

The received wisdom about the pain in fibromyalgia (FM) is that it’s being produced in the central nervous system. That idea was amplified very early in “Altered Pain in the Brainstem and Spinal Cord of Fibromyalgia Patients During the Anticipation and Experience of Experimental Pain” when the authors stated:
“Most evidence to date suggests that the abnormal pain responses in FM may be the result of central sensitization.”
Then came a caveat, though. Those nice imaging studies that demonstrated that FM patients’ brains are amping pain signals have tended to miss a rather significant part of the brain. As VanElzakker noted in a paper on ME/CFS, standard MRI studies don’t get a good picture of the brainstem. That’s a shame in a number of ways. For one, the brainstem determines how many pain signals the brain is going to get smacked with. For another, it’s a powerful regulator of the autonomic nervous system.
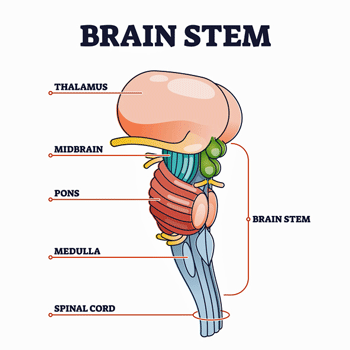
Importantly, the study also showed, though, that the pain signals in the brainstem and spinal cord were changing continuously even when no stimulus was expected; i.e. there was a kind of background level of brainstem activity that was being continuously modulated. They weren’t sure what was causing it, but given the brainstems’ ability to constantly fine-tune blood flows, these subtle background modulations have something to do with what we’re involved in at the moment; e.g. what we’re devoting our attention to, what our mood is, what we’re anticipating, etc.
Being able to sustain one’s attention on something is one way to reduce pain. In fact, the process of reducing pain by paying attention to something else or engaging in cognitive activity (reading, crossword puzzles, etc.) is called “attentional analgesia”.
A recent study found – to the researchers’ surprise – that the brainstem pathways that support attentional analgesia are still intact in FM. It’s hard to know what to make of that in a disease in which attention-deficit problems appear to run rampant. It appears that producing attentional analgesia is possible – but probably not easy in FM.
Next, the research team moved on to people with fibromyalgia. The same general study protocol was used. The subjects were given trials in which they received a small painful stimulus (heat applied to the hand) which were interspersed with trials in which no pain stimulus was given. As that was happening, blood flows in the brainstem and spinal cord were assessed. Special attention was given to regions of the brainstem involving autonomic nervous system regulation.
Pain and autonomic nervous system functioning was also assessed using various assessment tools (total FIQR and function, impact and symptom subscales), measures of autonomic function (total COMPASS and all subscales), and pain inventory scores (total MPQ).
Results
The expected parts of the brainstem became activated in both the healthy controls and FM patients, but the brainstems of the people with FM went further – parts of the brainstem that are generally associated with autonomic nervous system functioning were also activated. One connection, in particular – to the rather vividly named nucleus gigantocellularis or NGc – shot up in the people with FM.
Gigantic Neurons Activated
This “gigantocellularis” nucleus is aptly named as it contains some of the largest – and potentially most important – neurons in the brain. The connections this nucleus has to unusually large parts of the brain underscores just how significant it is. The authors didn’t mention it, but the NGc’s could impact arousal, pain sensitivity, autonomic nervous system activity, movement, and brain blood flows in FM.
Donald Pfaff, the leader of the Laboratory of Neuroscience and Behavior at The Rockefeller University in New York City, has said that the generalized arousal that the NGc’s trigger “is what wakes us up in the morning and keeps us aware and in touch with ourselves and our environment throughout our conscious hours”. Different parts of the NGc help to “awaken” also play a role in sleep, and assist with movement as well.
The Gist
- The brainstem is difficult to assess and most MRIs -which take a top-down approach – fail to capture it. Yet the brainstem controls the pain and sensory signals coming into the brain is an autonomic nervous system regulator, and clearly plays a role in pain. It’s even been called the “pain conductor” in the brain.
- In fact, the brainstem is such a fine-tuned instrument that it appears to be constantly modulating the blood flows within it – possibly reacting to our thoughts as we go through our day.
- Not many brainstem studies have been done in fibromyalgia (FM). This study used a series of trials – some involving a painful stimulus – and others not – to assess blood flows.
