

The 4th in a series of blogs on the 2022 IACFS/ME International Conference finds two new metabolomic studies that highlighted three problems that have cropped up recently in other studies: fatty acid metabolism, cell membrane damage, and energy production. The first comes from Maureen Hanson’s very vigorous NIH-funded research center, and the second from longtime ME/CFS and GWI researcher, James Baraniuk.
- Systrom’s Keynote Address Kicks off IACFS/ME ME/CFS and Long-COVID Conference
- The Hugely Predictive Factor for Long COVID…is also found in ME/CFS and Fibromyalgia: the IACFS/ME Conference II
- Myoglobin, Platelets, and Monocytes, Oh My: The IACFS/ME Conference Review #III
“Urine metabolomics exposes anomalous recovery after maximal exertion in ME/CFS female patients.” Katherine Glass PhD.
Not many urine metabolomic studies have been done. Metabolomics were done before baseline and 24 hours after maximal exercise. This was a pilot study – 9 healthy controls and 10 ME/CFS patients.
No differences were found at baseline. That, however, was not surprising given the small study size and the many metabolites measured. Twenty-four hours after exercise was a different story. Thirty-three metabolites popped up.

While approximately 400 metabolites were altered by exercise 24 hours in the healthy controls, none were in the ME/CFS patients: they showed no evidence of a “healthy metabolic response” to exercise at all. (from Wikimedia Commons)
The most eye-opening chart – which, unfortunately, can’t be shown because the study has not been published – showed the difference in metabolite levels before and after exercise in healthy controls and people with ME/CFS. The healthy controls show a veritable explosion in altered metabolite levels. Even 24 hours later, the levels of over 400 metabolites had been altered. Their systems had clearly responded quite dramatically to the maximal exercise bout.
Not so with the ME/CFS group. Their metabolites flatlined. In fact, no metabolites were significantly altered 24 hours after the exercise in ME/CFS. The “healthy metabolic response” to exercise, as Dr. Glass put it, had disappeared. This seems to jive perfectly with Germain’s presentation indicating the same.
Germain found that exercise triggered a much bigger change in the proteins found in the sedentary, but healthy, controls than in the ME/CFS patients. The healthy controls responded to the rigors of the second exercise test by scrambling their protein mix more. Lacking the same ability to do so, the ME/CFS patients did not.
Note that we now have – thanks to Maureen Hanson’s use of exercise stressors throughout her studies, that ME/CFS patients’ bodies respond, at two very fundamental levels – at the level of the proteins (which do the work of the cell) and at the level of metabolites – very poorly to exercise. The exercise period itself is problematic, but it’s in the post-exercise period that symptoms really crop up in ME/CFS, and Glass’s small study appeared to capture the dysfunction there in spades.
The levels of 33 metabolites were significantly different – most of them lower – in the ME/CFS patients post-exercise. The composition of those metabolites was telling: two of the three largest groups were lipids and amino acids, with unknowns making up the other category.
The lipids were involved in fatty acid metabolism – the same problem that the recent Lipkin study centered on, the same issue that the Fisher mitochondrial study picked up on, and the same issue that a recent fibromyalgia study highlighted. That all of them were found in significantly lower concentrations than the healthy controls fits the Lipkin study perfectly.
Next, they performed a ranking analysis called MEBA that ranked which metabolites were the most different between groups. Thirty-nine percent of the metabolites were unknown. Lipids and amino acids comprised, respectively, 29% and 10% of the top metabolites that were altered.
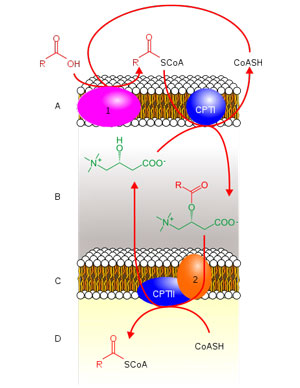
Acetyl-L-Carnitine moving Acy-CoA from the cytosol of the cell into the mitochondria.
The acyl-carnitine fatty acid metabolism pathway (six were shown) was the most altered sub-pathway affected by exercise in the ME/CFS patients. In all cases, the graphs showed dramatic increases in the metabolites associated with these pathways in the post-exercise period in the healthy controls but either no increases or slight reductions in the ME/CFS patients.
