

It was a small study, but small studies done in the right hands and pursued vigorously enough can make a real difference. Plus, there’s nothing like really digging into the brain – and this took actual digging – to learn what the SARS-CoV-2 virus is doing to this most obscure and difficult-to-access organ.
Nath’s findings suggested to him that leaky blood vessels may be sparking inflammation in the brains of long-COVID patients.
In a sense, the senior author of this study, Avindra Nath, has been here before. A year and a half ago, in Feb. 2021, Nath produced one of the first – and still the most interesting – brain findings in COVID. The “Microvascular Injury in the Brains of Patients with Covid-19” study highlighted a theme – small blood vessel damage – that has only grown over time. Nath’s early study found widespread punctate hyperintensities – similar to what was found in ME/CFS decades ago – and small blood vessel damage and leakage across the brain.
Proteins such as fibrinogen, C1q, IgG, and IgM – which should only be found in the blood – had leaked into the brain – triggering, no doubt, an immune reaction. The study findings suggested that damage to the blood-brain barrier that protects the brain had occurred.
A recent survey of the long-COVID literature found no less than 15 studies addressing blood vessel and blood clotting issues in COVID. None of them have addressed blood vessel problems in the brain, however.
In “Neurovascular injury with complement activation and inflammation in COVID-19“, Nath was back with a more detailed autopsy study that tells us much more about what this virus can do. The virus really displayed its potentially lethal character in this study: all the participants in this study died quite quickly after getting infected and, while they did experience respiratory symptoms, the virus did its deadly work in their brains. While the participants in the study died, Nath has, in interviews, stated that he believes their findings may well inform what’s happening with long COVID.
The study dug deep into the endothelial cells lining the blood vessels. Immune complexes produced by antibodies found on the endothelial cells indicated that an immune attack had occurred. IgG and IgM antibodies and platelet aggregates (i.e. small blood clots) were associated with proteins (C1q, C4d, and C5b-9) produced by the classical pathway of the complement branch of the immune system. Activation of the classical pathway occurs in many autoimmune and inflammatory disorders.
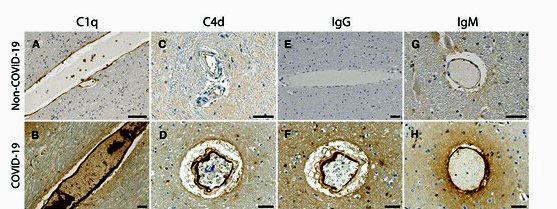
Staining shows high levels of different complement proteins in the COVID-19 patients (below) vs the controls (above).
Increased levels of an adhesion receptor on the surfaces of those cells suggested the damaged cells were attracting activated platelets to them. They produced a clotting cascade that damaged the endothelial cells, causing them to leak. (Gene expression data indicated that a coagulation cascade had begun and that a clotting event had occurred.) Increased levels of the von Willebrand factor, which is associated with damage to the endothelial cells lining the blood vessels, further cemented the fact that blood vessel damage had occurred.
The authors noted that COVID-19 lung studies have found similarly “strange” blood vessel findings including “unique vascular features consisting of endothelial damage, micro thrombosis (micro blood clots) and intussusception angiogenesis (‘splitting blood vessels’)”.
The all-important antigen – the molecule found on the endothelial cells – which had prompted the immune system to attack them remains unknown, but the authors suggested it could either come from the spike protein of the coronavirus or to the ACE-2 receptor the virus uses to penetrate the cells. Given the abnormal ACE-2 findings in chronic fatigue syndrome (ME/CFS), the latter would probably be more useful for people with ME/CFS.
An inflammatory response primarily involving the macrophages in the spaces surrounding the blood vessels reflected an attempt to repair the damage produced by the leaky blood vessels. With that, you have an ongoing inflammatory process taking place in the brain.
Widespread neuron loss and damage in the brainstem area, in particular, were found. While the study did not find evidence of infection there, they noted that “Involvement of the brainstem could have dire consequences since many vital functions are controlled by this region.” and that “five patients in our study died suddenly, most while sleeping”. The brainstem, of course, is of major interest in ME/CFS.
