

Andreas Goebel Ph.D. has been on the hunt for an autoimmune cause of chronic pain since he observed high levels of post-infectious antibodies in his patients.
It’s like a sapling putting out roots. Will it grow into a big tree or will it die on the vine? However, it turns out, Andreas Goebel Ph.D. has certainly planted some fascinating and possibly game-changing ideas about how fibromyalgia, ME/CFS, and long COVID occurs.
This blog focuses on a poster presented at the World Congress on Pain that recently took place in Toronto. (Thanks to Herbert for the tip :)).
Andreas Goebel has been on the hunt for a new way to explain pain ever since he found that his patients at the pain clinic at Würzburg University typically had low-level increases in post-infectious antibodies. (Hmm). He also knew that the current pain paradigms couldn’t explain a large group of chronic pain sufferers. How large? Potentially billions of people, large:
“In other words, symptoms that profoundly affect billions of people across the globe have remained unexplained. New approaches to their understanding are needed.” Goebel et. Al. 2022
A Different Kind of Pain
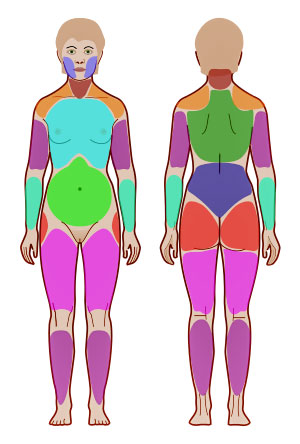
Fibromyalgia is considered a classic nociplastic disease. (Widespread pain index – JMarch Wikimedia Commons).
Chronic pain has long been believed to be the result of either tissue damage/inflammation (nociceptive pain) or damage to the nerves (neuropathic pain). In diseases like fibromyalgia, however, no evidence of tissue damage or widespread inflammation has been found, and the small fiber neuropathy damage thus far found can’t explain the disease.
Recently a new term, ‘nociplastic pain’, was developed to define people in chronic pain who have “structurally intact, but abnormally functioning” pain networks. Fibromyalgia is considered a classic example of this kind of disease. While this new characterization helped, it couldn’t explain how these “abnormally functioning” pain networks came about.
Enter Andreas Goebel – a German pain researcher working at the University of Liverpool. The Director of the Pain Research Institute in London, Goebel has been focused on understanding the role the adaptive immune system plays in causing severe ‘unexplained’ chronic pain, for the past 15 years.
A Different Kind of Autoimmunity
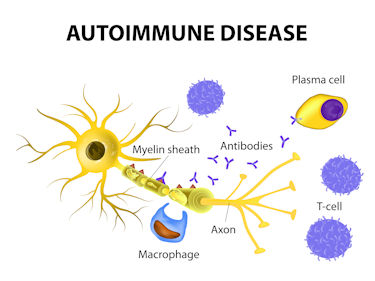
Goebel proposes fibromyalgia, ME/CFS, and long-COVID are autoimmune diseases that are characterized by localized (not systemic) inflammation.
In March of this year, Goebel and colleagues penned a review, “The autoimmune aetiology of unexplained chronic pain“, that explained that they’d uncovered “pain sensitizing autoantibodies” in no less than four chronic pain conditions (complex regional pain syndrome, fibromyalgia, chronic post-traumatic limb pain, and rheumatoid arthritis). Interestingly, the autoantibodies bound themselves to different sites in the 4 diseases – indicating that each appeared to be a different type of autoimmune disease. Goebel et al. stated that they showed that “functional, non-destructive biological processes can cause exquisite pain which severely affects daily living.”¹
Seven years ago Littlejohn proposed that CRPS, fibromyalgia, IBS, and migraine are all neuroinflammatory diseases driven by inflammation of the sensory and central nervous system nerves.
With fibromyalgia, Goebel injected purified IgG from people with FM and healthy control subjects into mice and then watched. Within two days, the mice given the IgG from the FM patients had become hypersensitive to pressure, cold, and pain, and reduced their grip strength while the mice given the IgG from the healthy controls remained fine. Goebel had apparently swiftly given mice fibromyalgia simply by giving them immune factors found in his FM patients’ blood.
Further digging indicated that the pain receptors on the nerves outside the spinal cord in the dorsal root ganglia had become hyperactivated in the FM mice. The dorsal root ganglia are like a way station that sensory and autonomic signals from the body pass through in order to enter the spinal cord and reach the central nervous system.

IgG antibodies produced a fibromyalgia-like state in mice.
