


It’s been a wild ride for Tonix and its TNX-102 SL drug for fibromyalgia.
If Tonix doesn’t get FDA approval for their new fibromyalgia (FM) drug, it won’t be for lack of trying. The small drug manufacturer’s flagship product, TNX-102 SL, has had more ups and downs than a rollercoaster. It’s been pretty much given up for dead twice but is now headed for its penultimate trial in FM – a 470-person, 31-site, randomized, parallel-group, double-blind, placebo-controlled, 14-week Phase III trial – which, if you’re living in the States – is probably coming to a city near you.
It’s sink or swim time. From its early Phase I to its present Phase III trials, TNX-102 SL has been in almost continuous trials in FM, with the Clinicaltrials.gov website listing no less than 8 trials since 2016. Drug trial costs vary widely, but with median Phase III drug trials clocking in at about $20 million, it’s clear that Tonix has pumped a lot of cash into this effort. There’s a good reason for that, though.
Lucrative Fibromyalgia Market Beckons
A lucrative fibromyalgia market beckons. The drug industry must have been salivating at the mouth when it saw three central nervous system-acting drugs approved by the FDA for fibromyalgia in just three years in the late 2000s.
The excitement fibromyalgia patients felt at finally getting an answer to their pain quickly faded, though, as it became clear that, while the drugs did help some, they didn’t help most and could come with a basketful of side effects. A 2020 review, “Current and Emerging Pharmacotherapy for Fibromyalgia“, stated:
“it must be acknowledged that pharmacological treatment has been met, in general, with rather modest rates of success in this area.”
That was polite researcher-speak for “it’s amazing how ineffective these drugs are”. A couple of paragraphs later, they changed that “modest” to strikingly modest.
“The strikingly modest progress in this field, as manifested by the surprisingly low compliance of patients,”
Strikingly modest then devolved into “only a minority of patients” can stand them.
“Real-life data published over recent years [3], together with the clinical experience of physicians dealing with this group of patients, all indicate that only a minority of fibromyalgia patients continue taking medications for more than a short period of time due to either lack of efficacy, side effects, or both.”
Why the drugs so under-delivered in the doctors’ office and why future efforts at developing drugs for FM have failed so far is unclear. The Emerging Pharmacotherapy review article cited above believes that FM (and other pain disorders) are simply very complex, difficult-to-treat disorders.
Whatever the reason, with the FM drug market in the U.S. in the billions, there appears to be plenty of room for an effective, or even moderately effective, drug that doesn’t produce significant side effects to sweep the market.
Up and Down History
The company may be simply glad to get TNX-102 SL decided one way or another. Excited to potentially add a sleep and pain improving drug to the lucrative FM drug market, Tonix started TNX-102’s phase III trial in FM before its phase II trial had ended.
Rally Trial Fades
That trial didn’t make its endpoints (wiping out $70 million in stock valuations), but then after the drug worked in PTSD at a higher dose, the company re-evaluated its data and began two larger phase III drug trials (RELIEF, RALLY) only to suspend the RALLY mid-trial in July of last year after an interim analysis suggested that it would make not make its primary endpoints. The drug appeared to be dead in the water.
The RALLY trial had started, though, in July 2020 as the coronavirus pandemic was in full swing. Not knowing whether the pandemic had affected the results, the company ceased enrollment but continued assessing the patients already in the trial.
Relief in Store
Meanwhile, the company had been engaged in an equally large RELIEF phase III trial it had started the year before. In December of last year, the company reported that the RELIEF drug trial succeeded; i.e. it had met its primary endpoint (reducing daily pain) and showed “activity” with regard to improved sleep, reduced fatigue, and functionality.
Decision Time
Now Tonix had two very big and almost contemporaneous drug trials – one that started during the pandemic, and which failed – and one that started before the pandemic, which succeeded.
Complete analyses of both trials indicated that 79% more people taking the drug dropped out of the study because of adverse events than in the RELIEF trial. Even more remarkably, 77% more people who were taking the placebo dropped out as well.
That might not have affected the trial outcome if not for the fact that the FDA requires an analytic method used called ‘multiple imputation’ (MI) to assess results. A look at the results shows that using the multiple imputation method changed the results dramatically. The p-value denotes the number of times the result could have been expected to occur by chance. Generally a p< .05 value, or a 5% probability that the result could have occurred due to chance, is required for a finding to be assessed as “significant”. Everything which doesn’t meet that criteria is discarded.
Using the imputation method (MMRM+MI*) rendered every test result except for one insignificant, while using a different method rendered every test result except for one significant with the sleep improvements, in particular, clearly being very consistently seen across the patient group. One analytic method indicated the drug was a failure, while the other indicated it was a screaming success.