The Model
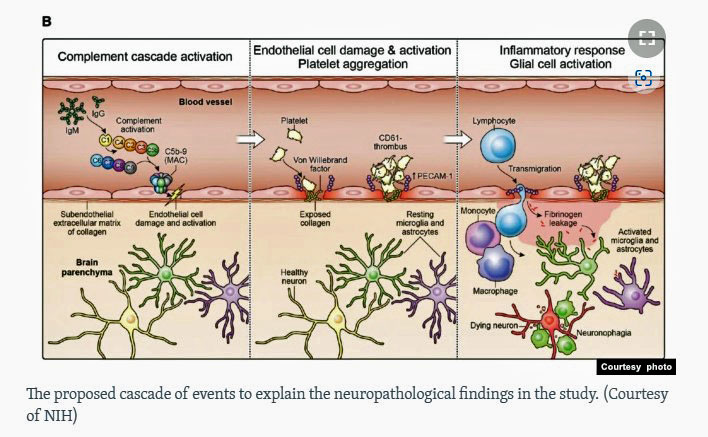
Nath’s model of endothelial cell damage in long COVID.
The Gist
- Over a year ago, Avindra Nath published the results of a small autopsy study which suggested that small blood vessel leaks in the brains of people with COVID-19 were causing inflammation in the brain.
- In an attempt to figure out how that happened, his most recent study took a closer look at the endothelial cells lining the blood vessels of the brain.
- Since that time, over a dozen studies have looked at blood vessel functioning in long COVID, and small blood vessel functioning has become a topic of great interest in long COVID and ME/CFS.
- Nath’s most recent study found that immune complexes associated with IgM and IgG antibodies were found on the endothelial cells lining the blood vessels. They indicated that the immune system had attacked the endothelial cells. These cells are responsible for opening/closing the blood vessels and play an important immune role.
- Nath was unable to determine what had attracted the immune system to these cells but posited that either the spike protein of the coronavirus, or damage to the ACE-II receptor that the virus uses to enter the cells, could be responsible. (Note that a couple of studies suggest damage to the ACE-II receptor has occurred in ME/CFS and POTS).
- The damage to the endothelial cells caused them to activate and produce more immune factors – including drawing platelets to the area and initiating a clotting cascade.
- The small blood clots that formed appear to have burst the tight junctions of the endothelial cells lining the blood vessels – spilling proteins into the perivascular spaces surrounding the blood vessels.
- Those proteins, and the immune response they elicited in the microglial cells, then produced an ongoing inflammatory state.
- The lack of lung involvement and the high degree of brain involvement seen in these patients – most of whom died without ever stepping into a hospital – led Nath to posit that if these patients had survived, they probably would have ended up with long COVID.
- A similar process may be occurring in ME/CFS. Thirty years ago, an ME/CFS study found widespread levels of “punctate hyperintensities” in ME/CFS patients that are believed to reflect small blood vessel dysfunction. Seven years ago, James Baraniuk’s cerebral spinal fluid proteome study pointed to the deposition of amyloidic proteins in endothelial cells, weakened brain blood vessel walls, and leakage into the brain.
- Nath suggested that treatment to tamp down the immune system activation could be helpful in long COVID and has begun a 40-person IVIG trial in long COVID.
- This is a hot field! More blogs on small blood vessel functioning in long COVID and ME/CFS are coming up.
The unwanted proteins then attract monocytes – which turn into macrophages – which then engulf the proteins in an attempt to clean up the area. Some proteins, though, made their way to the immune cells in the brain – the glial cells – as well as to the neurons they surround and support. Sensing an invader, the glial cells pounce on the proteins, but in doing so, damage the neurons – causing the macrophages to clean them up as well.
The patches of leaky brain blood vessels scattered across the brain, but apparently focused more in the hindbrain area where the brainstem resides produce an ongoing inflammatory response in the brain and nerve damage. The symptoms each person experiences depends on which part of the brain the blood vessel leakage occurs.
Long-COVID Connection?
The participants in this study had died, but the lack of lung involvement and the massive hit their brains took cried out long COVID to Nath. If these patients had survived, Nath felt they probably would have become long-COVID patients, stating “It is quite possible that this same immune response persists in long COVID patients, resulting in neuronal injury.”
Noting that immune-modulating therapies might help turn off the immune activation that may be causing so much trouble, Nath stated, “these findings have very important therapeutic implications.” Nath has begun a 40-person long-COVID IVIG/corticosteroid study.
ME/CFS Connection?
This model is all the more interesting because it appears to fit what some past ME/CFS studies have found. Baraniuk’s 2005 cerebrospinal proteome study found evidence of amyloid (misfolded protein) deposition in the blood vessels and a weakening of the blood vessel walls in the brain. Baraniuk speculated that localized bleeding caused by amyloid deposition into the blood vessels might be occurring throughout ME/CFS patients’ brains.
