Step by step Lipkin’s metabolomic study produces a coherent picture of mitochondrial dysfunction
The really neat thing about Ian Lipkin’s latest work on ME/CFS is how well all the results fit together. Health Rising reported on the preprint of this paper earlier, but the final version that recently came out has fleshed out some things.
This big NIH-funded Ian Lipkin study had several things going for it. It was larger, and assessed more metabolites than any other study; it used patients diagnosed by ME/CFS experts, and employed, in its words, “complete and cautious statistical approaches”; i.e. it’s a rigorous and trustworthy metabolomic study.
In short, it’s a major paper – and boy, were its findings interesting – not just because they fingered a potential new player – peroxisomes – but because of how well they all fit together – and that’s what this blog will focus on.
All Together Now
Metabolism is a big deal. In some ways, it’s the big deal. It’s concerned with nothing less than the “chemical processes that occur within a living organism in order to maintain life”. Breaking down foods to provide energy, proteins, or fats, etc. is metabolism. So is protein, carbohydrate, and fat synthesis. Any chemical reaction that transforms one compound into something else comes under the rubric of metabolism.
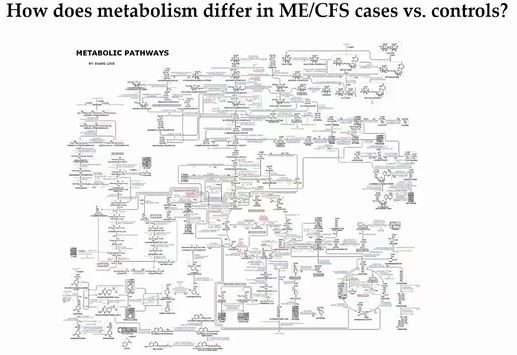
Possibly disturbed metabolic pathways in ME/CFS.
It’s metabolism that produces the sometimes huge, head-spinning diagrams of metabolic pathways. If some part of a pathway gets blocked – the upstream portions of the pathway build up – and the downstream parts of the pathway decline. Or, if a particular pathway is getting hit hard by something (infection, toxin, etc.) that will show up. It’s these kinds of abnormalities metabolomic studies are looking for.
Basically, with metabolomic studies, we’re looking for problems in the chemical reactions that sustain life. Since chemical reactions require energy, problems with energy production can show up big time in these studies – and in this study they did.
Study Findings
Small organelles show up in a big way. The peroxisome is the little purple circle at the bottom of the cell.
Fatty Acid Breakdown – Reductions in plasmalogens that protect the phospholipids which, in turn, support the all-important cellular membranes (the “skin” surrounding the cell) pointed an arrow at the peroxisomes – small organelles in the cells which manufacture them.
Peroxisomes do a lot more than produce plasmalogens, though. They also break down very long-chain fatty acids into the shorter chain fatty acids that our mitochondria use to produce energy. If those fatty acids aren’t broken down, the mitochondria become starved of an important energy source.
That’s precisely what this study suggests may be happening in ME/CFS. The authors believe the peroxisome-fatty acid-mitochondria connection has been cut.
“We posit that this crosstalk between mitochondria and peroxisomes plays an important role in maintaining energy homeostasis and that dysregulation contributes to the fatigue and cognitive dysfunction that are hallmarks of ME/CFS.”
That wasn’t all. The study also found reduced levels of carnitines. Carnitines play a key role in the transport of, yes, long-chain fatty acids from the cytoplasm of the cell to the mitochondria. This study already suggests that two processes involved in getting long chain fatty acids into the mitochondria have taken a hit in ME/CFS.
Several findings suggested the membranes surrounding the mitochondrial and other cells were suffering.
That’s not all carnitines do, though. Because carnitines also help to maintain cellular membranes, the low levels of carnitines found also threaten the stability of the all-important cellular membranes – leaving them more easily crippled by things like inflammation and oxidative stress.
The Gist
- We’re back for round 2 as we dig into the same paper twice – kind of. The formal publication of Ian Lipkin’s NIH-funded metabolomic study fleshed out the earlier preprint version of the same study.
- What was so striking about this study was its coherence. Step by step by step – the study results seemed to build on each other to an extraordinary degree – presenting, in the end, a picture of potentially damaged mitochondria struggling to get resources.