Germain’s recently published metabolomic study put a big “X” on the carnitines with its finding that “Many of the most altered metabolites contain carnitine.”. Sixty-five percent (13/20) of the lipids that were significantly altered by exercise in the ME/CFS patients contained carnitine. Plus the pathway analysis suggested that two problems with carnitines may exist “oxidation of branched-chain fatty acids” (that’s the peroxisomes) and “carnitine synthesis”.
If this work stands up – and Lipkin’s blood metabolomic study also highlighted reductions in carnitines – it may be pointing an arrow at a key feature of post-exertional malaise – the inability to produce sufficient amounts of carnitines.
Acyl-carnitines’ main function, as we learned in the Lipkin paper – is to transport long-chain fatty acids from the cytosol into the peroxisomes. Another analysis found that pathways involved in sugar metabolism – an important part of energy production – were also altered in ME/CFS.
The Hanson group is going to be digging into lipids in more detail soon and hope to expand the study greatly – to the 169 participants they have urine samples from – and to include males as well. If any study is crying out to be expanded, this one is. They also hope to use machine learning to then uncover diagnostic biomarkers at baseline.
Something Different from Dr. Baraniuk
“Dysfunctional cerebrospinal fluid metabolites and lipids infer white matter dysfunction in ME/CFS and mitochondrial dysfunction in Gulf War Illness (GWI).”
Dr. Baraniuk has been digging into both Gulf War Illness (GWI) and ME/CFS for quite some time. He’s come up with some differences. After a submaximal exercise test, for instance, people with ME/CFS showed activation of the midbrain during a cognitive test, while the people with GWI showed significantly reduced activity.
I found Dr. Baraniuk’s presentation hard to follow – hopefully, this overview is correct. It involved a metabolomic analysis of the cerebral spinal fluid before and after an exercise study. Be prepared – Baraniuk stated that metabolomic findings in the CSF can be quite different from those found in the plasma and urine – and indeed, some of them were.
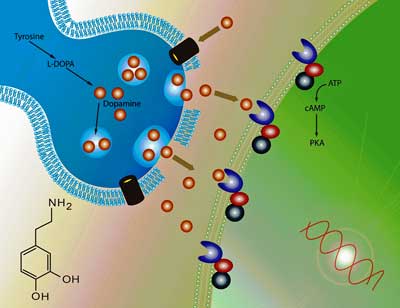
High dopamine levels in the cerebral spinal fluid led Baraniuk to suggest that “dopamine fatigue” may be present.
Dopamine Fatigue?
A ranked analysis pre-exercise that showed increases in serine, homocysteic acid, phenyalanine as well as dopamine left Dr. Baraniuk asking whether the elevated dopamine fits in with the dopamine fatigue hypothesis. The dopamine fatigue hypothesis – which was developed to explain the fatigue in multiple sclerosis – suggests that a dopamine imbalance causes a reduction in communication between the basal ganglia and the prefrontal cortex. One of the studies cited in that hypothesis was a 2006 ME/CFS study which found that methylphenidate (2 x 10mg/day) decreased fatigue.
Whither acetyl-carnitine?
The Gist
- First off was a small pilot exercise/urine metabolomic study that some really interesting results – like the ME/CFS patients were able to muster virtually no metabolic to exercise! That was shocking given . the dramatic response to exercise (the levels of approximately 400 metabolites were altered) found in the healthy controls.
- This finding jived really well with Germain’s recent study (also from the Hanson group) which found reduced levels of protein production in the ME/CFS patients as well.
- These studies suggest that the problem is not that the systems of ME/CFS patients over-respond to exercise but that they under-respond and that the healing, muscle repair, antioxidant, and whatever other systems that are supposed to respond to the stress of exercise just don’t kick in. It also shines a brigh light at what’s happening in the most mysterious period of all in ME/CFS – the post-exertional period.
- The three largest groups of altered metabolite levels were pretty familiar: lipids, amino acids, and unknown. That unknown category was the largest and the Hanson group thinks a lot of gold is buried in that group concerning ME/CFS.
- The lipids were involved in fatty acid metabolism – the same problem that the recent Lipkin study centered on, the same issue that the Fisher mitochondrial study picked up on, the same issue that a recent fibromyalgia study highlighted., and the same issue the recent Germain study found. One wonders if a core finding has finally shown up.