Back in 1992, a large study co-authored by Dr. Peterson, Dr. Cheney, Dr. Komaroff, and Dr. Paul Gallo, found widespread punctate hyperintensities in the brains of people with ME/CFS. The authors stated that these punctate hyperintensities corresponded to the perivascular spaces surrounding the cerebrospinal fluid and suggested that a similar process had occurred: leakage had ignited an inflammatory response in the brain that damaged the neurons. As with Nath’s findings, the location of the hyperintensities varied by patient. Punctate hyperintensities are now believed to be caused by microvascular disease; i.e. damage to the small blood vessels in the brain – exactly what Nath has found.
Hot Research Area
With over a dozen endothelial cell, blood vessel, and clotting studies popping up in long COVID, and similar studies popping up in ME/CFS, the blood vessels and the blood itself have become areas of intense interest. More on that is coming up soon.






I have had Mast Cell Activation Syndrome since the first two vaxes, for 15 months now. Mast cells are found in mucosal and epithelial tissues throughout the body. Perhaps mast cells may be responsible for the damage to the blood vessels.
This is so interesting because as I was looking through Baraniuk’s study from five years he mentioned histamine. I was surprised at that because 8 years ago I don’t think MCAS was considered a big deal in ME/CFS. He states that mast cells can regulate blood flows through the small blood vessels in the brain. Baraniuk is just very impressive – he doesn’t miss a beat.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1326206/
“Brain parenchymal mast cells may regulate brain microvascular permeability, possibly through histamine release [47]. If so, this could explain the benefits of tricyclic drugs such as doxepin and imipramine that have potent histamine receptor 1 antagonist activities [48]. Histamine receptor 1 antagonists that do not cross the blood brain barrier have no benefit in CFS.”
The recent blog on hyperarousal suggests that doxepin elixir may be helpful with sleep in ME/CFS. Cheney used it alot.
Thanks Cort for your great summary and your sharp eye, as always. That reference 47 really pulls together many strands of ME/CFS research!
Thanks for pointing that out. Theoharides is now working with Nancy Klimas at NovaSoutheastern.
Cort, excellent article. Re: histamine, during the Project EHCO Long COVID webinar “Practical Strategies for Symptom Management” three days ago a participant stated a “low histamine diet” was extremely helpful in reducing his symptoms. Are any ME/CFS clinicians including such a diet in their treatment protocols do you know?
How do you know you have mast cell activation? Have not heard of this. Had great deal of fatigue after 2nd and 3rd Pfizer shot and still not quite the same.
Thank you Cort, as always. I love reading these articles, they help me understand more about this mystery illness and help me keep hopeful!
Found this very interesting as I’m starting to show high amyloid levels in my labs. I haven’t covid yet…
I wouldn’t be surprised if we see more on amyloid proteins as I believe they’ve popped up recently. I just can’t remember where…
In all that research on clotting? And wasn’t there some paper on spike protein related amyloidogenesis?
(Don’t know much about amyloid so not sure if that’s the same).
Dear Cort,
I have experience chronic headaches for 16 months due to the covid vaccines. Do you think that I have this same thing going on which you wrote about here? If so, what should I be on to try to combat this injury? I would appreciate any and all help.
Same thing happened to my friend. Headaches for months after Moderna vaccine. Doctor put her on low dose amitryptaline amd no more headaches.
Thanks ‘Em, yes, I am on amitriptylin as well. Wish I could say the headaches went away.
Hi Cherie – you might want to check out what Long Covid people are doing for those symptoms. It looks to me like in some people the vaccine can bring on similar problems as the infection, but maybe not as severely?
Yes, I have been on the long covid protocol and now on the adverse reaction protocol. I have all the long covid symptoms. I just can’t seem to get over the headaches.
I heard that a combination of the following supplements a day can help neutralize the spike protein:
5,000mg Vitamin C
200 mcg selenium
600mg to 1,200 mg NAC ( N-Acetyl cysteine)
Apple Pectin
You can also add DMG (Dimethylglycine) 500mg to 1,500mg per day divided doses.
And If you are really adventurous you can start taking Ivermectin everyday and the dose would be .10mg for every ten pounds you weight. I weight 150 pounds so I take 15mg Ivermectin a day. If you take Ivermectin make sure and take zinc along with it. I don’t want to start out taking it everyday do it every 2 to 3 days.