- First, we see signs that the peroxisomes – little organelles in the cell – are not working well. Peroxisomes do two very important things: they break down long chain fatty acids into components the mitochondria can use – and they produce the compounds called phospholipids that protect the cellular membranes.
- The low levels of carnitines also found simply amplified those very same problems because carnitines transport fatty acids into the mitochondria and they also play a key role in the cellular membranes.
- Low levels of carnitines may turn out to be a big deal as they can also flip a switch that tells the peroxisomes to start producing carnitine – thus presumably inhibiting their ability to break down long chain fatty acids.
- Because peroxisomes also regulate the mopping up of free radicals (reactive oxygen species (ROS) the impaired peroxisome functioning could also contribute to a hot mess of inflammation.
- That brings us back to the membranes that protect the cells – including the mitochondria. Low levels of a major membranal component – phosphatidylcholine (PC) – potentially spell yet more trouble for the mitochondrial membranes and mitochondrial functioning.
- Plus, the low PC levels may also impair another transport mechanism into the mitochondria. Low PC’s can interrupt the transport of proteins into the mitochondria – further inhibiting mitochondrial production.
- Thus far we have evidence of starved mitochondria that are getting pummeled by free radicals.
- The authors weren’t done yet, though. Next came low levels of several compounds indicative of mitochondrial damage and/or damaged membranes.
- Finally, a not quite statistically significant decline in choline levels could contribute to autonomic nervous system dysfunction.
- The findings seemed pretty compelling given the rigorous nature of the study, and similar findings regarding problems with long chain fatty acids in ME/CFS and fibromyalgia. The authors cautioned, though, that it’s “imperative that the validity of novel findings reported here be independently tested in other cohorts” and that larger studies with more ME/CFS patients be done.”
- Let’s hope that’s done and Lipkin and others get a chance to study both ME/CFS and long COVID patients in bigger studies. With few long COVID studies exploring the mitochondria or the metabolome let’s also hope long COVID researchers – particularly the NIH’s RECOVER Initiative- are keeping an eye on ME/CFS studies.
- Meanwhile, the NIH is still somehow spending over a billion dollars on long COVID while spending virtually nothing on its sister disease ME/CFS. While it’s renewing the ME/CFS research centers, they’re being funded at the same paltry levels as they were five years ago. Hopefully, at some point that will change.
Low levels of carnitines may turn out to be a big deal as they can also flip a switch that tells the peroxisomes to start producing carnitine – thus presumably inhibiting their ability to break down long chain fatty acids.
Because peroxisomes also regulate the mopping up of free radicals (reactive oxygen species (ROS)), the impaired peroxisome functioning could also contribute to a hot mess of inflammation.
That brings us back to the cellular membranes that protect the cell – a prime target of free radicals. Thus far, we’ve two potential hits to them (low plasmologen and carnitine levels), and now comes a third: low levels of an important membranal component – phosphatidylcholine (PC).
The PC depletion suggests that yet another important transport mechanism into the mitochondria has been disturbed in ME/CFS. Low PCs can interrupt the transport of proteins into the mitochondria – further inhibiting mitochondrial production.
That’s not all. Low PC levels can also impair the ability of protein translocases which shuttle proteins through the various membranes in the mitochondria and direct them to their proper place. Problems with these protein translocases have been directly shown to impact the ability of the mitochondria to produce ATP or energy.
Look how quickly a potential suite of hits to the mitochondria have shown up:
- Problems breaking down long chain fatty acids so that the mitochondria can use them,
- Problems transporting the fatty acids into the mitochondria,
- Damage to the cellular membranes that protect the mitochondria (and other cells) from damage,
- Problems shuttling crucial proteins into the various compartments of the mitochondria.
More was coming, though.
The authors reported that the low levels of lysophosphatidylcholines, phospholipid ethers, and prostaglandins (D2, F2α) have been associated with mitochondrial damage and/or increased oxidative stress which, given the poor state the mitochondrial membranes appear to be in, is not a happy situation.
About those membranes… the low levels of PCs, ceramides, sphingomyelins, and phospholipid ethers provide further evidence of membrane damage, as they all play important roles found in the membranes as well. Cells get their marching orders through receptors found in their membranes. Because ceramides play an important role in the propagation of signals through the membranes, the low ceramide levels found could render a cell dead in the water unable to respond to the signals it’s getting.