Fibromyalgia researchers have mapped out all sorts of central nervous system abnormalities, but it’s never been clear if they were the result of central nervous system problems or if they were simply the result of central nervous system overload from an unrelenting barrage of signals that were hitting it.
Goebel’s findings suggest the second: the IgG from the FM patients never made it to the spinal cord or the brain; instead, it mostly accumulated in the glial cells surrounding the dorsal root ganglia. While the glial cells just outside the spinal cord became hyperactive, the activity of the glial cells inside the spinal cord remained at normal levels.
That meant that after all the focus on the central nervous system in FM, Goebel was able to reproduce a fibromyalgia-like condition in mice without ever directly impacting the central nervous system at all. Nor did evidence of systemic inflammation crop up. Whatever the antibodies were doing, they were doing it very locally.
Goebel’s findings are potentially so exciting because they could explain one of the great mysteries in FM and ME/CFS – why we don’t see enormous spikes of inflammation. Goebel’s findings suggest that small amounts of inflammation are probably occurring very close to nerves. (Van Elzakker, in his Vagus Nerve Hypothesis, proposed something similar for ME/CFS.)
Well aware of the implications of their findings, the authors wrote their results may “transform future research and facilitate development of mechanism-based therapeutic interventions (in fibromyalgia)”.
A Different Look at Long COVID
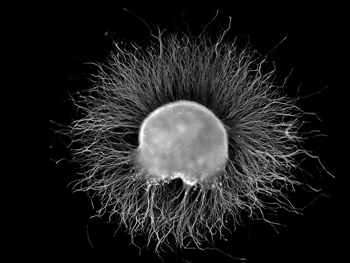
IgG antibodies attacked the glial cells surrounding the dorsal root ganglia (pictured – Image from Wikimedia- Ganglia Category).
Recently, Goebel reached outside of the chronic pain arena when he did a different experiment on long-COVID patients. He and his team took purified IgG antibodies from-long COVID patients experiencing widespread pain, from people who’d recovered from COVID-19, and from an FM patient, and then planted them in cultures of glial cells from the dorsal root ganglia of mice.
They then did an inflorescence study to see if the antibodies from the patients or healthy controls were binding to or attacking those glial cells – thus activating them and causing them to begin spewing out pro-inflammatory factors that were tweaking the nerves and causing increased pain.
The Gist
- About ten years ago, Andreas Goebel saw that some of his chronic pain patients had high levels of post-infectious antibodie,s and began searching for an autoimmune cause of it.
- Since then, he’s shown that putting IgG antibodies from people with fibromyalgia, rheumatoid arthritis, chronic regional pain syndrome, or post-traumatic limb pain into mice appeared to cause the mice to come down with similar conditions. Interestingly, the IgG antibodies appeared to attack different parts of the body in each condition.
- Goebel found that the antibodies from the fibromyalgia patients had gathered in the glial cells covering the dorsal root ganglia (DRG). The DRG are the last way station for the sensory signals before they enter the spinal cord. The glial cells are immune cells that, when activated, spew out proinflammatory cytokines that could cause the DRG to become hypersensitized to pain signals.
- Goebel believes that the reason that systemic inflammation is not found in FM or ME/CFS is because the autoimmune attack is very localized and is focused on these nerve bodies found just outside the spinal cord. (Herpesviruses are known to infect these nerve bodies.)
- Goebel has said he plans to test ME/CFS and included ME/CFS in a list of autoimmune chronic pain conditions but moved forward first with long-COVID patients who were experiencing significant pain.
- Using a culture experiment, Goebel found that the IgG from the long-COVID patients attacked the glial cells covering the dorsal root ganglia – suggesting that the same process occurring in fibromyalgia is occurring in long COVID.
- Goebel proposed that long COVID is a subtype of ME/CFS and fibromyalgia.
- He also noted that, at least in mice, the condition is reversible if one can mop up the autoantibodies. Plasmapheresis, the BC007 aptamer, or a drug that blocks the autoantibodies in question could do that.
- Much more work clearly needs to be done, but if Goebel is right, he would clear up several problems in these diseases – why not a lot of inflammation or evidence of trauma or injury is found.
These findings were clearly preliminary, and more work needs to be done, but they suggest – as some other studies have – that an abnormal antibody (e.g. autoimmune) response is present in long COVID and that it may be an autoimmune disease. Importantly, fibromyalgia – which, for some reason, has been almost totally outside of the long-COVID discussion – now becomes the third leg of a possible ME/CFS/FM/Long-COVID triad.