I take all those things I mentioned everyday and I’m still here and doing better.
By the way DMG is supposed to be good for Chronic Fatigue Syndrome. I’ve noticed an increase in energy with DMG. It’s also good for your liver and NAC is also good for your liver and lungs. Hospitals use NAC to detox people with Tylenol poisoning because NAC increases glutathione in your liver.
Research all these supplements for yourself and decide what you want to do.
Another supplement I heard might help CFS is Dihydroquercetin. I haven’t taken it yet. I’m also going to start taking Magnesium Taurate which is supposed to help with heart rate and blood pressure and Afib and anxiety and with sleep.
Good luck and I just want to say that I have been taking Ivermectin on a consistent basis for a year now I’m just fine . I know lot’s of people taking Ivermectin and they are OK. I know lots of people who have taken it for Covid and they are just fine.
Thank you Jude for your comment. I have been taking everything you suggested here except I’m not on the dose of Selenium you suggested. I am on NAC. I will try the DMG. I was on ivermectin months ago and it didn’t help as well as prednisone which didn’t help. I’m on hydroxychloriquine now. I haven’t tried the apple pectin but drank the pine needle tea for months which didn’t seem to help. All along I have gotten somewhat better. I take celebrex and tylenol in the morning and half a sumatriptan at dinner time if the headache is bad, or just tylenol if it’s not too bad. Ice pack on my head every night. Have you heard of photophobia? This came on at the beginning along with the migraines which are now more like chronic headache. I take a ton of supplements and am on a special detox diet avoiding any foods which would cause more inflammation.
hmmm…. maybe check out Angela Staton and what they do for migraines… nutritional.
I get headaches when I haven’t eaten enough calories / carbs, low on CO2, etc.
Hmm… the headaches may also be caused or made worse by one of the many supplements you are taking, or foods you are not eating or eating.
I saw the webinar on solve from jarred younger: how can we see neuro inflammation in the brain. he talked also about treatments. I do not understand the link between what they found in the study of ME/cfs and the webinar of jarred younger. It is as if there are different kinds that causes neuro inflammation. If i remember correctly, jarred younger talked about 2 or 3, i thought 3 possible causes. It is al so confusing with this on top. do you understand it Cort? does anyboddy understand it?
I haven’t seen the webinar yet but plan to check it out.
it is really interesting and hopefull in many ways!!! even a scan never done before and only now in (they had to do first a few healthy people) the first ME/cfs suferer. And if neuroinflamation, lots of allready excistent drugs wich he will try verry fast, one after the other. and he urges the ME field to make more work from clinical trials, faster, he sais 400 clinical trials for long covid (they do not wait!) and gave the number and a list for ME. he wants to move whole the ME field way faster! and that i am glad for!!!
I liked the part in Jarred’s video about brain temperature being too high and contributing to symptoms. It gave me something to try since I get a low-grade fever every relapse. I tried maximum strength tylenol on the last relapse I had and it seemed to shorten it quite a bit, from 5 days to 2. The tachycardias and brain fog stopped in less than a day. Then the fatigue was gone after two days. I’m keeping my fingers crossed that it was real and not my imagination. Shortest relapse I ever had so far. I’m keeping an eye on my temperature now, hoping to pre-empt relapses. Maybe it will help?
Hmmm… My mother had ME/CFS for years and then vascular dementia. Sounds related. It’s looking like my near-future, too.
Another study showing endothelial problems in long covid/ ME i really feel we are starting to focus in on the nub of the problem now.
Wonder does this tie in with Carmen’s paper on GPCR antibodies and if happening in the brain surely it is happening all over the body. Would immunoadsorption work while waiting for the long overdue trials of BC007.
I started out with Hypothyroid in 8th grade. Then Fibromyalgia, moderate to severe a few yrs later. Never had any allergies but several yrs later added MCAS to become bed-bound. shortly thereafter, I developed full blown ME/CFS after first being diagnosed erroneously with Parkinson’s.
This may be odd but where can you donate your body/brain for ME/CFS specific research?
The process is complicated, and it seems more suited for people in hospice (as everyone around you needs to be aware of your status and react quickly), but this place is accepting brains: Human Brain and Spinal Fluid Resource Center.
I was sent a ridiculously long application packet by James Watkins, NIH Brain Bank, Donor Coordinator, 310-268-3536 (Office)
When they note the similarity of the punctate hyperintensities to strokes, it makes me wonder how similar or different are the effects of repeated leakage (as found here) vs repeated blockage (as in micro-strokes).
