

Friendly Fire?
Recent studies featuring potentially game-changing blood vessel problems in ME/CFS/FM and long COVID have been illuminating but have lacked a crucial component: how it all started.
A July study led by Edward Yang and fellow Yale researchers published in the Nature journal provides one possible answer. In the “Diverse functional autoantibodies in patients with COVID-19” study, Wang used a high-throughput autoantibody discovery method known as rapid extracellular antigen profiling (REAP) to assess the production of autoantibodies produced during and after COVID-19.

An antibody-producing plasma B-cell.
In their zeal to get rid of a pathogen or foreign substance, sometimes the B-cells make a mistake and produce autoantibodies that attack our own tissues – causing an autoimmune disease.
Autoantibodies aren’t necessarily or even mostly dangerous. A recent study comparing post-intensive care non-COVID patients with COVID-19 patients suggested that any serious illness is likely to result in increased levels of autoantibodies. Avindra Nath has stated that autoantibodies are found in large numbers in every neurological illness and doesn’t seem to think they are of great consequence.
The Wang study suggested, though, that the SARS-CoV-2 infection had produced an explosion of rare and uncommon autoantibodies. Three general classes of autoantibodies were found:
- autoantibodies that were likely present before the infection and were doing no harm;
- autoantibodies whose presence rose as the B-cell (IgG) response) mounted but then declined and produced mischief for a short while;
- autoantibodies that rose as the B-cell response mounted and remained high afterward – and could be implicated in long COVID.
Immune System and Vascular and Connective Tissues Hit
Autoantibodies targeting the interferon antiviral system suggested that the B-cell response had mistakenly knocked out one of the most potent antiviral responses in the body – resulting in severe illness.
Laboratory (in vitro) tests suggested that the autoantibodies were indeed having an effect and were associated with reductions in cytokine levels and natural killer cells and monocytes.
Another large set of autoantibodies targeted the vascular system (blood vessel cells, coagulation factors), connective tissues, and organs including the central nervous system compartment, skin, gastrointestinal tract as well as others.

Antibodies attacking the coronavirus. Large numbers of rare and uncommon autoantibodies were found during and after a coronavirus infection.
Another study found that COVID-19 spurred the production of a certain slice of autoantibodies focused on the skin, smooth and skeletal muscles, and cardiac tissues. That was an intriguing finding given other studies that have suggested that blood vessel functioning is impaired in long COVID.
This wouldn’t be the first-time autoantibodies have been proposed to affect blood flows, hearts, and gastrointestinal systems in people with post-infectious illnesses. Over the last decade or so, researchers have been asking whether a similar process is occurring in postural orthostatic tachycardia syndrome (POTS) and chronic fatigue syndrome (ME/CFS).
The fuss all revolves around a certain group of receptors.
G-protein Receptors
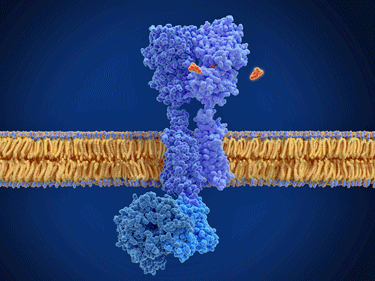
The binding of a G-protein receptor (red marks).
The autoantibodies in question in ME/CFS, POTS, and long COVID are all focused on the G-protein receptors that control autonomic nervous system functioning.
The receptor question is complicated and detailed and many people may want to skip ahead or go straight to the Gist at this point.
The crucial thing to get, though, is that the receptors these autoantibodies may be affecting govern things like blood flows, heart rate, gut motility, and energy production. As such, they play a particularly important role in our ability to exercise and stand without symptoms. Given that, the question whether autoantibodies triggered by an infection could be affecting these receptors in these diseases certainly makes sense.
The two main kinds of receptors in question are the adrenergic receptors and the muscarinic receptors.
Alpha and Beta-Adrenergic Receptors
Sympathetic nervous system (SNS) produced hormones like epinephrine and norepinephrine use the adrenergic receptors on the cells in the heart, blood vessels, gut, etc. to make things happen.