Next came low levels of choline – essential for the production of phosphatidylcholines (PC) – which, as was previously noted, were low. The low levels of choline did not meet the statistical criteria needed for significance – but it was getting close.
Because choline also enhances the functioning of the G-protein coupled receptors that regulate autonomic nervous system (ANS) functioning and play a role in the production of epinephrine, as well, the authors proposed that the lower choline levels might be impacting ANS functioning, resulting in problems with blood flows and oxygen supply to the tissues.
Conclusion
It was the coherence of its findings that made this study so intriguing – every major finding seemed to fit together in some way. What we really want out of a study is the ability to tell a biological tale, and this one certainly did regarding the mitochondria.
Of course, we must remember that correlation is not causation, and that the body is very complex and can and does frequently fool us. The authors said so in so many words. While noting the strengths of the study (rigorously defined patients, a “complete and cautious statistical approach”, etc.), the authors stated that it was “imperative that the validity of novel findings reported here be independently tested in other cohorts”, and that larger studies with more ME/CFS patients be done.
Still, the emergence of such a coherent pattern of dysfunction is nothing if not encouraging, particularly since one of the central findings – problems with long chain fatty acid metabolism – was also found by the Fisher research group in Australia, and has popped up in fibromyalgia as well.
The funding for the NIH-funded ME/CFS research centers has been renewed – at the same pitiful levels they began with – and which caused some researchers to shy away.
I don’t know how much longer the NIH can keep up the farce of spending a billion-plus dollars on long COVID while ignoring ME/CFS, but let’s hope that studies like this one from Lipkin and those from Maureen Hanson’s NIH-funded centers will change its mind, and Lipkin will be able to get the funding to do a larger study containing both ME/CFS and long-COVID patients, one which is able to get definitive results.
The ME/CFS field, after all, is way ahead of the pack regarding the mitochondria and metabolomics. ME/CFS researchers latched onto metabolomics about six years ago, and since then many studies have been done, yet few metabolomic studies have been done in long COVID, and none that approaches the sophistication of this one. Nor have the mitochondria received much study in long COVID. One hopes that long COVID and RECOVER Initiative researchers are keeping an eye on the mitochondrial and metabolomic findings in ME/CFS.
The long-COVID studies that have been done suggest the mitochondria have been impacted, and a recent hypothesis paper proposed that “NAD+ metabolome disruption” plays a central role in the condition, suggesting that intravenous NAD+ trials begin.






We will always get the best from Ian Lipkin known as the virus hunter. He is brilliant. NIH, GIVE UP THE $$$.
How long can they continue to act as if ME/CFS isn’t intimately connected to long COVID. Not forever I don’t think and not too much longer, I’ll bet. One good bit of news – the RECOVER program does appear to have a real interest in what’s going on with ME/CFS. That’s the first crucial step.
Cort, thank you for the in-depth, recent information.
Also could be considered, Gulf War syndrome and Fibromyalgia. I’m unaware of how they are funded but also have similarities to MECFS
Do you think that taking L-carnitine and Acetylcarnitine supplements may help a patient?
I have no idea actually. I would think its worth a try but my laymen’s guess is that ME/CFS is a multidimensional disease affecting the mitochondria, the blood vessels and other things and it will take a range of treatments working together to really be effective.
i just read about l’carnitine and it is not recommend with low thyroid as it can further lower it. Secondly check out the diet for reducing long chain fatty acids-it seems counter intuitive to me because i seem to do well on most of the foods it says to eliminate and the alternative of eating more carbs does not seem to be rec for me/cfs nor would that work for me.
Roy, I’ve gotten good results from “ATP 360”.
what is atp 360? live in belgium
It’s this: https://www.researchednutritionals.com/product/atp-360-mitochondria-support/
they have been used to treat cfs for a very long time
I think it does help some people with me/cfs (there’s a summary of the research into it here https://me-pedia.org/wiki/Carnitine), but I’ve generally found it makes me ‘tired-wired’, perhaps because by upregulating the carnitine-related pathways, I’m putting more strain on other dysfunctional pathways?