I believe many ME patients might report sometimes experiencing events that might be suspicious for a transient ischemic attack (TIA). This work makes me wonder if leakage and blockage might be considered two pathways to TIAs; or if they’re more fundamentally different in their implications (even if some transiently shared symptoms).
If only researchers paid attention to what has gone before. I was diagnosed with “Chronic Encephalopathy and Immune Deficiency” in 1984 by two immunologists, one a practicing doctor who was very prominent in the American Academy of Allergy and Immunology and the other a professor of immunology at the University of Central Florida. 1984 was the year that the illness that would eventually be called ME/CFS was first reported in San Francisco and in other sites around the country. (Oslers Web)
My original doctors put me on the heparinoid, dextran sulfate, obtained on orphan drug status from Germany and transfer factor, white blood cells from healthy patients. These were enormously helpful, but my doctors never promised a cure…just more good days than bad days.
Since dextran sulfate is a mast-cell stabilizer, it really helped with the “allergies to everything” I had developed.
In the beginning, I had horrid headaches that could go on for days. A shot of heparin at the doctor’s office would knock these out.
(David Berg also had a protocol using heparin and/or other blood thinners for treatment of ME/CFS.)
My first doctors were on the cutting edge of something and I think they knew more about what this is than they ever told me.
My blood is so thick that it is almost impossible to do a blood draw and the blood clots in the drawing tube. It puzzles me why ME/CFS specialists today don’t try the heparin protocol when they know there are blood clotting issues in both long Covid and ME/CFS.
Betty. I saw that illness in San Francisco in 1983.
The one Carol Jessop was investigating.
But it was sporadic and not outbreak style.
This led to furious debate between Dr Straus and Dr Cheney because the Lake Tahoe outbreak was exactly that. A wildfire contagious illness that went from person to person with a four to seven day lag time.
Thanks to Dr Straus’s influence and desire to keep EBV dominant in the debate, the Holmes committee created a vague new syndrome that omitted the difference between the widespread sporadic “flu that never goes away” and the “Tahoe Types” which is what Dr Paul Levine called the outbreak “cluster” kind of illness.
Researchers never came back to discuss the differences between the “EBV Syndrome” and the Lake Tahoe Mystery Disease.
They just left this confusion in place.
Betty, my doctor reached out to Berg’s clinic in Phoenix in the early 2000s to set up a blood draw while I was visiting my mom in assisted living. I had mild levels of hypercoagulation, enough that Berg recommended low dose heparin. After two weeks of daily belly injections, I felt so much better. My muscle pain reduced, especially in the “coat hanger” region of my upper back and shoulders where I still experience my fibromyalgia and myofascial pain the most.
I did low dose heparin injections for about six months before the bleeding problems began. Minor bumps would produce dark painful bruises 10x larger than previously. I’ve always been easy to bruise with occasional spontaneous bruising, which worsened with the heparin. What got me off it was a nasty fall on a staircase that left a deep contusion the size of my hand on my outer thigh that took almost a year to completely disappear. After my dentist told me a dental reconstruction horror story about a patient on heparin who tripped and fell while crossing the street, hitting her mouth/upper jaw on the curb, I swore to never use heparin again.
Hi Judi, I am so sorry that you had bleeding problems on low-dose heparin. The medication I took is somewhat different and not available in the U.S. I took DS for a year with no bleeding problems. It is interesting that this overview of Berg’s protocol also recommended Transfer Factor, an immune booster that is rarely mentioned today. https://www.anapsid.org/cnd/diffdx/hypercoagulation.html
Hi Judi, I am so sorry that you had bleeding problems on low-dose heparin. The medication I took is somewhat different and not available in the U.S. I took DS for a year with no bleeding problems. It is interesting that this overview of Berg’s protocol also recommended Transfer Factor, an immune booster that is rarely mentioned today. https://www.anapsid.org/cnd/diffdx/hypercoagulation.html
Some doctors are prescribing blood thinners to Covid patients. It seems to help them. https://www.upmc.com/media/news/080421-pitt-activ4a-nejm
The 1992 “study” by Cheney, Peterson and Komaroff showing lesions on an MRI scan is actually the 1986 study initiated by Dr Paul Cheney when the first MRI scanner was installed in Reno.