- The Baraniuk cerebral spinal fluid metabolomic exercise study also highlighted acetyl-carnitine but instead of low levels, he found high levels in his ME/CFS patients. Baraniuk explained that metabolomic findings in the cerebral spinal fluid can differ from those in the plasma or urine and asserted that most important thing about the finding was that it was different.
- High dopamine levels in the cerebral spinal fluid lead Baraniuk to suggest that “dopamine fatigue” may be present.
- Baraniuk also found evidence of lipid problems (altered serine, lipid levels before or after exercise), altered levels of metabolites involved in energy production, and increased levels of dopamine which he potentially linked with the “dopamine fatigue” hypothesis generated to explain the fatigue in multiple sclerosis.
- As other studies have – and as Germain’s recent study did – Baraniuk also found evidence that instead of employing more efficient energy pathways, people with ME/CFS were breaking down amino acids to produce energy.
- Finally, given his acetyl-carnitine and lipid findings, Baraniuk suggested that something had happened to cellular membranes; either they were damaged or the cells were being broken up.
- Lots of interesting strands showed up in these presentation including the long chain fatty acid/peroxisome/carnitine connection that Lipkin found showed up in spades as well as problems with energy metabolism. Time will tell how this all works out but it certainly seems that, right now, things are cohering rather nicely.
Post-exercise – which involved a smaller group – increased serine, homocysteic acid, and phenylalanine again, showed up again and, this time, some lipids were decreased in ME/CFS.
A Metaboanalyst analysis that again plucked out serine suggested that exercise had caused reductions in phosphatidylglycerols (a small component of membranes) and glutathione in ME/CFS. A linear model that pooled all the analytes found in the various tests together found significantly altered levels of 26 metabolites. Again, Baraniuk found higher acetyl-carnitine levels.
In conclusion, in the pre-exercise period, serine, which is important for lipid metabolism and mitochondrial functioning, was highlighted, as were several energy-related metabolites (creatine) and several lipids, most prominently acetyl-carnitine. Exercise resulted in drops in phosphatidylglycerols (fats) and reduced glutathione indicating oxidant injury.
Mentioning the Warburg effect, Baraniuk stated that the results from his and other studies “pretty conclusively” show that people with ME/CFS are metabolizing incorrectly; i.e. they are bypassing the regular glucose pathways and finding other ways to produce energy. Germain’s recent study also mentioned the Warburg effect and we can now add it to the list of studies that have found a strange and more inefficient pattern of energy production in ME/CFS. This finding seems ever more solid as time moves on.
Baraniuk was asked if the elevated acetyl-carnitine levels indicated problems with beta-oxidation. Noting that carnitines carry long-chain fatty acids from the cytosol into the peroxisomes, Baraniuk stated that anything that disrupts that system will interrupt the metabolism of the fats.
While other studies have found reduced acetyl-carnitines in the urine and plasma, and his study found it increased in the CSF, Baraniuk stated that the important fact was that acetyl-carnitine levels were significantly different from the controls.
The fats, he stated, are supposed to stay in the cell in the membranes and if they are higher in the cerebral spinal fluid – you have to ask why. Has something gone wrong with the membranes, are the cells being chopped up or what?
Germain’s recent metabolomics study from Maureen Hanson’s group – which is really knocking it out of the park right now – The effects of exertion in the ME/CFS cohort predominantly highlighted lipid-related as well as energy-related pathways and chemical structure clusters, which were disparately affected by the first and second exercise sessions.
Conclusion
With two more metabolomic exercise studies pointing fingers at problems with energy production, the lipids (cellular membranes), and, in particular, fatty acid metabolism, one wonders if we’re getting closer to some core problem in ME/CFS.
With another study suggesting that ME/CFS patients’ systems essentially flatline after exercise and are unable to produce a “healthy metabolic response” to it, we also may be getting a handle on what’s causing post-exertional malaise (PEM). It’s not that the system is overreacting to exercise – it’s quite the opposite – it’s simply not responding – which suggests that the muscle repair and antioxidants and whatever other systems that should be ramping up to ameliorate and heal are on vacation in ME/CFS.
The lipids – the fats found in the cellular membranes – are also showing up big time. Problems with the cellular membranes could be rendering cells unresponsive, leaving the mitochondria unable to produce normal amounts of energy. On that note, it’s good to see that the Hanson group plans to be digging deep into the lipids in ME/CFS in the future. Since these are potentially very basic problems that could be affecting many cells, they could be affecting many systems in ME/CFS.