During exercise, the alpha-adrenergic receptors induce the blood vessels to constrict in order to force more blood into the muscles. Meanwhile, the beta-adrenergic receptors are causing the blood vessels associated with the intestines, skin, and kidneys to relax – diverting blood away from those organs in order to make more blood available to the muscles.
Adrenergic Receptors
Two major types of adrenergic receptors exist – the alpha and beta-adrenergic.
Alpha-adrenergic receptors (A1AR and A2AR) – mainly occur in the smooth muscles lining the blood vessels and adjacent tissues. They stimulate smooth muscle contraction, trigger vasoconstriction, intestinal relaxation. When you think of overly narrowed blood vessels – think of the alpha-adrenergic receptors.
Beta-adrenergic receptors (B1AR, B2AR) mainly occur in the bronchial, heart, and uterine muscles. They cause the smooth muscles lining the blood vessels, gut, etc. to relax resulting in reduced blood flows, increased relaxation of the bronchial tubes and gut, and increased heart rate.
They also induce the liver and other tissues to produce glucose in an attempt to make that essential energy source more available to the muscles. They also activate the renin-angiotensin-aldosterone system (RAAS or RAS). Increased angiotensin II levels induce vasoconstriction thus increasing blood pressure and driving more blood flows to the muscles.
By turning down blood flows to the skin, gut, etc. the beta-adrenergic receptors ensure that enough blood is available for the muscles during exercise. They also help provide more energy by promoting the production of glucose.
Receptor Subtypes
Of course, it’s not as simple as that. Different receptor subtypes produce different effects. In order to decipher what the studies have found, we need to know what these specific receptors do. (You may REALLY want to skip this part.)
Alpha Adrenergic Receptor Subtypes
- A1AR – induces vasoconstriction of the blood vessels, increased heart rate.
- A2AR – induces vasoconstriction of the arterial and venous blood vessels, inhibits norepinephrine release, relaxes gut muscles, reduces gut motility, and fatty cell breakdown.
Beta-Adrenergic Receptor Subtypes
- B1AR – increases heart rate, heart contractility, fat breakdown.
- B2AR – blood vessel vasodilation, bronchial tube dilation, bladder wall relaxation, liver glucose production (gluconeogenesis and glycogenolysis), inhibits histamine release from mast cells.
Muscarinic Receptors
The last two receptors of interest concern the muscarinic receptors that regulate the other side of the autonomic nervous system – the parasympathetic nervous system (PNS). These receptors respond to acetylcholine – the main neurotransmitter of the PNS.
Since the PNS keeps the sympathetic nervous system (SNS) in check, and since SNS activation appears to be increased in ME/CFS, it would not be surprising to find that problems with these receptors occur in ME/CFS/POTS – and some studies suggest they do.
- M3 receptor – Interestingly, activation of this causes a constriction in the smooth muscles lining the bronchial tubes and vasodilation of the smooth muscles lining the blood vessels. This receptor also increases stomach and salivary gland secretions and regulates insulin secretion. Autoantibodies to the M3 receptor would presumably reduce vasodilation of the blood vessels – leaving them narrowed and unable to sustain normal blood flows.
- M4 receptor – Activation of M4 receptor, on the other hand, inhibits acetylcholine release. Damage to this receptor caused by autoantibodies would then, one might think, increase acetylcholine release – thus producing too much vasodilation – and reduce blood flows that way.
Postural Orthostatic Tachycardia Syndrome (POTS)
As the adrenergic and muscarinic autoantibody question has been investigated longer in POTS than in ME/CFS, it makes sense to tackle POTS first.
Note that damage to different receptors in these diseases may be evoking very different responses. For instance, damaged or dysfunctional B1AR receptors in POTS may prevent the blood vessels from tightening down enough and providing the pressure needed to drive the blood into the tissues. In an effort to get those receptors working again, it’s speculated that very high levels of the stress hormone norepinephrine are leaving POTS patients “wired and tired”.