- It found that contrary to the healthy people the brainstems of people with FM were activated even when they were at rest. The FM patient’s brainstems also sent blood to areas of the brainstem involved in autonomic nervous system functioning – potentially linking this deep, primordial part of the brain to the autonomic nervous system problems found in FM. That’s a potentially important link as the autonomic nervous system is looking more and more like a key player in chronic pain.
- The FM brainstems also sent blood to an unusual part of the brainstem called the gigantocellularis neurons; huge neurons that connect to unusually large portions of the brain.
- These neurons play a big role in arousal and wakefulness, pain sensitivity and blood flows in the brain. They play such a powerful role in arousal that researchers have been able to quickly pull laboratory animals out of a chemically-induced coma by activating them.
- It’s not known why these neurons are being activated in FM but they play such a critical role in the brain that their appearance in FM is nothing if not interesting.
- Other studies have found brainstem issues in FM. One even suggested that the wind-up phenomenon which causes the nerves to accelerate pain sensitivity over time instead of reducing it may originate in the brainstem. Others have found the brainstem connecting more actively with areas of the brain known to produce pain.
- Seemingly every part of the pain processing pathways – from the small nerves in the skin, to the neurons in the dorsal horn outside the spinal cord, to the brainstem, to the pain processing centers in the brain have taken a hit FM. In fact, one wonders if any parts of the pain processing system have NOT been deranged in this disease. If the brainstem – in its role as the main conduit between the spinal cord and the brain, plays a major in FM, deep brain modulating techniques might help.
When Pfaff’s team assessed the genes being expressed in these giant neurons, they were shocked to find genes that regulate brain blood flows popping up – a potentially interesting connection given reductions in brain blood flows seen in FM and ME/CFS. These are the only neurons in the brain known to express these genes.
Reduced blood flows in the brain could be causing some unusual mischief. For instance, mice injected with a substance that knocks out these genes had trouble calming down after being exposed to with novel scents. – an interesting finding given the problems with scents, light, and other stimuli often found in these diseases. It’s no wonder that the NGc has been called “a central integrator for pain and cardiovascular-related functions“.
Why these huge neurons have become activated in FM is unclear. Is the brainstem is trying to get the NGc to wake up or something else? We’ll see in an upcoming blog that the big problem with the autonomic nervous system in FM may be that it’s fallen asleep.
Back to the Study
Getting back to the first paper, the striking thing was how amplified the brainstem circuits of FM patients were before the pain stimulus was applied. While the anticipation of pain further heightened activity in these circuits in people with FM, those circuits were amplified at rest. The outcome was the opposite seen in the healthy controls; their brainstem blunted their pain levels, but the brainstem activity in the FM patients left them in more pain.
Plus, connections to parts of the brainstem involved in producing pain and regulating the autonomic nervous system (locus coeruleus, parabrachial nuclei, and hypothalamus) were particularly activated as well. Their increased COMPASS (ANS symptom) scores suggested that the brainstem-autonomic nervous system connection the imaging study found in FM was valid, and that the autonomic nervous system had been dragged into the pain amplification process. The authors proposed that the “convergence of autonomic regulation and pain modulation systems” at the brainstem was causing problems in FM.
An earlier study found that the brainstems of FM patients are communicating more actively with pain-producing regions of the brain (insula, anterior cingulate cortex, anterior prefrontal cortex) that also, regulate autonomic nervous system activity. The higher the level of communication, the greater the pain.
Connectivity between brain regions may also influence how effective drugs are. Milnacipran was effective in FM patients whose brainstems were connecting less with the insular cortex but not as effective in patients whose brainstems were communicating more. The drug was not powerful enough to overcome that stream of signals.
A recent “windup” study amplified how important the brainstem may be. It traced how pain signals get amplified in FM (instead of being ratcheted down) and examined activity up and down the spinal cord in conjunction with pain. The brainstem was the only region to show accelerated activity.
While not a lot of work has been done on the brainstem in FM, it is increasingly being seen to play a central component in chronic pain. Imaging advances are allowing us to study the brainstem in much greater detail. It’s recently been shown, for instance, that the placebo effect may originate in the brainstem.
A recent review article called it “the pain conductor” and proposed that our understanding of its role will lead to “better optimized pain-relief strategies”. Those include the new forms of “deep brain stimulation” that get down to the bottom of the brain.
That another pain processing center has gone awry in FM shouldn’t come as a surprise. With similar findings in nerves leading to the spinal cord, in the brainstem, and in several pain processing pathways in the brain, we have to wonder if there are any places pain signals are NOT getting amplified in FM.