Could some pieces be fitting together? Time will tell…
These are pretty new themes and time will tell if fatty acid metabolism really is a core problem, if the peroxisomes represent some sort of ground zero for ME/CFS, or if cell membrane issues play a large role in ME/CFS.
One of the more interesting findings from the Hanson metabolomic studies is how many unknown metabolites showed up in ME/CFS. Germain et. al. noted that determining the identity of those metabolites will have “far-reaching consequences in our ability to decode ME/CFS“. So long as these big studies keep coming, surprises are surely in store.
Lastly, let’s hope the RECOVER crowd is keeping an eye on this intriguing ME/CFS research as few metabolomic studies have been done in long COVID thus far.
- Coming up – an interview with Suzanne Vernon on the central role that peroxisomal dysfunction (fatty acid metabolism) could be playing in ME/CFS.






Great summary Cort. Agreed that this is very important work.
Thanks again Cort 🙂 I had a very mild fatty acid oxidation disorder that suddenly became severe when I got ME/CFS. I was originally diagnosed with late-onset mitochondrial disease. But it was weird in a fatty acid oxidation pathway. Very unusual. I had to go on a low-fat diet and take a lot of acetyl-l-carnitine and riboflavin to keep from having severe headaches and muscle cramps. Right after I got ME, I also developed sets of matching lipomas down my spine which disappeared later with the FOD treatment. They are really common in FOD’s. So now it looks like ME is an ACQUIRED mitochondrial disease and fatty acid oxidation disorder. Crazy. I always wonder how many adult-onset mitochondrial disease patients are misdiagnosed (sort of) and actually have ME/CFS, like me.
I like the idea of ME patients under-responding to exercise. It feels like a real breakthrough. Thank you for the explanation and summary.
How did you get diagnosed with the mitochondrial disease?
With a genetic test for mitochondrial and related metabolic diseases.
Chris Bilton, which type is this called the Mitochondria Disease Fatty Acid oxidation disorder?
Is this Kearns-Sayer Syndrome (KSS)?
I’m heterozygous for LCHAD and HADH deficiencies, both in the fatty acid oxidation pathways. HADH also causes hypoglycemia when you eat too much protein at once. I had a little trouble with hypoglycemia and muscle cramping before ME. But after ME it was suddenly everyday until I learned about the treatments I mentioned.
I have CPT2 deficiency so I cannot metabolize fat. Addressing this issue did nothing to change my pain or fatigue. Moreover I am in a Facebook group with others who have CPT2 deficiency and most do not have ME/CFS or any of the associated conditions such as pots or MCAS.🤷🏻♀️
What is CPT2 Deficiency?
What doses am/pm of Acetyl L Carnitine daily & B2 amounts? I looked up LCHAD on Invitae I could not find this type on there?
I found HADH. FOD treatment do you mean Fatty Oxidation Disease diets? thanks, Christyne xx
Thank you for the summary. Reading this series of reports, I’m finally starting to feel like there is hope around the corner, if not for my age bracket, for the younger sufferes. Woo-hoo!
So should we take more
L-carnitine? I’ve been taking 1.25mg, but have just increased it to 2.5mg. (on an empty stomach, first thing).
This stuff is so complex and there are so many things going on in ME/CFS that I don’t know. I would imagine its worth a try though and I’m going to try it.
It gives me insomnia even at a tiny dose. Could that be because it’s already high in spinal fluid,
I’m wondering whether the reason high carnitine was found in the spinal fluid (and so far nowhere else) is because the lymph is attempting to move it out with waste such as toxins? It fits with Perrin’s theory that there’s a toxic build up in the spine, though if that’s true I don’t know why the body would be getting rid of it?
Maybe taking it earlier in the day try between meals or with meals. I wonder if all the B Vitamins Dr. Dmitry Kats is saying to use them is why it helps the Riboflavin Deficiency B2
If they can identify some of the metabolites…..does that mean they will have a diagnostic test for ME/CFS?
That was a stated goal! Let’s hope 🙂
Cort, so often your blogs bring so much more than is easily found online to light, and gives us the most up to date info.
Alain Moreau in Quebec has a similar goal re: diagnostic test using (metabolomics/metabolites?–but with blood samples.