Dr Cheney and Dr Peterson spent $200,000.00 on brain scans to prove to the Center for Disease Control that the Tahoe Mystery Illness was real and serious.
This worked and the CDC was scared into convening the Holmes committee which created a new syndrome.
Insurance was not covering this “experimental” diagnostic.
Dr Cheney and Dr Peterson counted on proving their case and having the CDC/NIH reimburse them. This did not happen and Dr Peterson was stuck with paying the bill.
The story is in Osler’s Web.
Yes Erik , and my 1992 brain scan by Peterson was part of this ! Thank you for posting this.
Thanks for the historical perspective. 🙂
The 1992 “Reeves Letter”
Dr William Reeves letter to Dr Komaroff Cheney Peterson and Buchwald explaining why the newly discovered HHV6 with its cytopathic effect should not be researched.
And in spite of being the very virus that scared the Holmes committee into coining the CFS syndrome, this virus should not be considered part of the CFS syndrome.
——————————————————————————————-
The Chronic Fatigue Syndrome Controversy
To the Editors: Buchwald and colleagues (1) conclude that
the chronic fatigue observed in their patients may reflect an
immunologically mediated inflammatory process of the central
nervous system and may be associated with human herpesvirus
6 (HHV-6). The authors, however, failed to consider organic
causes of chronic fatigue for analysis as a separate category
and referred to neurologic symptoms without specifying diagnostic criteria. The study also tacked appropriate controls; this
was not a cohort study with matched controls, as stated in the
abstract, but a case series with variously selected nonmatched
controls. Controls were not characterized, and each major
comparison used different controls. Furthermore, the case series included two different groups of patients who were not
separated for major analyses; no comparison included more
than two thirds of the patients; and those tested for HHV-6
and by magnetic resonance imaging were more ill than those
not tested. Moreover, the authors did not present statistical
analyses to elucidate observed differences, did not address
possible interactions between risk factors, and did not control
for possible confounding.
The cardinal finding, frequent isolation of HHV-6 from patients, cannot be independently reproduced because the methods are inadequately described. The presence of HHV-6 was
inferred from the appearance of “giant cells” that showed
diffuse immunofluorescence, using partially characterized test
and control sera. The possibility exists that an agent other than
HHV-6 was detected. Of the confirmatory assays, only the
monoclonal antibody test is unambiguous. Confirmatory tests
used only a tew selected patients; each assay used different
uncharacterized controls; and the three negative monoclonal
antibody controls appear to have been tested in a different
laboratory than the positive controls. The passage experiments
are uninterpretable because cytopathic effect alone is inadequate for diagnosis, in addition, the primer sequences used in
the polymerase chain reaction were not described. The work
therefore cannot be replicated, and the appropriate test of
primer specificity, demonstration that unrelated sequences cannot be amplified by the primers, was not done.
Although the term the “chronic fatigue syndrome” is not
used in the article title, the first two paragraphs of the discussion implicate this syndrome as the condition affecting their
patients. Because the chronic fatigue syndrome has no known
cause or specific treatment and it exists in a highly charged.
medical, social, and political atmosphere, new potential causes
and treatments are accepted by many as gospel before scientific confirmation. Accordingly, published studies on this syndrome must be as precise as possible. We conclude that the disease Buchwald and coworkers described is not the chronic
fatigue syndrome or any other clinical entity and that they
showed no association with active HHV-6 replication.
Wiliam C. Reeves, MD, MSPH
Philip E. Pellett, PhD
Howard Gary, Jr., PhD
Centers for Disease Control
Atlanta, GA 30333
Reference
1. Buchwald D, Cheney PR, Peterson DL, Heni? B, Wormsley SB,
Geiger A, et a). A chronic illness characterized by fatigue, neurologic
and immunologic disorders, and active human herpesvirus type 6
infection. Ann Intern Med. 1992;116:IO3-13
@Erik Johnson,
it seems to me that the hyper-intensities in the study only showed up when a special mri machine that could show much smaller areas more precisely was used.
I thought they stated (somewhere?) that the regular mri machines can’t show small enough areas to be useful for making the punctate hyperintensities show up?
are you aware of anyone a person or doctor’s office could send mri’s to that would be knowledgeable enough to spot hyperintensities if they are there?
and what kind of mri machine needs to be used?
sincerely, sunie
This MRI study was a real demarcation point in the history of CFS.