I’m curious to know the whether the brainstem can be affected/irritated by nearby bone structure. I have neck stiffness and scoliosis – but do not suspect cranial instability.
I don’t know. My understanding of CCI is that the ligaments are failing to keep skull up off the brainstem – and in doing so – can produce all the symptoms of ME/CFS/FM. I imagine the brainstem is a very sensitive piece of our anatomy but I don’t know if there are other ways to impact it.
It’s fake. There is no such thing. It is there to make surgeons money,
Is this just more about chiari malformation they were discussing in the 90’s?
Very interesting study! This deep dive into the brainstem made me think about Jen Brea’s story (CCI-related brain stem issues). It could be a real clue for causation of ME and FM.
Jen Brea is a fraud. She must have had hypothyroidism because it states on her webpage she had a thyroidectomy before her CCI surgery. She is just after making money. avoid.
ME is caused by an enterovirus infection of the CNS and PNS. Thanks
Why does anyone think this? Jen Brea – through thick and thin- has made an enormous difference for ME/CFS. I guess it’s part of being a public figure.
If you’ve read her story you know her thyroidectomy made her worse – much worse – and guess what – the surgery made her better = end of story. Given that she’s not surgeon I have no idea how she could possibly make money off of this.
She is dishonest whatever she had because she deleted her mid page showing a picture of her back and saying she has atypical poliomyelitis.
She has also stepped down from ME Action which has a UK arm too and yet is not registered as a charity in the UK. I can not find the charities register in the UK but they need auditing that ME Action do toute de suite. Whatever Jen Brea had, she is dishonest and played the victim whenever anyone criticised her.
Last year I had hip replacement surgery. I am 73 and have had fibromyalgia for over thirty-five years. Prior to surgery I had a battery of tests including a cardiac test in order to be declared fit for the surgery. During surgery my blood pressure dropped to 50/30, and adrenaline was administered. I had to stay overnight due to concerns with the low blood pressure. Autonomic nervous system problem? My surgeon also said I had very little bleeding during surgery, probably due to low blood volume. It took about three weeks for my blood pressure to rebound, but intense pain in my neck, left shoulder and head followed, particularly the base of the skull. I decided to try some of the supplements recommended here including NAC, NAD, , fisetin and B1 and CQ 10. I do not feel the intense pressure in my neck and head that I felt before. I don’t know how it works, but the combination did give me some welcome relief, even though my sleep remains fairly poor.
Please continue taking those supplements. Healthy to keep brain balanced.
A hefty nightly dose of melatonin is essential. Like 80mg
It makes sense-I always say my brain doesn’t quit sending pain signals. Nor does it go into sleep signal. All I can think whilereading this-in high pain -is if they do tests on one I hope they have something to help calm that already ramped up pain back down 😬. It would be heavenly to find help
Cort can you answer my tweet please RE R NAse L. I thought it had been proven by Sodalnik et al that this antiviral pathway was not functioning properly.
That’s what I thought at well but VInce Lombardi who worked on it told me it turned out to be an artefact. If I remember correctly doing the test made the enzyme break apart. It was kind of stunning blow – so much work was done on it – and several prominent ME/CFS specialists used it for year. De Meirleir even wrote a dense book on it.
Through the years you ask yourself 101 questions about the cause. Included in that list for us was the fact that there was mild scoliosis in some members of the family. NUCCA chiropractors position the skull to stay in position. If you have adjustments by them and find that your skull does not stay positioned properly that may be a clue. My daughter found the adjustments helpful for her pain but her head did not stay in position after the adjustments.
Cort- I’ve been going for upper cervical chiropractor for years- be it Atlas Orthogonal or NUCCA ( Nat’l Upper Cervical Chiro Assoc) . Both techniques necessitate accurately measuring the position ( via X-Ray) of the Atlas ( top cervical vertebra) and adjusting it accordingly. When Atlas is out, it puts pressure on the brainstem, blocking the brain’s healing messages. It’s effective for pain, MS, TBI and much more.
I’ve also had Gonstead Chiropractic (very different from general chiropractic) weekly. He adjusts different vertebrae depending on what my current symptoms are. Has helped very much – along with a weekly massage. My life is much better than it’s been in the entire 55 years I’ve been living with FM.