Hoping his work will be the subject of a blog at some time.
An encouraging report and a good synopsis – TY Cort. The findings here resonate with me personally, as I have always had a sluggish metabolism (lower than average BP, heart rate and temps). After developing symptoms of CFS and Fibro at age 30, I also tested my hormonal (Cortisol and ACTH) reactions to exercise, which stayed the same or decreased (instead of increasing as expected). In addition, hormonal supplementation trials (with various forms of thyroid, both with and without cortisol supplementation) did not increase my BP, heart rate, temp or energy levels (metabolism). At very high (but slowly ramped) supplementation levels, they simply made me feel anxious or “wired”, with no benefits. Lastly, some in-depth testing showed my amino acid levels to be low, which perplexed me as I have always had a high protein diet. Perhaps my amino acids are low if they are being used as fuel (as suggested herein). The findings and hypotheses of this report provide potential explanations for all of these abnormalities – the cause(s) of which were not understood when the tests were done. I hope this research gets attention and follow-up as it seems very promising to me.
Dear Cort, I was confused about the first part of the article – it says “In fact, no metabolites were significantly altered 24 hours after the exercise in ME/CFS”, but then says 33 metabolites were significantly different. Were you talking about different studies here? Thanks!
My recollection is that no metabolites were significantly different between the first exercise bout and the second exercise bout in the ME/CFS group but 33 metabolites were different between the ME/CFS group and the healthy controls. I hope I got that right 🙂
Thank you, Cort!
I have often wondered why adrenaline can make temporarily much more functional, but result in vicious crashes later. I’ve wondered if (& if so, how) adrenaline mobilises a kind of extra emergency energy, maybe switches to (or amps up) a different energy metabolism pathway – one that somehow “digs into the substance” of the body.
I wish ME/CFS researchers would look into this too, as I’ve seen the “adrenaline effect” reported by various patients. It could provide additional clues about energy metabolism in ME/CFS patients’ bodies and PEM. There seems to be previous research on adrenaline and energy, for example this 2016 paper https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4831313/#:~:text=Adrenaline%20is%20now%20increasingly%20recognized,or%20for%20recovery%20from%20hypoglycaemia. mentioning that “Adrenaline is a hormone that has profound actions on the cardiovascular system and (…) is now increasingly recognized as an important metabolic hormone that helps mobilize energy stores in the form of glucose and free fatty acids in preparation for physical activity or for recovery from hypoglycaemia.”
Is cortisol the factor you’re looking for? This steroid, part of the stress hormonal response, has a number of implications in this physiology (inflammation, sugar and fat metabolism, BP, circadian rhythm, many of our more notorious issues have cortisol in common) , and has been the subject of many recent inquiries.
But then the adrenaline can do so only for a limited time (which can even be for a couple of days), suggesting that maybe by then some reserve has been used up?
Hi – can you recall if the urine samples were spot or 24-hour collection?
Have they been standardized to creatinine?
This would make results look different from what they are – whatever that means – i.e. falsely lower of higher, because what has changed really is the creatinine secretion, which is what is used to standardize urine samples.
Whomever came up with this system believed creatinine secretion is constant – it’s not.
It’s involved in energy metabolism, so anytime this goes wonk… (I.e. this would include for example diabetes and hypothyroidism)
Hansen and her team mentioned something on one of their papedz on blood tests results coming up screwy, and them using a different process to establish values.
The Australian team also wrote something to the same effect, they used relative vs absolute values. That was blood. I can’t recall urine (they had tested both).
Very interesting. I have MCAS & neuropathy symptoms, my daughter probably has ME. We are both carriers for MCAD deficiency. My father probably was, also. He had multiple system atrophy and towards the end of his illness he suffered a bout of rhabdomyolysis which his doctors attributed to MSA – but AFAIK no case of rhabdo due to (or occurring with) MSA has been reported in the literature. My daughter suffered a bout of neuroleptic malignant syndrome from Reglan. I requested a blood acylcarnitine profile when she was hospitalized and it was in normal range. I get bouts of tremor if I eat coconut oil; I have not had my acylcarnitine profile checked.. Seems like something is up with dopamine & fatty acid metabolism in my family.
Re: cell membranes being chopped up. I Exercise cause releases of extracellular vesicles / exosomes – could there be a problem with this process? Perhaps the vesicles are forming improperly and breaking up.