Autoantibodies to a receptor not discussed, the angiotensin II type 1 receptor, may also be preventing the blood vessels in the legs from narrowing enough to stop blood pooling in the lower body upon standing.
The autoantibody situation in POTS is promising, but not entirely clear as different studies have, at times, found problems with different receptors.
A 2017 study found autoantibodies to several adrenergic receptors (α1AR, β1AR, and β2AR) in most POTS patients. The study also produced an important finding when the IgG serum from the patients was shown to impact the receptors in question.
Elevated autoantibodies to the B1 and B2AR, A2AR, and M3AR receptors were found in POTS in another study. By 2018 a University of Oklahoma group had demonstrated, in mostly small studies, the presence of four different autoantibodies (α1AR, β1/2AR) in POTS patients.
A larger 2019 study found that while many POTS patients had increased levels of autoantibodies against the A1AR receptor, a significant number also had autoantibodies against the M4 muscarinic receptor (53%). That strange combination suggested that problems with both too much vasoconstriction and too much vasodilation might be present.
The fact that the study found only a weak correlation of symptom severity with these autoantibodies, though, cast some doubt on the role they were playing.
Overall, the study results suggest that autoantibodies may indeed be affecting these receptors – and POTS patient’s health – but larger studies are clearly needed to validate the findings.
Chronic Fatigue Syndrome (ME/CFS)
Wirth and Scheibenbogen believe that elevated autoantibodies to the B2 adrenoreceptor (ß2AdR) and the M3 acetylcholine receptor play a crucial role in ME/CFS. They speculate that damage to these receptors has impaired the ability of the blood vessels to vasodilate – leaving them in a cramped narrowed state that’s impairing blood flows to the tissues. A massive attempt to open those blood vessels via the production of pain and fatigue-causing vasodilators is then producing the symptoms found in ME/CFS.
Several studies suggest that autoantibodies to these blood vessel, heart, and gut-affecting receptors may indeed be contributing to ME/CFS in at least in a subset of patients.
A large 2016 trial found that antibodies against β2, M3, and M4 receptors were significantly elevated in about 30% of ME/CFS patients. A 2020 paper potentially linked autoantibodies to the two beta-adrenergic receptors to abnormal findings regarding connections in two parts of the brain.
Recently, Carmen Scheibenbogen’s group has produced several autoantibody studies. A large (n=116) 2021 study found that fatigue was significantly correlated with a different immune measure (IgG/autoantibodies) of almost every autoantibody tested (A1AR, A2AR, B1AR, B2AR, B3AR, M3AChR, M4AChR, AT1-R-, ETA/B-R). This study used a different methodology (IgG/autoantibody ratio vs autoantibody levels) to assess the impact of these autoantibodies on symptoms. If that is indeed a more effective way to measure autoantibody impact, it’s a pretty dramatic finding.
The Gist
Could autoantibodies be causing mischief in ME/CFS, POTS, and long-COVID.
- Two studies have found that the coronavirus infection unleashes a wide variety of rare and uncommon autoantibodies – some of which stick around after the infection has been resolved.
- The autoantibodies mostly affect the immune cells, smooth and skeletal muscles, the blood vessels, coagulation, and the connective tissues.
- A similar question concerning autoantibodies that attack the autonomic nervous system potentially causing problems with blood flows, heart rates, blood pressure, etc. has arisen in postural orthostatic tachycardia syndrome (POTS) and ME/CFS.
- Studies have found increased levels of autoantibodies to the adrenergic and muscarinic receptors that control blood flows, etc. in some patients. These autoantibodies may be causing the blood vessels to dilate too much or too little, cause increased heart rates, etc.
- Similar autoantibodies recently found in long-COVID patients suggest that POTS, ME/CFS, and long COVID could result from an autoimmune reaction that targets autonomic nervous system receptors.
- While the findings are promising, larger, more comprehensive studies are needed, though, before conclusions can be drawn.