Doctors fought with the new EBV serology test. Denied that they saw sick people. Said they couldn’t find swollen glands and lymph nodes. Couldn’t see the sore throats and oral thrush. Claimed there was nothing strange about teachers unable to remember their subject. That dark circles under the eyes and pale skin wasn’t proof of illness.
They fought and fought. Did everything to hide and deny the outbreak.
The early CT scans snowed nothing. Then the new MRI was set up in Reno. The Unidentified Bright Objects were similar to what was seen in MS and AIDS. This was on the first machines, so I guess they were not special MRI’s.
Lakeside Hospital, just across the street from the Cheney Peterson office, hated people with the “mystery malady” so much that they wouldn’t see any mystery patients. Only for a broken bone or something obvious. Those MRI’s made all the difference. Suddenly the disease was “real” and they ceased their denial.
But they did not drop their opposition to Cheney and Peterson.
Instead they switched to saying something WAS going wrong, but it was inappropriate for Cheney and Peterson to have said anything in public or gone to the newspapers, as this was destroying the Lake Tahoe economy.
In this way, the derision, doubt and denial remained embedded in the newspapers and in the public consciousness as if it had never been overturned.
We achieved “local belief” for ourselves but the appearance to the rest of the world remained that the outbreak was nothing more than hysteria.
Showing these scans to the vast majority of doctors won’t do any good because most of them use the logic “Yes, we see them but we don’t know what they mean. Therefore they don’t mean anything”
That is what they did to us, and the rest of the doctor population is doing the same thing to sick people the world over.
Old song – “There’s a Hole in the bucket, dear Liza, dear Liza,
there’s a hole in the bucket, dear Liza , a Hole…”
While interesting, I’m curious whether the participants used in the study had been vaccinated or not? It’s known that the vaccines caused clotting and shedding. Was that taken into consideration or mentioned in the study to make certain as to the causation?
I think this was pre-vaccine – earlier in the pandemic.
Seems like a logical fallacy tbh. Yes, the vaccine can create clotting and shedding. But it only creates clotting and shedding bc the virus itself does it first and the vaccine is derived from it. So it doesnt matter. Same with ppl claiming dont get the vaccine bc it creates heart inflammation. Yes, what a surprise, thats bc corona does it 10x more often if you get it.
False the Covid vaccines are not made from a live virus. The vaccines are a synthetic spike protein manufacturing potion.. It’s gene therapy. This technology was developed to deliver cancer drugs. This is new technology. We are all being experimented on. I don’t want to scare anyone but I know two people who died from the Pfizer shot. They died from myocarditis. Two healthy men working full time who received the first shot and the very next day they ended up in the hospital and they were dead within a couple months. Very sad and totally unnecessary.
Come on the vaccines don’t work to keep you from getting Covid and they are dangerous.
The shots are causing the blood clots and the heart attacks. Not only that but the shots are also causing shingles and Bells Palsy and all kinds of other nasty things.
At a recent wedding I attended 5 people came down with Covid and ALL of them were vaccinated and boosted.
You can choose to focus on anecdotal reports or the science. Our brains have evolved to make much of anecdotal reports and reject the science. As a species I would say the more we evolve to accept the science – in whatever field we’re talking about – the better off we will be.
The studies and the tens if not hundreds of thousands of people who have taken part in them have again and again found that the vaccines are very effective in preventing hospitalization and death. Recent studies that compare the death rates amongst the vaccinated vs the unvaccinated confirm that.
You, of course, have the option of rejecting the science that’s designed to produce objective results and cut out subjective assessments- in favor of your own personal and highly subjective experience. You should be clear, though, that you’re rejecting the very foundation of medicine that’s allowed it to make so much progress over time.
The problem is when science becomes politicized
This is a nice overview of the history
https://www.bbc.co.uk/blogs/adamcurtis/entries/a2094c9d-9864-348e-a241-7aa93adf0c09
And recordings one and two
https://www.npr.org/2005/04/22/4612464/freuds-nephew-and-the-origins-of-public-relations
Our experiences are very important in forming knowledge – an empirical world.
Yes, this is a question that should be addressed in every study. Otherwise, there is no real way to see whether covid by itself, the vax by itself, or a combination of both are at play in the various findings.
Thanks again Cort! I hope Nath comes up with something in his IVIG trial, something to stop post exertional malaise. I’m going to let my rheumatologist know of the possibility of IVIG treatment when I see him next month – just in case something good comes of it in the trial.
Hopefully he’ll be able to identify who benefits from who does not – and can apply that to ME/CFS.
