this study, which results do you think are leading us in the right direction? Or all all the findings likely related somehow?
Do those with ME/CFS/FM have low levels of cortisol?
When my herpes started interfering with my daily life. And my lips always bother me every damn day. I remember going to the hospital when I was around 25 years old cuz my whole lips were burning like hell. And a nurse came to the waiting lobby and said sorry there are no doctors here. So I had to go back home. My lips were burning like hell and I felt so uncomfortable that night so I turned to the internet where I found a drortizthenauturopathic . com. I’m a man. I don’t know what would have healed me from this virus if not for the product. I took a comprehensive STD blood test last week again and everything came out negative ,thanks to drortizthenauturopathic . com.
Much of this information has already been known for decades. Dr. George Watson wrote a book back in 1972 called ‘Nutrition and Your MInd’ about how differences in the body’s metabolic rate and it’s connection to various mental disorders. But the real genius was his work on how various vitamins and minerals could either speed up or slow down metabolism – in other words – metabolism is malleable. Watson’s work was the foundation for Dr. Paul Eck’s program called Nutritional Balancing (aka mineral balancing). Dr. Eck work used a specific type of hair mineral analysis (that didn’t alter water soluble minerals) to determine the body’s metabolic rate, and used a combination of vitamins and minerals to move the body to a more efficient metabolic state (producing more ATP).
In summary, you could say that in slow metabolism (called a slow oxidation rate), the body does not break down enough sugars to effectively utilize fats in the Krebs energy cycle. And in fast metabolism sugars are utilized too quickly, and more fat breakdown is needed for the optimal energy levels.
It is shockingly easy to see how dis-regulated metabolism can become by looking at hair mineral levels (considered a cellular biopsy). While blood levels of minerals are regulated to maintain consistent reading, cellular levels can vary by hundreds of %. In slow metabolism, calcium & magnesium levels will go up and sodium and potassium levels will go down – and vice versa for fast metabolism. In slow metabolism, these changes in minerals levels make the cells less permeable so that thyroid, adrenal, and insulin have a lower affect compounding the low metabolic problems in a domino type of effect.
I have personally been on a Nutritional Balancing program for a number of years and have almost completely recovered from a severe case of CFS. When I added exercises to calm my nervous system (vagus nerve exercises) my progress increased dramatically. That is because the body tends to accumulate minerals and toxic metals that have a stimulating affect on the nervous system to offset the loss of energy from poor metabolic output. As energy increases those stimulating elements are eliminated (detoxed) which can be difficult at times, overstimulating the nervous system as they circulate prior to being excreted. The vagus nerve exercises help tamp down the nervous system and allow for better sleep and quicker healing.
The (late) Dr. Paul Eck set up Analytical Research Labs in Phoenix AZ to do the hair mineral analysis tests for those that are interested. Their website has many good articles to read. You need a Nutritional Balancing practitioner to submit the test and assist with the evaluation, you can search online for one. This was the only test that ever accurately diagnosed my ME/CFS. It never ceases to amaze me that it is not used extensively by both patients and researchers – so much time and money is wasted by not utilizing what is already known and building on that foundation.
What about the theories that PUFAs (especially Linoleic acid) is creating Torpor (hiberation) in people?
Keywords for searching: Ray peat diet, croissant diet, reddit StopEatingSeedOils.
I’m still with the saying: Let food be thy medicine and I’m sure at least a percentage of CFSers can be helped by this.
Thank you Cort for your article about these findings!
One week of bed rest results in 1,5 kg of muscle mass decrease for healthy. Something that I think about sometimes is why I apparently do not lose muscle mass after 20 years of inactivity because of ME. My thought has been, maybe because my body senses that I exercise because of increase of metabolites (e.g. lactic acid, oxidative stress, …) due to ME.
I would love to know why fatty acids are considered “dirty fuel” and carbs are not.
Dr Sarah Myhill’s work led me to believe that fat was a superior fuel source.
I think they’re referring to something other than diet. The results suggest that high fat, higher protein diets are better for ME/CFS than high carbohydrate.
Interesting. There is other work in which evidence is presented, where defective mitochondria give rise to autoimmune problems. https://factor.niehs.nih.gov/2021/3/papers/autoimmunity/index.htm
I think ME/CFS fits to a certain extent in the list with Sjogren, Lupus, etc described in the paper in the link.