As in POTS, the results thus far are promising, but larger, more comprehensive studies are clearly needed to validate the autoantibody findings and to demonstrate the effects they’re having.
Long-COVID Connection
A German group reported in August 2021 that they’d found “functionally active antibodies” targeting the G-protein receptors in 31 long-COVID patients. Several of those receptors, the β2-adrenoceptor (β2AR), the α1-adrenoceptor (A1AR), the angiotensin II AT1-receptor (AT1-fAAB) have become of interest over time in ME/CFS.
Speaking in superlatives not usually seen in a scientific paper, they reported an “astonishing finding” of an “unusually high number” of autoantibodies to G-protein receptors and immediately commented on the similar antibodies found in ME/CFS and long COVID. The authors reported, in fact, that almost all the sera from the long-COVID patients contained similar autoantibodies being assessed in ME/CFS.
The presence of autoantibodies directed against receptors (angiotensin II AT1 receptor, angiotensin (1–7) MAS) that regulate the renin-aldosterone-angiotensin (RAS) system made sense as well given that the RAS system appears to be dysfunctional in ME/CFS/POTS as well as long COVID. In both diseases, the anti-inflammatory aspects of the RAS system appear to be inhibited, while the pro-inflammatory and vasoconstricting aspects are being exacerbated.
While the authors were a bit excitable, it was intriguing to see some of the same autoantibodies being assessed in ME/CFS patients show up in long COVID as well.
Conclusions
The hypothesis that an infectious event that has unleashed autoantibodies that are affecting receptors that control blood flows, heart rates, digestion, and energy production makes sense.
Two COVID/long-COVID studies found that the infection spurred the production of surprisingly high rates of autoantibodies, some of which remained at high levels. The study’s findings were particularly interesting given that the autoantibodies mainly targeted systems of particular interest in ME/CFS/POTS and long COVID such as the immune system, the vascular system, the blood, the skeletal muscles, and the heart.
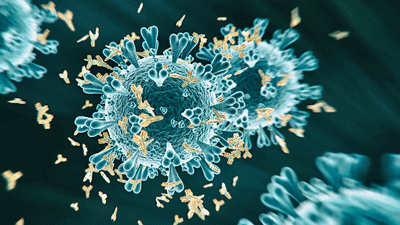
One study found that the autoantibodies unleashed by COVID-19 particularly targeted immune cells and blood vessels.
In fact, interest in autoantibodies in ME/CFS and related diseases such as POTS far predates the COVID-19 pandemic. Studies in these diseases have suggested that autoantibodies to alpha and beta-adrenergic and muscarinic receptors may be interfering with blood flows in different ways in at least a subset of ME/CFS, POTS, and long-COVID patients.
While the studies have mostly been small and the results somewhat variable, the results have generally been favorable. Small treatment trials and case reports have bolstered those results, but clearly much more work needs to be done for us to be confident that autoantibodies are playing a crucial role in these diseases.
The fact that autoantibodies are getting attention in long COVID provides that this intriguing area of ME/CFS, POTS, and long COVID will get some much-deserved attention.
Note that the autoantibody approach and Bruce Patterson’s statin approach attack long COVID in entirely different ways. One focuses on the humoral branch of the immune system (B-cells) while the other focuses on the innate immune system.
The most intriguing recent treatment possibility comes from Germany where a drug designed to wipe out these autoantibodies has reportedly worked well in some patients. A blog on that is coming up next
- Next up – BCOO7 – the Berlin Cures Drug and other potential autoantibody treatment approaches.
BIG (little) Donation Drive Update
Thanks to everyone who has contributed!

If getting deep into the weeds gets your receptors humming please support us.
This blog demonstrates another of Health Rising’s strengths (and one of its challenges) – a commitment to dig into the details. Those details – the different receptor findings in ME/CFS, POTS, and long COVID – were kind of numbing but they did show something: autoantibodies to the same general groups of receptors – with some differences – are showing up in these diseases.
If that kind of attention to detail turns your receptors on, please support us!