Reversible Illness?
The fact that the mice returned to normal when the FM IgG levels declined suggested that the illness is not permanent and could be reversed by removing the autoantibodies. Goebel suggested that IgG-reducing therapies such as plasmapheresis or immunoadsorption could be helpful. Of course, some success has been found in small studies with immunoadsorption in ME/CFS, and the BC 007 aptamer is another candidate. Approaches that block the autoreactive IgGs could be effective as well.
Lastly – in my favorite part of the poster – they suggested – rightly in my opinion – that long COVID is not a separate condition but is a subtype of FM and chronic fatigue syndrome (ME/CFS).
“Our results lend credence to the hypothesis that long-COVID is an infection-triggered subtype of FMS and chronic fatigue syndrome.”
And there you have it – a possible infection-triggered autoimmune response that sends the glial cells that are wrapped around the main sensory processing neurons at the spinal cord into a tizzy.
Time will tell if Goebel’s sapling grows into a mighty tree and redefines how we understand fibromyalgia, long COVID, ME/CFS, and others. His autoimmune hypothesis seems to me, at least, like just the thing the NIH RECOVER Initiative would be interested in. Let’s hope they can give it some water and fertilizer and see if it can grow.







This sounds so promising. I hope many staff at the NIH are reading your blog.
If it is autoimmune, by the time the slugs at the FDA approve a treatment, we’ll be dead.
Wow. Terrific news (and very well-done presentation, Cort. Thanks.) After 23 years of illness, I tend to take a “wait and see” approach to new findings. Including this one. But this one checks so many of my boxes, that I’m excited about the possibilities. Your conclusion is terrific: Let’s hope that the NIH sees the potential here, and gives it the food and water to help it grow. Come on!
Let’s hope. Instead of Goebels digging away at this himself for the next five or ten years I hope the NIH and other groups jump on it so that we can tell fairly quickly whether he’s right or not.
Cort, You are truly our injection of hope every week. THank you so much for this article. Now, how do we gather together to influence the ginormous but normally unhelpful medical community so that they will help in this much needed process of alleviating crippling pain?
That is such exciting news. I’ve also been reading many medical papers that are connecting long Covid to ME/CFS. I never thought I’d see the day and unfortunately we had to go through a pandemic for the health care system to start connecting the dots. They’re too slow though, I’m getting old and would love to experience life as it should be.
This is so interesting. I first strongly suspected that FM was an autoimmune disorder from a massive flareup in response to an AstraZeneca vaccination on 29 January 2021.
This suspicion was confirmed in July, when the results were published of the Karolinska Institutes’s study injecting IgG antibodies from FM patients into mice.
So was it the adenovirus base, or the engineered spike proteins — or both — that provoked a severe FM reaction? And I had virtually no reaction to a second AZ shot three months later.
This is fantastic news! I’ve had FM & ME/CFS for many years & my daughter was dx with CRPS (complex regional pain syndrome) about 10 years ago when she was 9. Knowing there are identified similarities & hopefully treatments for both almost makes me cry. Thank you for this!!!
Interesting – both are usually initiated by an infection. In CRPS’s case, if I have it right, it’s initiated by an infection plus an injury of some sort. Both also feature autonomic nervous system problems and similar parts of the brain are affected. Seven years ago Littlejohn proposed that CRPS and FM exist on opposite ends of the same neuroinflammatory spectrum. He also proposed that migraine, IBS, and asthma are part of that spectrum. All feature, he believes, inflammation of the nerves.
https://www.healthrising.org/blog/2015/08/17/fibromyalgia-and-complex-regional-pain-syndrome-the-neurogenic-inflammation-connection/
I wonder how this relates to the work of Bruce Patterson (the Stanford guy who formed his own company to test and treat specific autoantibodies).
I also wonder if Bruce Patterson’s papers have/are coming out. Over the summer, he was everywhere promoting his testing/treatments, and referring to papers coming out soon.
I’ll try Googling him.
I’ve been suffering from long Covid for a year now and discovered Dr Patterson back in June. He put me on hiv meds. Definitely improved some after but still not back to normal yet. Both of these Drs seem to be on the right track.
Thanks for reporting! Gives me hope! I’m a 60 years guy and can trace my fibromyalgia as far back as I can remember.