Yes, exactly! Same here.
Japanese researchers were looking at carnitine and CFS more than 20 years. I tried supplementing it at the time, did nothing for me.
Mh, feels like a nothing burger. Member when Lipkins said in 2015 he will crack Cfs in less than 5 years ? yea, i member.
That was conditional on more funding – which we haven’t gotten. Lipkin remarked on how poor the funding was for the NIH-funded ME/CFS research centers, for instance. I wouldn’t count Lipkin out – he’s a well-known researcher who;s made a difference with various diseases and thankfully has decided to turn his attention to ME/CFS.
Since 2015 evidence has (slowly) accumulated regarding problems with energy production and the mitochondria in ME/CFS. While we don’t have a smoking gun it’s very nice to see more and more data pointing in that direction.
Yeah I agree.
One of many researchers who showed real promise but have never delivered.
Speaking of which, how about Ron Davis?
If they had funding this would change.
Ron Davis wants a cure more than anyone as his son has cfs and is totally bedbound.
They also had to shut everything down with covid.
They’re just starting up again.
Of additional interest to me is the finding of low prostaglandin D2. This substance is an absolute requirement for sleep. I think this is a major finding even if it was a minor part of the study.
On supplements, the critical question is not whether or not they help, but whether or not they will harm if they do not work as intended. Such supplements, and choline and others, have been tried repeatedly to no avail in many patients. I think there was a study some time ago that showed that carnitine had some small benefit in a small cohort. Its imperative we have a clinical trial that tracks the relevant metabolites on specific supplements. Objective outcome markers are very important. Its always more studies, bigger studies, and more funding, but this study gives us a direction.
Alt docs have been prescribing NT Factor for decades – it was one of the first things I heard about when I first got ME. Glad to have the clarity, but is this really anything new?
There are lots of ways the mitochondria can go wrong. I don’t remember anything about problems with long chain fatty acid metabolism until the last year or so – so yes, this is actually quite new because instead of saying “something is wrong with themitochondria it’s actually pointing to a potential cause – problems with breaking down these fatty acids and transporting them into the mitochondria.
It’s great if NT factor helps but its truly a stab in the dark – a blunt instrument trying to somehow fix what’s gone wrong in the very complex mitochondria . Until we know what’s causing the problems in the mitochondria we won’t know how to fix them. It might not even be the mitochondria – it could be problems getting oxygen to them. Or perhaps people with ME/CFS have trouble producing carnitine or have trouble getting it to the cells or maybe their peroxisomes have been damaged.
Multiple options exist but you can’t even begin to trace them down until you have some idea what’s gone wrong. That’s why this study is exciting to me – it’s past the “oh the mitochondria are malfunctioning’ but is getting down in the weeds and postulating something distinct like the peroxisomes is the problem. Plus it was nice that all these metabolomic findings (the phospholipids, carnitine and the rest) seemed to fit together nicely.
It’s a long search for sure but it does potentially lead somewhere.
Pretty heavy sledding for our cohort of brain-foggers this study. As a great and largely comprehensible back ground study of cellular metabolism check out the new release by microbiologist Nick Lane… Transformer..it belongs on the bookshelf of all searchers for a more profound understanding of our curse . Also recent book by anthropologist Roy Richard Grinker…. “Nobody’s Normal, How culture created stigma of mental illness” The title speaks for itself and offers insights into the shadow history of FM ME/CFS
It is heavy sledding! I hope most people check out the GIST. Thanks for the recommendations. 🙂
I have been taking L-Carnitine and omegas for years and also did a several month stint with Phospholipieds including Phosphotidylcholine and didn’t notice much improvement.
As my comment in Cort’s last blog, I am now using Flexeril at night and I do notice longer and better rest. Now, I’m a girl who tries all kinds of things and in the last couple of months I have gone off milk and have been using two supplements which are blends of proteolytic/fibrolynic enzymes (Serracor-NK, InflamaZyme) and I am noticing improvement and especially, especially in my skin! I’ve had bad skin my entire life and it is healing and my scars are disappearing. I’m feeling more energy too. I can’t go as far as saying I’m headed toward being cured, but these changes are very noticeable. Usually effects are way too subtle.