I found this review on how fatty acid oxidation disorders cause neurological harm interesting:
Amaral et al. (2017) ‘Mechanistic Bases of Neurotoxicity Provoked by Fatty Acids Accumulating in MCAD and LCHAD Deficiencies’
https://journals.sagepub.com/doi/10.1177/2326409817701472
I wonder what they would find in these base line tests of ME/CFS people who are severe and unable to do any form of exercise.
The finding that people with ME/CFS under-respond to exercise made me think of Naviaux’s hypothesis, suggesting there’s a dauer / hibernative state going on in ME/CFS. Cort, do you know anything about the Naviaux trial of suramin that was due to take place this year at all? Just wondering what might’ve been found.
Because all ME/CFS participants in these studies won’t have changed their diets collectively; so many unknown metabolites might mean we are eating our own (muscle) tissue for the energy needed for CPET and that’s what might cause PEM?
Thanks for the info Christyne Bliton xx
“Creatinine has been used to standardize urinary metabolite concentrations to account for variations in urinary accumulation and dilution factors. In this instance we deemed it an inappropriate method as creatinine was trending towards a significant decrease within the absolute concentration blood data of ME/CFS patients. In this study when the urinary data was normalized to urinary creatinine all metabolites were significantly altered, which we concluded was an artificial result. To remove the influence of dilution factor on both the urine and the blood we normalized each sample to the total metabolite concentration, therefore producing relative abundance data. This method of normalization focuses analysis on the ratio of metabolites within either the blood or urine.” *
Is Hansen and her team doing this?
IF NOT, you can see how their findings could be wrong, and subsequent interpretations too.
In fact – is everyone involved in ME/CFS research, and long covid at that, doing this too?
You can see how this can make results messy, and possibly incongrous between teams. With interpretations following.
There are quite a few pitfalls like this when using blood and urine to analyze quantities of substances…
* From a 2015 study on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome [ME/CFS]
”Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients”:
https://www.researchgate.net/publication/277979239_Metabolic_profiling_reveals_anomalous_energy_metabolism_and_oxidative_stress_pathways_in_chronic_fatigue_syndrome_patients
– “Dysfunctional energy metabolism appears to have impacted creatinine and its elevation in urine…”
I don’t cease to be baffled at how this remark on this paper has gone under the radar. It should spark discussion amongst all the teams involved and coordination. Say, the Open Medicine Foundation. Unless they know it too and justbdon’t make it public.
Hansen mentions on a more recent paper how they adjusted blood values.
I haven’t read anyone else do the same.
I question metabolomics when this is not taken into account. And also, when blood volume changes – how does this affect test results too.
That is why Genetics are the diagnostic tests, not biopsy urine or blood sample testing its Genetics that confirms the diagnosis. Everyone is interested in Patents making money
I have noticed they keep lots of secrets, they even said at one point they would release the Family data on DNA testing I never saw this.
ME/CFS patients, we need full genome & metabolic & mitochondria DNA tests I have said this for the longest time & we all also need (HATS) copies of genetic tryptase done mouth swab DNA
test Hereditary Alpha Tryptasemia genetic test done here $169.00
http://www.genebygene.com
This is not a test of the effects of exercise, but of the effects of an exercise test designed to push people to max effort.. Therefore results are meaningless in the real eorld.
ME/CFS sufferers are, as we know, “exercise intolerant”. This is because they are aerobically deficient and even the slightest effort pushes them to anaerobic system, lactate production and associated hydrogen ion acidosis.
A pointless study.
The question is whether a strenuous exercise test can reflect the mild exertion that causes PEM in people. I imagine we will get there at some point but its very important that our researchers have validated test points and the maximal exercise test will do that.
You might note, Simon, that the same type of exercise test was used to demonstrate the aerobic energy production problems that you apparently do accept. I’m afraid you have to either accept them both or neither.
I think the main effect of this study is to broaden the impact that exercise has on people with ME/CFS – even the immune cells are not responding. As Herbert noted we now have to look for some way to explain similar findings found across multiple compartments. Is a system-wide problem with mitochondrial functioning present? Or as Herbert suggested – perhaps it’s something in the brain? I don’t know but this finding expands the problem.
I might also note that it also shows how the immune system could be producing or exacerbating PEM in the after exercise period.