I was pretty sure IVIG has been tried for CFS before, so I googled and this Health Rising article came up: https://www.healthrising.org/treating-chronic-fatigue-syndrome/drugs/ivig-intravenous-gamma-globulin-therapy/
This was the concern that I raised a year ago when people were tying CFS with long COVID. Long COVID may turn out to be “CFS with cause”, like those with heavy metal poisoning or other toxins, and equating long COVID with CFS may lead CFS research astray for a while. I think the takeaway of this study to CFS community should be that the brain inflammation from the leakage causes CFS-like symptoms in long COVID patients, and leave it at that rather than conjecturing further to tie it to CFS.
Endothelial dysfunction in the context of CFS, by the way, was about reduced blood flow, not leakage, if I remember correctly. I’m not sure if you can tie that endothelial dysfunction theory of CFS with this particular study other than that both involve blood vessels.
Btw, BBB leak hypothesis for CFS had been around for a while. Maybe it’ll prove to be more relevant to long COVID, but my guess is that there is nothing there for CFS if nothing came out of it in 20 years. https://pubmed.ncbi.nlm.nih.gov/11461179/
Cort, isnt’t an idea to start a blog with the view of ME/CFS/POTS-patiënts what they think is the cause of this disease? Or what is wrong. It must be short and straight not to much words.
Thought I’d share this podcast (and written version) about a new investigational drug to deal with long-Covid;
https://www.medpagetoday.com/podcasts/healthwatch/99751?xid=nl_mpt_DHE_2022-07-17&eun=g1240599d0r&utm_source=Sailthru&utm_medium=email&utm_campaign=Daily%20Headlines%20Evening%202022-07-17&utm_term=NL_Daily_DHE_dual-gmail-definition
Kindly scroll down to the appropriate section on microtubules.
The drug does something called microtubule disruption and so far it has shown efficacy. Apparently some studies show reservoirs of Covid spike proteins which some think are fueling long Covid. This new drug disrupts that and the scientists who developed it think that it could be applied to other maladies.
Have any thoughts on this?
“Hot in the field”
Can I just say that it angers me that Nath can suddenly “speed-research” when something is ‘hot’?
I read about complement activation, monocytes & macrophages, blood clotting (platelets up/red blood cells abnormal?), persistent immune activation, Neuro consequences, IVIG trial …
All (!) these things can be found in my medical records.
Unexplained monocytosis, leukocytosis, high complement factors, platelets & red blood cells abnormal, cd19 & HLA-DR (pointing to some kind of auto-immune activation), cytokines up, elastase, nagalase, GPCR Aabs, quinolinic acid, ammonia, lactic acid D & L, brain scans abnormal, …
Decades we’ve been practically screaming to study us properly.
Our lives lost & in constant agony. Disbelieved.
What did they do for us?
What will Nath finally do for us?
Does he mention ME in this covid study? The overlap?
You mention ME Cort, but does he?
Or is ME ‘totally NOT hot” & still best to be avoided? No prices to win if you solve ME. Prices to win if you solve long covid?
We can’t even get IVIG or plasma exchange or whatever therapy that could really help some of us.
After +9000 research papers (!).
I’m glad the covid community sees research going full force.
Perhaps help on the horizon for them.
But for us?
To read studies where you know after decades of tests & misery, you ‘fit’ this profile too?
Quite frustrating. Maddening even. Given we lost so many friends in our community. Not at once. Not within a year. One at a time …
Did he forget about the autopsies on ME patients? That the brain is where he should have looked a looong time ago?
“Basal root ganglia inflammation … “
Sorry. Had to say this.
No worries. Good questions. Nath has become quite a source for the media – he is often giving interviews – and he almost always seems to find a way to plug ME/CFS. He’s actually one of our best advocates.
By hot I meant that this topic is getting a lot of interest in both conditions. I think prizes go to whoever solves either. The fact that we all have to struggle with is that long COVID is getting a ton of interest and money while ME/CFS still is not and researchers go where they can get funding. (Nath was studying ME/CFS before long COVID showed up).
There was little money or interest in doing autopsies in ME/CFS. He couldn’t have done them if he tried. With long COVID it’s different.
The more ME/CFS is mentioned with long COVID, though, the more it should benefit. Just think – hundreds of researchers are now studying long COVID – and they should all be interested in ME/CFS. What a great jolt to ME/CFS research long COVID should be for us. Hopefully, it will float all boats. Is it fair? No. Will it be helpful – almost certainly so.