NIce to hear and congrats on the creative approach to supplements that seems to be helping. Hopefully, a more effective form of flexeril will soon be available.
Hopefully we’ll find out at some point what is the up with the mitochondria but I would never assume that we have the answer for whatever it is right now. In fact, I would assume that we don’t – that if we need more choline – we would need something else to go along with it – and that something else could have a wide range. Maybe its the gut in some people; blood clots in others….I don’t think there’s going to be a one-shot solution.
I was struck by the paper suggesting NAD+ IV’s be trialed in long COVID. I didn’t even know those existed…
i was taking a supplement with a hefty does of nadh, 20 mg. had to reduce it. it also had coq10 and carnitine in it. then i came across a few articles that said nahd or nad can encourage the growth of cancers, particularly gioblastoma. certainly don’t want that. i’ve had 2 kinds of cancer so far so i am scared to keep taking it. there were articles refuting this of course. i just hate to find something that helps and not be able to take it but it won’t be the first time. i took citicholine when i was doing a cancer dose of cannabis oil because it was supposed to make me less stoned. i read it causes depression in some.
How does this hypothesis add up condierling the Davis et al Hypothesis explaining that cells take a shortcut in methabolism. f.ex in the brain the cells “eat” aminoacids, bc they cannot “eat” what thyare supposed to (sugar and fat). To me lack of GABA (mostly) and GLUTAMATE, as a possible cosequence after this “brainmeal” gives sence, as I think lack of GABA can explain many sympthoms.
Are these two analysis/findungs mutually exclusive, can they supply each other?
And does annonse have a clue on how this ev.t Can be treatet? HE.L P-apharesis,plasmapharesis, FMT, supplement, immunotherapies?
All or none? Where are we regarding hope based on this Lipkin study and Ron and Dr. Phairs work (the cellmetabolism-shortcut, driven by an acute respinse to “danger” that gets cronic?)?
Los of Q-s. Hipe some have thoughts regarrding som of the A-s.
Thansk
So many questions 🙂 but its good that there are a lot of questions to be asked. In general I think Lipkin’s findings do fit other findings that mitochondria are using unusual substrates to power them.
I would be shocked, given the sleep problems in ME/CFS, if GABA didn’t play a role somewhere. As to the treatment possibilities I’m glad that more are cropping up and I really have no idea but I do imagine that it will take a combination of them.
I thought this was study was interesting and possibly related: https://www.frontiersin.org/articles/10.3389/fphys.2021.668330/full
This is a very important study !!!
Peroxisomal dysfunction may be the link between immune dysfunction and neuroinflammation – which in ME/CFS apparently includes white matter changes / myelin defects (i.e., oligodendrocyte dysfunction) which in turn may explain the connectivity defects seen in several brain imaging studies of ME/CFS patients. Peroxisomes are heavily enriched in all neuroglial cell populations, including oligodendrocytes and are thus paramount for the preservation of axonal integrity. Here, Lipkin´s work goes well with Arnaud Germain´s/Maureen Hanson´s work (https://pubmed.ncbi.nlm.nih.gov/33572894/) and also Akiko Eguchi´s analyses (https://pubmed.ncbi.nlm.nih.gov/31759091/) which both highlighted axonal guidance pathways as possibly dysfunctional in ME/CFS.
Of note, the membrane damage associated with peroxisomal dysfunction may also play a role in (or even explain?) the channelopathies observed in ME/CFS (see, for instance, the recent work by Sonya Marshall-Gradisnik).
If only on a speculative note, in the largest ME/CFS genome-wide association study to date, the most significant risk loci association was found for the tubulin polymerization promoting protein (TPPP) gene region:(https://pubmed.ncbi.nlm.nih.gov/35318112/). (TPPP is expressed in brain tissues and plays a central role in myelination).
So I guess this venue should be explored further, especially as it may also be a boon for biomarker research.
Again, this is a very important study. Thank you, Cort, for sharing!