I’m interested in seeing if specifically infected ganglia affect specific parts of the body I.e. ganglia that connect from the spine outward to the lungs affect breathing, and ganglia that branch to the gut results in gut issues. I sure hope this line of study goes somewhere!
150 million people and one drug would help, no one investing? Joke…
No money to be made off the healthy!
ooo yes, that’s interesting. Makes sense.
Would Lyrica help people with FM and what would be the side effects?
I have been with FM & CFS since 1979.
I started Gabapentin 3 weeks ago for my Fibromyelgia cause it had helped both my daughters with Fibromyelgia. I have since stopped my overnight Long acting Tramadol and my Noratryptline. I now take 200mgs Gabapetin 3 times a day and only one dose of Panadol 2 and Tramadol 50mg in morning. Still early days as I also have Osteo Arthritis and Sciatica in Right Leg.
Many drugs specifically for FM find FDA approval for showing a marked difference in the reported pain of 1/3 of the patients in studies. So you see it isn’t helping the majority and of those feeling being helped, it’s not a lot. I do know we FM’ers would take any help, but side effects are real. I was on for less than a month and I found it hard to retrieve correct words when speaking, my keyboard typing was dyslexic upon dyslexic. Short term memory difficulties and felt high all day. The weight gain was close to 20# and though it helped me sleep and helped my co morbid back pain it wasn’t worth it to have my mind altered to the extent it was. Many couldn’t read and retain so that was lost, we were made more drowsy in the daytime and concentrating was made close to nil to even balance a checkbook. For many gambapentin stopped working after a while while being thrust into zombie land. I was pushed to 1800/day and stopped the upper titrations when my house filled with things to do but no energy to see through. Not everyone or at least the majority of FM folk get relief from those meds out there proven to help by FDA standards. Cymbalta did help me for 3 years than started to wane over the next two. I and many will try all of the meds we can, but end up with a huge weight gain and that nothing works. The best things so far out there are learning to pace and eliminate many of the things we do with our energy. Getting better rest even adapting to a rest/nap for 20-25 min/daily can help. Not at all what a working and or parent can easily do I might add.
Incredible, thank you Cort!
rusticwoodpecker@gmail.com
Wow!!
and what about schebenbogens her auto antiboddys for ME?
That GPCR autoantibodies which are part of IgG autoantibodies
This is super exciting news! It engenders a feeling of hope I’ve not felt about ME-CFS in years.
Thank you Cort, I really think we are getting nearer to the answers with this. I wish that there would be more studies into this area. It certainly does ring bells with me as pain is such a big part of my illness. I am praying that I am not too old now to see some light at the end of the tunnel.
The issue with Immunadsorption and Plasmapherese is that the autoantibodies are getting reproduced in most patients. BC007 was somehow able to neutralize the autoantibodies for a longer periode.
Se sabe algo nuevo sobre la investigación del BC007? Sigue adelante?
Gracais
si, vivo en Alemania y de momento no va a llevarse a cabo. El Gobierno simplemente boicotea que se apruebe este medicamento. Berlin Cures no obtiene respuestas hace meses del ministro de Sanidad.
And I bet the cost is prohibitive. I doubt insurance would ever pay for it and I doubt any clinics offer a cash option. Please let me know if you know of any
Timo do you have more information on the immunoabsorbtion autoantibodies being reproduced? I have been thinking of doing this although it’s very expense
I find it interesting that it did not happen with igG from people who had pain-free long-Covid. I suggest that the process of igG hypersensitising neurons in the dorsal root ganglia is a part of the process of central sensitisation and is initiated by the peripheral drivers of widespread trigger points. Hypersensitising the dorsal root ganglia would increase levels of substance P which gives weight to my theory that widespread trigger points are present in fm and that central sensitisation is just a wake-up call to the conscious brain to do something about the trigger points. This also explains why the brain filters are faulty and letting through too many nerve impulses.