Thank you! What is sometimes forgotten, is that the target-receptors of the auto-antibodies mentioned are not just to be found in the vessels but also on immune cells and in the brain. The autoantibodies in the blood can in some circumstances (like endothelial dysfunction or neuroinflammation) also cross the blood brain barrier and cause dysfunction in the central nervous system. This may explain why we so often encounter this “bad perfusion-bad brain” combo.
Another point to remember. Tests for auto-antibodies vary in methodology/sensitivity. It´s therefore hard to say what percentage of ME/CFS patients have these aabs – with one method it may be 30 percent, with a more sensitive one it may be 90 %
My gosh, testing variability again! My recollection is that most studies suggest that a significant percentage but certainly not all people with ME/CFS may have some of these autoantibodies.
Thanks for the reminder concerning the brain. I was wondering why the Japanese study looked at the brain!
yes! I have been trying to get a neuro to work with me 2 years now… the neurological issues/decline I have had is pretty significant. A Neuropsychologist said I am within the top 80-90% but that doesn’t mean I am not suffering. I estimate I am 65-70% of what I was if I am lucky… but they wouldn’t continue addressing anything further. He even didn’t acknowledge my request for imaging.
What I find interesting (but is so far only my own speculation) is the coincidence that a new symptom of mine is chronic bronchial hypersensitivity.
I’ve seen another ME/CFS patient mention chronic bronchial hypersensitiity as a symptom on twitter, and it also exists as a separate syndrome. According to my pneumologist, the exact mechanism of chronic bronchial hypersensitivity is not known but it can be initiated by a lot of factors including allergies, infections, stress (to me this quietly screams autoimmune, as I think a lot of autoimmune diseases have a range of onset factors which all include an activation of immune system). Now that I have it, it sometimes behaves in a similar manner as ME/CFS e.g. getting worse when going to bed too late.
If beta-adrenergic receptors modulate bronchial muscles, it would be very interesting to see if beta-adrenergic antibodies could possibly be implicated in chronic bronchial hypersensitivity.
I have not done an antibody test so far, so this is just an idea because I find the coincidence noteworthy.
@ JR your chronic bronchial hypersensitivity how was it tested please? and may i ask if you have astma or copd or somthing else? I struggle long time with bronchi, but also ears, throat, lympnodes but the brochi (had long time ago when i still could) 5 pneumologist who all gave me another diagnose. even for astma, if i had to nhale a product, my parameters of alveolar volume got worse!! they al did not understood a thing about it. then it was copd, then it was a verry bit of emphysema, then it was just to weak peripher and respiratoir, etc how does it feel for you, wich symptoms? and can they do something about it? thanks!!!
Dear konjin, thank you for your reply.
I think the exact term for it is Chronische Bronchiale Hyperreagibilität (in German), and probably bronchial hyperresponsiveness or hyperreactivity in English.
I think there is no test for it, but the pneunomologist diagnosed it by asking questions about the symptoms and circumstances of onset.
I think she also did breathing tests which I believe can help support such a diagnosis, but even though for me they were very good showing no narrowing or clenching of the airways, she still supported the diagnosis based on the circumstances, and my GP said she’d thought the same.
I my case, the main symptom is a slightly burning, sickly feeling in my chest area that also feels energy-draining.
It gets worse e.g. when I stay up too long and possibly also when I am exposed to laundry fragrance. Currently I am having an episode of that general infectious feeling with ME/CFS including a worsening of chest symptoms with budesonid helping less than usual. According to the Doc a worsening during allergy season would also not be untypical.
Pneumologist explained that first line treatment is to try corticoids, starting as a first choice with beclomethasone (which did not help plus I did not tolerate it), then as a second choice budesonid (which I tolerate and helps some), and as a third choice Formatris (= Formoterol).
For all of these, the more regularly they are taken, the better the treatment effect according to the doc, but she said if taken irregularly there is some effect too.
What sometimes also helps a bit is a product called Soledum = Cineol.
(For me it seems to be fairly typical though that the effect of some medications varies from day to day and depending on whether I am in a crash or not.)