Thank you for sharing Herbert – I had no idea about the potential link between the Germain/Hanson paper (https://pubmed.ncbi.nlm.nih.gov/33572894/) and Akiko Eguchi´s analyses (https://pubmed.ncbi.nlm.nih.gov/31759091/) – which both highlighted axonal guidance pathways as possibly dysfunctional in ME/CFS. Thanks so much for pointing it out. I’m still a bit agog over the idea that this little organelle which we’d basically heard nothing about until the last year or so might play a big role in ME/CFS and possibly fibromyalgia. I guess we should expect surprises, though. 🙂
Interesting, but so what?
What is the causative mechanism? And how is it treated?
I am running out of patience. We have had many of these kinds of interesting studies, yet they never seem to lead to anything.
I would submit that they are leading to something – they are digging deeper into the processes under play in these diseases and learning more about them which hopefully brings us closer to a target and a treatment. They’re not producing a target or a treatment now, though. We need strong advocacy to get us more funding and I can’t imagine that long COVID research won’t dramatically speed things up.
The brain controls everything in our body. It controls the electrical brain signal by transmitting it to the organ through the neural network. When the brain signal is interfered with by external electromagnetic waves, the information is altered and the organ malfunctions and enters a disease state. CFS is mostly out of control. Treatment is by controlling the electromagnetic environment and brain-body tuning to control the brain to control it normally. The most important thing is that the brain breaks down due to disturbance like an electronic device, and it needs to be repaired like an electronic device.
and how do we do that
FYI in case this helps anyone else. I have tried phosphatidylcholine supplements and also sunflower lecithin but my body has a bad reaction it actually increases pain. Any oil derived from seeds it creates more inflammation. I don’t have this issue when I take choline bitartrate. I’m wondering if anyone else has this issue?
Had the same adverse reactions. I use (raw) egg yolk instead : it is rich in choline AND in fatty acids, so hopefully quite nourishing for the cells.
I’ve been taking egg yolk lecithin for years. It was one of Rich Van Konynenberg’s ideas.
Mitochondrial turnover: Researchers discover what causes cell ‘batteries’ to run down:
https://phys.org/news/2022-08-mitochondrial-turnover-cell-batteries.html
“When certain nuclear encoded proteins aren’t brought into mitochondria, the mitochondria are removed.”
That seems like quite the connection, Ann. Check this out: This is from the paper you linked to:
This is from my overview of some of the issues the Lipkin paper found:
Problems with transporting stuff into the mitochondria may be rife in ME/CFS.
Plus the Simmaron Research team is hot on the trail of autophagy – a blog on that is coming up.
Thanks!
“How mitochondrial dysfunction leads to premature aging and disease”
https://phys.org/news/2022-08-mitochondrial-dysfunction-premature-aging-disease.html
“This paper links, for the first time, mitochondrial dysfunction to the shortening of telomeres, a key biomarker of premature aging.”
“Telomeres are specialized DNA sequences that act as caps that stabilize the ends of chromosomes,” explained Taosheng Huang, MD, Ph.D., professor and chief of the Division of Genetics in the Department of Pediatrics in the Jacobs School of Medicine and Biomedical Sciences at U of Buffalo.
“The shortening of telomeres is generally regarded as an important biomarker of aging, but for a long time, no one knew the mechanism. Now we are able to link mitochondrial dysfunction directly to the shortening of telomeres,”
“By utilizing optogenetics to force a physical interaction between mitochondria and another cellular component, the lysosome, we were able to restore the mitochondria to a more normal size while also improving their energy production functions,” explained Huang. “We believe that this new finding could be used as the basis for future diagnosis and treatments for this group of diseases.”
Thanks again, Ann. Lots of juicy connections – shortened telomeres have been found in both ME/CFS and long COVID.
https://www.healthrising.org/blog/2021/07/29/long-covid-chronic-fatigue-syndrome-hypothesis-merging/
I tried levocarnitine earlier this year for a few months but didn’t really notice much of a difference, it did seem to give me some acid reflux and tachycardia at times from what I remember. At least I didn’t smell like fish.
Thanks, Cort. I very much appreciate this blog. As a result of reading everything that pops up here, I have added NMN, Ashwagandha, and 7-keto DHEA to my daily regimen. I can report that I have experienced a significant improvement in the symptoms of MCAS.
I have no family doctor but that’s ok, they wouldn’t understand this anyway. I have a naturopath who fortunately has read Dr. Afrin’s book, “Never Bet Against Occam” and simply writes prescriptions as I ask for them.