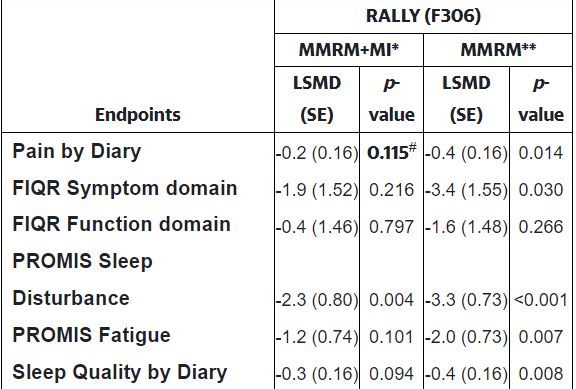
Check out the p-values. The FDA-required method is on the left; the other method is on the right.
A similar analysis that was done on the RELIEF (pre-pandemic) study found that both methods agreed – the trial was a success. For some reason, the multiple imputation method heavily downgraded the effectiveness of the drug even if both arms of the trial (placebo and drug) experienced high dropout rates; i.e. this method is uniquely vulnerable to miscalculate the results when something like a pandemic comes along.
Decisive Trial Beginning
Tonix recognized its trial results were actually quite good and has pressed forward with its next, and surely last, and aptly named RESILIENT phase III trial for TNX-102 SL. The trial was posted to the clinicaltrials.gov website yesterday and the locations have been identified but aren’t yet recruiting.
The Biggest Fail
This isn’t the first time that trials have potentially faltered because of methodological issues. The biggest fibromyalgia clinical trial in history may have failed because of them. The company, Daiichi Sankyo, was so confident in its Lyrica upgrade, Mirogabalin, that it skipped the phase II trial and went straight to 3,600 person phase III trials. Instead of staggering its phase III trials as Tonix did – giving it room to adjust them if necessary – Daiichi conducted them simultaneously.
Daiichi researchers claimed, though, that the FDA decision to require using the worst daily pain score – which they proposed was subject to catastrophic assessments – instead of the average daily pain score, basically cost Daiichi its shot at getting its upgrade to Lyrica to drug approved. Under one method, the drug did reasonably well; under the other, it bombed.
Boom Year for Fibromyalgia Drugs?

With several major drug trials underway 2022 could be a turning point for FM.
With three drugs approved in three years, the late 2000s were a heyday for getting drugs approved for FM. The next couple of years might top that if a couple of more effective drugs get approved.
With its solid results, Tonix looks well-placed to add a 4th drug to the list. Its TNX-102 SL drug is notable for its ability to improve both sleep and pain.
That’s not all, though. A bevy of other big fibromyalgia trials are underway. Skip Pridgen’s Phase III antiviral drug trial, a large ketamine trial in Europe, a neuroplasticity drug NYX-2925 that could also help with sleep and pain, and a large anti-CGRP (anti-migraine) drug trial are all underway in FM this year, and some were projected to be completed soon.
Notably, instead of the overt emphasis on the neurotransmitters in the first batch of FM drugs, several of these drugs attempt to get at FM in a novel ways indicating that the FM field is evolving and broadening.






Whoa – that’s a wild ride for Tonix!
It’ll be interesting to see if FM patients can be helped with these new offerings.
I really hope this pans out. I strongly believe there are a lot of problems that make it less likely for drugs that would benefit fibro patients to show that. I think the patients who end up included in these trials do not all have fibromyalgia as many are likely misdiagnosed and have other things like arthritis. I also think the patient population is just overall really heterogeneous and likely has many subgroups within it, which again means some drugs will only work for certain subsets but without knowing that we can’t analyze it or show that. And also, I think we don’t have good clinical endpoints to accurately measure pain (see your reference to the Daichii Sankyo trial of mirogabalin). Then the stats methods just muddy the waters because what might only ever be relatively small effects become non-significant. Until we have a more reliable method to diagnose fibro (some of the blood tests or better biomarkers) or understand anything more about the underlying pathophysiology, it seems to me that we will only get some drugs approved every so often that are repurposed from other indications that just might happen to tangentially do something in fibro.