I think what probably helps me more than medication is lifestyle i.e. when I sleep more, pace better, have more energy and less stress, the bronchial problems get better. What might also help is to have less exposure to laundry fragrance, which I cannot avoid though because it wafts in from the neighbours all the time.
I do not have asthma or COPD but hay fever. My eyes are often a bit dry & itchy as well.
I am afraid I cannot really help with your adverse reaction of alveolal volume because my volumes are good. But now you mention it, I think my problem actually got worse from beclomethasone. The pneumologist confirmed that not every patient reacts well to every product, but may tolerate another one better. In trying out products, she mentioned the abovementioned order of priorities. So maybe you could give other products a try …
Should I put a disclaimer “this is a report of patient experience and does not constitute medical advise, please consult with your doctors etc…” 🙂 ?
All the best for you & Merry Christmas!
Just to clarify about my reply: Feeling of bronchial irritations seemed to be worse AS LONG AS I was taking beclomethasone.
hi cort,
you wrote:
“The most intriguing recent treatment possibility comes from Germany where a drug designed to wipe out these autoantibodies has reportedly worked well in some patients. A blog on that is coming up next
Next up – BCOO7 – the Berlin Cures Drug and other potential autoantibody treatment approaches.”
when will treatment be available at bed? now scheibenbogen is only taking patients from berlin and 1 place around.
i thought omf did also, time ago, but with an other produce a “wash out” for scheibenbogens antiboddies with succes. but i could never understand why they not replicated it because they had more succes at that time then scheibenbogen on her own, why they did not a larger study. as with so much, think of the low dose abillify open acces study, sumarin, etc. All those promises from nancy klimas to long time ago even ian lipkin, the cd38 from bateman, etc it hurts. nothing moves for us. it is years and years and years… how long do we still have to wait on verry long time ago promised treatments???? is this the so manyest treatmentpromise that never happens????
Maybe Cort knows more on Klimas’s studies. Is it 5 or 6 years now that she has been talking about them.
It’s frustrating, and probably means it’s not promising.
What I know or have heard 🙂
Berlin Cures – did get some money to do a small clinical trial in long COVID. More on that in the next blog.
Nancy Klimas – the ME/CFS study took a while in part because she had to raise private funding for it. (The DOD will fund GWI clinical trials; the NIH will not fund ME/CFS clinical trials) They were literally about to start it when the pandemic hit. I believe it has started.
I do wonder about the similar GWI study. It did start and I haven’t heard of any results.
CD 38 – don’t know about that.
When last heard from the Pridgen clinical trial in FM was going to start.
There’s also Cortene, Naviaux’s trial of Suramin, I think it is.
The biggest thing, though, is the NIH’s ‘urgent” call for long COVID treatment trials. No word on what’s been funded but that will hopefully draw in a lot.
This makes so much sense to me. I had my autoantibodies tested (in Germany!) for all of the ones mentioned above and was highly positive for 8 out of 11 I believe. This also makes sense as to why Rutiximab works (for some people) since it depletes B cells. It also makes sense as to why when they put the antibodies from fibromyalgia patients into mice, the mice acted exactly like they had fibromyalgia until the antibodies died off. It can also explain the wide range of symptoms experienced across various people with the same disorders – maybe some people produce more of one autoantibody and less of another depending on type of virus, genetic predisposition, or some other factor.
Interestingly, I just had an endoscopy today because my throat is constantly “swollen” or feeling constricted.
Thank you for this article. I can’t speak for others, but I really think this makes a lot of sense for my own experience in terms of why this happened and what’s causing my symptoms.
Nice link! I had completely forgotten about the antibody study in FM.
https://www.healthrising.org/blog/2021/07/02/blood-cause-fibromyalgia-autoantibodies/
If it is something in the plasma causing ME symptoms ( as Davis, Prusty, and others say)
then giving iv bolus of ME patient’s plasma to scid mice should also cause symptoms of ME in the mice.