Tests would only be misinterpreted. My approach is to TRY EVERYTHING one at a time and see what works and what doesn’t. Sometimes I go back after rejecting one supplement or drug and add it again. It’s all individual anyway. I am gardening, shopping and walking the dog again and I’m pleased. Some way to go yet, but improving.
I’m with you on the try everything approach. I have a long car trip coming up and I’m going to pack in the mitochondrial supplements and see what happens 🙂
How does this study relate to, fit with, or contradict the study that showed me/CFS patients fall into 3 clear categories of macronutrients we burn?
As I recall, some burn amino acids, others fatty acids, and the third cohort was more similar to the control group.
How can a group be using fatty acids for metabolism if the peroxisomes don’t work?
Dr Daniel Peterson’s very first foray into writing a paper was on an L Carnitine deficiency in the 1985 Lake Tahoe Mystery Disease.
Errr… how is this flying so under the radar?
There is a doctor that has been treating strokes and traumatic brain injury and pain with a drug originally intended for rheumatoid arthritis. it’s mode of action is to bring neuroinflammation down.
Been thinking with the similarities with TBI, this has got to be a candidate for ME/CFS.
Turns out he has been trying it on long covid and it is working.
Not only that – it’s one of the two drugs Nancy Klimas is using in her trials for GWI.
Wouldn’t that be something, a person who comes up with a potential cure/treatment for ME/CSF is someone outside the field…. I’ve been seeing this a lot lately, when you have people with scientific background that get ME/CFS, they get lost in the mire of details… It’s like they are too close to the subject to see things from another view / differently.
Etarnecept.
Google it.
A TV channel has been tracking this doctor’s work for over 7 years. They are trying to get it accepted as on-label use there for TBI/Stroke.
My thoughts are: it probably doesn’t need to be injected, even rubbed on the skin can get action in.
This and rapamycin and other macrolides…
Stop/reduce the inflammation / LPS in the blood stream.
Australian TV on youtubes
I’m trying to understand what this substance is (and its safety)
What strikes me is how just one-time administration of it leads to
like it lifts whatever was going on and normal processes can resume
I must say I have felt and experienced this with a few substances – foods and hormones. Immediate effects. When you are missing something, and have missed it for a loooong time, it is amazing what the effect can be when you provide it to our body again.
I appreciate all the info Cort. All these studies after studies, and there’s just no real meat on the bone.
What would constitute meat for you? Studies that are zeroing in some central problem at least constitute beef jerky for me 🙂
aged meat cures best? 😉
This is really very complex research. The more research, the more questions. The enemy still doesn’t show up. Only small parts on the battlefield become visible. Thanks for your good explanation(s) Cort, keep on walking the battle field to complete the puzzle. I’ve lost the overview.
This could be the cause here & a Treatment Cure. He even treats the Negative blood samples he goes after symptoms of ME/CFS & other illnesses. He says the blood test is not reliable because the parasite hides.
It comes from Cats, meat, water, fruit, vegetables & other animals. It could explain why so many women are sick who own Cats.
Toxoplasma Gondii his book manual for Doctors & patients is called Shadow Disease on Amazon. The back cover explains it all
https://en.toxoplasmachronic.com
A recent study linking T. Gondii to mitochondrial dysfunction: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733060/
Very interesting study! Yes, it is “heavy lifting” for my rapidly fatigued brain but I am glad that you put the effort into writing an article on these findings. I may have to read it over and over again just to retain a fraction of the information, but I understand it as I’m reading and I clearly see how important these findings are. Ian Lipkin is like a dog with a bone when he’s studying something (just like Ron Davis) and they’re both brilliant. But without funding, the most brilliant minds can’t conduct the level of research needed to unravel this disease. I’m grateful for the researchers that keep trying to get funding and haven’t just thrown in the towel. Maybe Ian Lipkin is a big enough name in the research game to get the NIH to finally concede and approve appropriate funding for ME/CFS research? Maybe the long-Covid researchers will take note of the findings? It’s been a long time waiting for funding, but I can’t give up hope. Also, as a patient, I want to know what is going on regarding studies but just can’t search these things out on my own, so I’m grateful for your articles! I actually have a doctor that wants to know where I’m finding the studies I tell him about. Unfortunately, I always draw a blank when he asks. This time I typed up the title of your article and the link to share it with him as I know I’ll forget your name and the name of this site the second I close it out.