Many thanks to all those who are working for Treatment for FM as my heart goes out for those who have to endure Fatigue, Insomnia. Pain & all the horrible Symptoms FM brings daily. It is so sad that Prescription MEDS for FM bring horrible side effects [: Myself thankfully have managed all symptoms without Meds. I WISH TO SHARE MY STORY OF GREAT PROGRESS…a few yrs ago when I was diagnosed with FM & was prescribed MEDS, I instantly took a decision to change my life habits & life style. I managed by doing extensive research. The 1st thing i did was a Food Intolerance test. I started eating what my body needs. By doing so I controlled my Heartburn, IBS, lost weight, bloating & controlled my Eczema I WAS DETERMINED TO GET BETTER. I take OMEGA 3 Supplements [if Omega average 3:6 is unbalanced, that could be reason of some symptoms we endure], Megnesium, VIT D3, Calcium [for post MENOPAUSE] & daily PRE/PROBIOTICS. I run my Blood Tests every six mths to ensure my blood levels are in tact. Also i ingest FLAXSEEDS, TURMERIC, CHAI SEEDS & GINGER, Lemon & fresh MINT. My diet includes many VEGs [mostly dark greens], High fibre, White Meats, Sweet potatoes, Avacado, 2 to 3 times weeks OILY FISH [like Salmon, Sardines, Tuna & Seabass, DAIRY FREE [plant based], UNREFINED SUGAR, GF & Wheat Free, UNREFINED FLOUR, a FODMAP Diet & Anti inflammatory Diet. I use only a good OLIVE OIL, EXTRA VIRGIN OIL & COCONUT OIL [cold pressed]. Also I Drink more than 2 ltrs of LEMON/GINGER 7 MINT Water. I DETOX 4 times a wk. My daily b’fast is OATS, FLAXSEEDS, BERRIES, HALF BANANA & KEFIR. It gives me alot of energy & prevents Acid Reflux :] The past 2 yrs I don’t recognize the person I was who was always in Pain, couldn’t drive or walk, couldn’t keep my eyes open but at the same time couldn’t sleep, terrible Stomach reflux & IBS, Sleepless nights, irritability & Brian fog. I had lost more than half my Hair …& the list of Symptoms goes on forever [: I WAS DETERMINED TO GET BETTER without Prescription Meds. What really helped are OMEGA 3 Supplements [if Omega average 3:6 is unbalanced, that could be reason of some symptoms], Megnesium, VIT D3. Also i take FLAXSEEDS, TURMERIC, CHAI SEEDS & GINGER & lead a HEALTHY DIET which includes plenty VEGs [many dark greens], High fibre, White Meats, 2 to 3 times weeks OILY FISH [like Salmon, Sardines, Tuna & Seabass, [DAIRY FREE, UNREFINED SUGAR. GF & Wheat Free diet & UNREFINED FLOUR, a FODMAP Diet & Anti inflammatory Diet. Also I Drink more than 2 ltrs of water daily & exercise 5-6 hrs a week which includes STRETCHING 7 dys a week, before Training & after, CARDIO & MUSCLE STRENGTHENING. Of course I managed STRESS which triggers Symptoms & retained a POSITIVE ATTITUDE which is the initial step to recovery. I was determined to get better without MEDS & WITH A CHANGE OF LIFE STYLE & today I am so proud of the HUGE IMPROVEMENT I MADE. Earlier on I described how i used to feel a couple of yrs ago & today I jog, carry weights, extremely energetic, always with a smile, sleep pretty well [dream practically all night which I had forgotten], very rarely have heartburn or IBS symptoms, very rarely have a headache, NO BODY PAIN [maybe slightly swore after training which lasts a few hrs & is pretty normal]. TODAY after 2 yrs I have a Head full of hair much more than I had in the past :] FROM MY EXPERIENCE I FEEL COMPELLED TO HELP OTHERS BUT I DO NOT KNOW HOW ! If I managed I am 100% certain that many other sufferers with determination & a positive attitude can feel as good as I feel today :]
Not everyone slapped with fibromyalgia diagnosis has same underlying condition at all. Some of us were young snd in cery great shape. I am sick of the testimonials of being determined, positive, and reducing stress and chsnging lifestyle. How many years and how much money and effort snd it still must be your fault. As a 20 year old, I did not at all identify with the overweight 40 year old fibro patient who likes warm water for their pain. It would fatigue me and hurt. Their legs were not turning colorsm They were not sinking to floor. Their legs were not swelling. Keep in mind a lot of us were thrown in the same trash can because the questionairre at rhuemetologist is very rigid and does not even adress certain symptoms or even the main complaint. So superficially we look the same on paper. My muscle contractions were not from pain. The fibers the PT noticed getting worse each visit are not stress related.
I think we all need to keep in mind that the ME/CFS and fibromyalgia fields are full of very different recovery stories. What works for one person often does not work for another.
I really appreciate Nadine’s willingness to publicly tell her story – which was not focused on stress reduction, by the way.
Even if Nadine’s recovery was accomplished ENTIRELY through stress reduction it would still be a legitimate comment because if it worked for her it might work for others. Providing as many potential avenues for recovery is a core value for Health Rising.
So
I remember decades ago that there was a little alternative practice in California that was trialing ketamine. They said if it would help a specific individual, they’d know in a very short time–at the same visit. Did anyone ever hear what happened with that? I’d say shut down in U.S. because it was ketamine. But other countries have used ketamine comas for healing.
Quite a few ketamine clinics have opened up – which is a bit scary actually. If the study gets good results and ketamine does indeed get FDA approved for FM, we should see ketamine becoming available in doctors offices/hospitals.
What happend to the Duffy and Pridigan combined drug discovery. It was successful with great results
They’re on their Phase III trial right now – and are starting a long COVID trial.
Fibromyalgia and ME are not the dame disease even though there are similarities. A French doctor, Dr Seignalet, tested the diet mentioned by Nadine on more than 100 patients. The results were positive only for fibromyalgia…
I wonder how much of the (positive) action of any drug/supplement formulated with HCl is actually due to the HCl.
After all, most people with degenerative health problems have low stomach acid…
This goes even for Betaine HCl, thiamine HCl, etc
Improved nutrition due to improved digestion of food, reduced endotoxin, reduced leaky gut, reduced digesfive distress, etc