I have a condition known as Klippel-Feil (apparently shared with Tutankhamun!) in which two vertebrae at the top of my neck are fused together from birth. Apparently this results in no problems unless there is head trauma. ( I had a serious head trauma after an accident which s why I know about the effects of the condition). The vertebrae immediately above and below the fusion are hyper-mobile to compensate for lack of movement. My long term Fibromyalgia which started fifteen years ago soon after the accident, is made worse by the inherent instability of my neck, as confirmed by several doctors. I have often wondered firstly what percentage of the FM population has such a hidden birth defect, and secondly in view of your last blog, how much it affects FM sufferers.
My slow journey into the FM wilderness has coincided with menopause years, the onset of osteopenia and and fast increase of my scoliosis curve which is now in the severe bracket. It often feels like my head is too heavy now.
I have wondered about the possible connection.
( have followed Jenn Brea’s story closely)
I also have a spinal deformity from birth: reverse curve. It was discovered in grade school about 65 years ago but there is nothing to be done. Whereas most people have an inward curve in the lumbar region and an outward curve in the thoracic region, I have the opposite – my spine curves inward at the level of the ribs, and outward at the lower back region. Looks a bit funny, but my father had the same thing and swore that it made him have a strong back. I agreed with him, I have an incredibly strong back for lifting heavy things. But maybe this interferes with the positioning of the cervical spine. Then in 1990 I crashed my motorcycle and had a slight concussion and a cracked cheekbone. Another insult to the neck?
On the other hand, it was my mother that had MCAS, not my father! And now my daughter has it, too.
The spine is part of the brainstem ‘trunkline’ for metaboloc regulation. Immune/neuro/endrocrine imbalances caused by Pollutions are key in scoliosis, me/cfs, and all modern illnesses. World pollutions affect each creature uniquely
Cort Johnson :o>
http://www.forbes.com%2Fsites%2Fwilliamhaseltine%2F2022%2F05%2F09%2Fscientists-discover-genetic-cause-of-lupus-findings-may-help-research-on-long-covid%2F&usg=AOvVaw09ejuPYr1MktHQG7_CYELi
Toxic exposures deforming different genes in each creature >
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8580522/
https://pubmed.ncbi.nlm.nih.gov/33064832/
This makes sense since the beginning of my problems began after a car accident where I rolled the car and landed on my head. I had a lot of neck and shoulder problems that progressed to a fibromyalgia diagnosis. But during that time I was also exposed to a moldy house and worked in a hair salon around chemicals. I’ve also been tested positive for previous mycoplasma, Epstein Barr, and other infections, but treating them with anti virals only helped so much. It’s like a perfect storm of conditions that keep me sick with flu like symptoms and fatigue, intolerance to my environment, and chronic pain. I’m discovering that vagus nerve stimulation and meditation is helping a lot to quiet down my hyper responses.
Glad to hear you found something and thanks for passing it on.
My fibromyalgia appeared shortly after a car accident I was in. I was in coma for awhile with concussion. When I came out if it, it was discovered that I lost muscle control of my right leg. With therapy it improved. I am positive this caused my problems. Now I have Non -tuberculous Mycobacterial Avium Complex, B12 deficiency, PA, Bronchiectasis, and still have fibro. I have disc problems in my cervical spine. I get stiffness and popping and creaking in my neck. The doctors told me to never have a chiropractor touch my neck.
About 6 years ago I found out my daughter had Chiri Malformation. I had already been diagnosed with Fibromyalgia by this time. It is when your brainstem protrudes pass your skull. It is quite dangerous being if you were to get hit in the back of the neck or head too hard you could die. Many of the symptoms are like Fibromyalgia which was interesting to me because then I was like maybe I have Chiri. I asked if when I had my brain MRI if they checked for that and they said yes. Because it is hereditary. My MRI was for migraines so not really sure if I believe the hospital or not. If the symptoms get too bad you have to get brain surgery. Another thing to me that was interesting was my cousin had back surgery and ended up getting spinal fluid leak. That too has alot of the same symptoms as Fibromyalgia. One of my Therapist asked if I had an epidural with my two children and I did. She said that seems to be linked to Fibromyalgia. So does that mean spinal fluid leak? I mean every doctor I go too just seems not to really care. So I love to see new research into Fibromyalgia!!!
I’ll have to read it a few times to understand it due to my attention and concentration deficits, due to fibromyalgia and cervical. That’s what I’m trying to confirm as I don’t get any help from doctors unfortunately