( @Cort, Why haven’t more Fibromyalgia researchers jumped all over the study you linked?)
Also, some researchers are trying for inexpensive test. Don’t they know that ANY test would be helpful?
There used to be a saying years ago “the rabbit died” to mean a woman’s pregnancy test was positive.
How about having a patient’s plasma put to mice, and when effects seen, considered ‘positive’ result?
And what a boon for fibromyalgia to have fibromyalgia mice— to try meds and see which improves mouse symptoms?
I thought I would attach my Cell Trend results (from Germany) if anyone is interested:
Anti AT1R Antibodies
17.0 U/ml: positive
My result: 17.3 (positive)
Anti ETAR Antibodies
17.0 U/ml: positive
My result: 21.4 (positive)
Anti -1-adrenergic Antibodies
11.0 U/ml: positive
My result: 12.6 (positive)
Anti -2-adrenergic Antibodies
15.0 U/ml: positive
My result: 19.6 (positive)
Anti -1-adrenergic Antibodies
15.0 U/ml: positive
My result: 29.6 (positive)
Anti -2-adrenergic Antibodies
14.0 U/ml: positive
My result: 38.9 (positive)
anti-Muscarinic Cholinergic Receptor-1-Antibodies
9.0 U/ml: positive
My result: 11.2 (negative)
anti-Muscarinic Cholinergic Receptor-2-Antibodies
9.0 U/ml: positive
My result: 19.8 (positive)
anti-Muscarinic Cholinergic Receptor-3-Antibodies
10.0 U/ml: positive
My result: 7.1(at risk)
anti-Muscarinic Cholinergic Receptor-4-Antibodies
10.7 U/ml: positive
My result: 10.1 (negative)
anti-Muscarinic Cholinergic Receptor-5-Antibodies
14.2 U/ml: positive
My result: 15.1(positive)
Anti-FGF Receptor-3-Antibodies
12.0 U/ml: positive
My result: 10.8 (negative)
anti-TSHDS-IgM-Antibodies
9.0 U/ml: positive
My result: 30.9 (positive)
Did you have Covid?
I have positive AABs, were tested at Berlin Cures. Wanted to validate these results and sent an additional blood probe to Cell Trend last week, shoulb be ready next year. They use completely different method though. Berlin can prove that antibodies have a functional effect by testing them on spontaneously beating cardiomyocites and then inhibiting the effect by a specific antagonist.
Cell Trend cannot do this, they estimate a measured value and also a “background” value of a healthy cohort, that’s why I think their test should be interpreted with caution. At least this is my understanding.
No I didn’t have COVID (that I know of). That is very interesting about Cell Trend vs. Berlin Cures testing. How did you access the Berlin Cures testing?
Oh, that is interesting, if possible you could post a comparison of both Berlin Cures and Celltrend values when you’ve got them?
About your description of Celltrend’s approach, I don’t KNOW, but I think that their approach as you describe it is not very different to how healthy and sick values are being established for any other lab values in blood tests. I think Schreibenbogen uses Celltrend too.
And I would not expect that Berlin Cures proving that antibodies have a functional effect would contribute a lot to distinguishing between sick and healthy groups, because maybe auto-antibodies always have a bad functional effect even in healthy individuals like a kind of “background noise”, but due to the small amount of auto-antibodies in healthy individuals it is not enough to cause sickness.
I would be interested in knowing more about how both testing methods work.
What I would also be interested in understanding is the difference in sensitivy between Berlin Cures and Celltrend tests, which I understand as: does one test simply find more of the antibodies than the other, and so are their results comparable at all or do they need to apply different benchmark values to distinguish between sick and healthy? I think Herbert Renz-Polster commented on sensitivity too on this blog.
Also, if one of the tests shows antibodies, how sure can I be the amount found really has a pathological relevance? (How well do test results really distinguish between/predict healthy or sick individuals in practice – i.e. how meaningful are test results really and how well invested is my money in such a test?) (If I remember the terms from Covid tests, maybe this question refers to “specifity”?).