Thank you, Cort!
To me, these type of studies really seem to discredit the Gupta, DNRS brain training programs. It seems the people who got better from them must not have had true CFS. Am I wrong in thinking that?
Or are those programs the only things getting to the core issue itself…a dysfunctional brain from chronic stressors?
Someone said on Twitter fenofibrate helps them at times of PEM. According to WIkipedia, “Fenofibrate is (…) used to treat abnormal blood lipid levels.” in order to treat “hypercholesterolemia or mixed dyslipidemia.”
I have high cholesterol – so could that possibly be related to an impaired lipid metabolism in ME/CFS? Also, my gut does not do well with eating too much lipids.
What’s even more interesting (can’t tell if that could somehow be related, too?), the German Wikipedia entry on fenofibrate states that: “In July 2020, research teams from Israel and the US reported that fenofibrate counteracts changes in lipid metabolism induced by SARS-CoV-2 in vitro. Among other things, fenofibrate was able to inhibit the accumulation of phospholipids in lung cells and increase glycolysis. These effects lead to inhibition of virus replication and the virus could no longer be detected with the methods used after five days. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3650499 “.
It seems fenofibrate can have possible serious side effects though and is contraindicated in hypothyreodism (this article about one such case says hypothyreodism is also a frequent cause of high cholesterol https://pubmed.ncbi.nlm.nih.gov/17278784/). I don’t know if this refers only to untreated hypothyreodism or any.
As always, Cort, your tireless work is greatly appreciated.
The results from Lipkin et al. reminded me that in 2015 I put out a very short, readable paper on how we could greatly expedite the understanding/elucidation of this complicated disease. It can be found here: https://www.kylemcnease.com/uploads/1/0/6/5/10651553/a_different_approach_for_understanding_cfs.pdf
What’s worth noting is that I’ve been advocating for a computational approach for over a decade now. And if you notice in that short paper, the mitochondria is the emphasis. I question how it’s possible for Sherpas to be so efficient at altitude despite possessing less mitochondrial volume density than a sedentary person at sea-level.
Synthesizing the research from endurance athletes is crucial because it informs the mean. It provides the opposite end of the continuum—from highest functioning (which may end up simply meaning most efficient because ME/CFS is potentially the least metabolically efficient state for energy production) to Severe ME/CFS. I’m talking ultra marathoners & Sherpas on one end and people like me during my worst days at the other end.
It makes sense if one thinks of the extant literature that demonstrates how much of the brain is activated in a ME/CFS brain while tasked with cognitive activities. Intuition might lead one to think “hey, more areas of their brain are being recruited for even simple tasks. If they’re so fatigued why is their brain so active?” Someone who is physically strong and well-conditioned doesn’t need to recruit many muscle groups to move something quite light. Over-recruiting is a sign of inefficiently. More of our brain was recruited because it took more effort than it otherwise should. It’s like one of the daily Sophie’s choice conundrums for those living with ME/CFS (and now post-Covid19): do I have a basic conversation today or take a shower and that be the one thing I do this week?
Take aways: 1. Computational approaches would get us there much faster because we’re modeling, then outsourcing the biology to AI platforms (like DeepMind’s AlphaFold) where therapeutics/drugs/treatment modalities can be identified and tested. 2. In the meantime, finding a workaround for energy production is a must. We could easily fund studies on photobiomodulation starting today. That intervention may not get us all the way there, but it would likely help by presenting photons directly to the mitochondria vs the now obvious problems identified in the Krebs Cycle/CAC & electron transport chain.
Doing a search on NAD IV as just listened to a Craig Konifer MD on Andrew Huberman talking about how wonderful peptides are but then he said his greatest supplement success is in a NAD but given IV – says that orally it’s just not absorbed he also gives it sublingual but says the IV is the best. He had a patient who was in bed all the time on disability gave him an infusion and a few days later the man’s wife came in crying telling him how much better her husband felt.It’s discouraging that all of this isn’t mainstream medicine. I’m on a hunt to find an infusion but doubt will find in my medium sized town.