Also, an interesting question to me: Would antibody levels vary dependent on whether I am in a crash or not? (I know that for example for Hashimoto’s disease, autoantibody levels are known to vary. For ME/CFS, is autoantibody production a potentially continuous process too, or is there (in cases of sudden post-infectious onset) only an initial infection-related production of auto-antibodies? But why then does ME/CFS sometimes get worse over time?)
Another interesting question: Do the tests measure only antibodies that are circulating in the blood? If autoantibodies are bound to nerve receptors, how can a test still detect them circulating in the blood? How meaningful is the test with regard to the total amount of autoantibodies (including both circulating and receptor-bound?) Could there still be a lot of auto-antibodies bound to receptors, even when only few are detected in the blood?)
I didn’t have Covid either, but I have health issues that started 4 days after my second Biontech vaccine in July…
As I live in Germany I sent a blood sample to Berlin Cures.
There is a publication of Berlin Cures!, where thay do a comparison..
“Difference between beta1-adrenoceptor autoantibodies of human and animal origin— Limitations detecting beta1-adrenoceptor autoantibodies using peptide based ELISA technology”
This is also intersting:
“β1-Adrenoreceptor Autoantibodies in Heart Failure”
@JR: Excellent questions, I would also like to know the answers…
Thanks for diving into the weeds for us, Cort. Your writing and thinking here are impressively clear given the daunting complexity of the systems involved.
Herbert, you made a great point about the different sensitivities of measuring methods contributing even more confusion.
If autoantibodies to receptors in circulatory/sympathetic/ parasympathetic systems really are a common mechanism in both ME/CFS and long Covid, I wonder what that implies about the danger of Covid infection to ME/CFS patients. Would we be more vulnerable to exacerbation above and beyond our usual inability to deal with a stressor like an infection, because the existing plasma cells which produce the autoantibodies would be boosted during the infection (scary but likely)? Or alternatively, if these particular autoantibodies are already wreaking their havoc in us on the various systems and saturating the various receptors (many kinds of receptors are internalized by cells when bound), maybe the the boost from Covid infection wouldn’t make that much of a difference?
If the former, then we’d expect ME/CFS patients to present with long Covid symptoms both more severe than non-ME/CFS patients and more severe than each of our usual responses to infectious illness. If the latter, then we would expect that after the initial post-infection crash we would each return to whatever level of functionality was usual for us.
I’m sorry, I haven’t followed enough discussion to know which is happening now in the ME/CFS community. Does anyone else have a sense of this?
In any case, the potential for exacerbation implied in this write-up has renewed my motivation to be extra-careful at a moment when my
motivation was flagging, so thanks for that also, Cort.
Anecdotally (I am in the Long Covid world) there are some people I’ve seen in my support group and online that had ME/CFS prior and they indeed experienced a worsening of symptoms from their baseline, for some (possibly even most?) of them it remained that way and they struggled to return to previous baseline.
I have ME (10 years) and just got Covid. It was awful for about a week, now back to baseline. I am surprised by how well my body handled it, but I was also double vaccinated when got it.
Thanks Alexis
Thanks also AM. I’m so glad that you weathered Covid well!
Some time ago I was in contact with a professor of immunology. He told me that elevated autoantibodies are also seen in healthy people. This in itself doesn’t say much. These studies do show that there is something going on with the immune system. He said.
By the way, autoantibodies can block a receptor but can also stimulate it. To do its job.
Yes, as the blog noted, the functional autoantibodies study found autoantibodies in healthy people – it found many more in people with COVID-19 and long COVID. It also showed the autoantibodies also tended to be focused on the immune system, smooth and skeletal muscles, etc.
One of the studies also associated some of the autoantibodies with alterations in the immune system.
Thanks about the stimulation. If I remember the autoantibodies Scheibenbogen has been concerned with are stimulatory and might cause increased vasoconstriction of the blood vessels.
Does anyone know if autoantibodies can wane over time? Assuming someone with ME improves their health over time, could these autoantibodies reduce of their own accord without specific treatment?