Tricia, I think you misunderstood the methods. They compare patients with painful Long Covid to painfree patients fully recovered from COVID:
See their poster here:
https://www.researchgate.net/publication/363660932_Patients_with_Painful_Long-COVID_Syndrome_Produce_Immunoglobulin_G_Autoantibodies_which_Stain_Dorsal_Root_Ganglion_Satellite_Glial_Cells_Similarly_to_Patients_with_Fibromyalgia_Syndrome
I obviously misinterpreted Cort’s sentence. However, pain free recovered or not recovered does not matter (and I suspect there are long Covid people for whom pain is not the main problem) as that is not the main point I was trying to convey. Most people have latent trigger points of some sort and viruses activate trigger points causing pain. If the body overcomes the virus, the trigger points deactivate and the pain recedes but if the body cannot fully overcome the virus, the pain will continue. If the pain is present long enough, the process of central sensitisation will commence. I am suggesting that the igG activating the satellite glial cells of the dorsal root glanglia is not the cause of pain, rather that this is happening because of pain and is the starting point of central sensitisation. Inflaming the glia which are densely packed around the sensory nerves, would cause the sensory nerves to produce substance P which heads to the brain through the cerebrospinal fluid to continue the process of central sensitisation. Treating the trigger points will help to ease the situation. We have an opioid crisis on our hands because trigger points are not recognised and treated.
Is BC700 available on the market noe?
Of not anf if pactitioners findes pharesis a too big try, what medicine/precedure could remove antobodies? Assume we are not back to rituximan, but another kind of treatment -does anyone know what mlght work? What can remlve antiautobodieS?
Thanks Cort!
will never be available, you can wait 30 years. They just do not want to give us BC007. We need to start taking legal actions against the people who are responsable we dont get treatments.
My understanding is that the BC007 long COVID trial is moving forward but that one cause for the delay is that the company has never done a trial like this and it’s taking some time for them to put it on.
I wondered the same and researched a little earlier – the form of LDN Jarred Younger was impressed by a while ago called dextro-naltrexone came up. Apparently it inhibits TLR4 which activate the microglia. I’m in no way an expert so may not fully understand but it sounded interesting! And reservatrol and curcumin came up as possible natural alternatives. Would be interested to know if anyone has experience with dextro-naltrexone?
TLR4=endotoxin
I haven’t tried the dextro-naltrexone, just the LDN. It helped a little, but the nightmares were too much for me. But I think Younger started the whole microglia cell role in Fibro. Will have to look further into the dextro-naltrexone. But again, this would probably only provide temporary relief, not a cure. If we can address the actual autoantibodies then we have potential for cure.
The researchers behind the rituximab study, is about to get started with a new one. They will use Daratumumab (Darzalex) this time, which targets plasma cells. They still believe in the autoimmunity hypothesis, at least for a subgroup of patients.
Good to hear! Thanks for relaying that. 🙂
The working hypothesis is that ME is a variant of an autoimmune disease, an autoantibody mediated respons that disturbs the the blood flow. Which leads to hypoxia etc. And that rituximab failed because it does not affect the mature long-lived plasma cells at all. Good news is they think it is totally reversible, and found no tissue damage or inflammation in deceased patients. Well,time will tell I guess!
Thanks Cort for sharing this great update. I’m very grateful that Andreas Goebels is focussing his attention on the FM/ME/LC pain areas. It – and your reporting – gives me great hope. I wonder how these autoantibodies findings link in with the microclots findings in LC and ME (and possibly FM too). Are they a separate phenomenon or all part of the same issue? And the issue as with all autoantibody mediated illness, as Timo and others note, is how to remove the antibodies without them coming back quickly or causing too much harm. I wonder if there are any LC patients with pain on rituximab etc
The most interesting part of this report was this.
Goebel believes that the reason that systemic inflammation is not found in FM or ME/CFS is because the autoimmune attack is very localized and is focused on these nerve bodies found just outside the spinal cord. (Herpesviruses are known to infect these nerve bodies.)
Before his death, I asked Dr. Paul Cheney if he thought HHV6A was the cause of CFS. He agreed with me that this was the most likely candidate.
If this is true, treatment has to center around ways to prevent HHV6A from wreaking havoc on the body when it reactivates. You are never going to get rid of it since, like all herpes family viruses, it lives in your cells forever.
I have even wondered if Long Covid was causing a reactivation of HHV6A because the symptoms are so similar to ME/CFS.
HHV6 A & B were discovered in the Gallo Aids lab in 1986. First named HBLV for the viruses’ ability to kill B cells, the name was changed to HHV6 when they discovered it could also kill T cells. Then it was subdivided into HHV6 B, a common cause of childhood roseola, and the more dangerous HHV6A which can only be reliably tested for at a specialty lab. Why in 36 years has a reliable and easily accessible test for HHV6A not been developed? How many of you have been tested for HHV6A and I don’t mean the Quest test which doesn’t separate A & B?
Fibromyalgia is NOT autoimmune. Check out the guaifenesin protocol
I’m fortunate to have a primary care doc that’s interested in me/cfs and hhv6. However he didn’t test me beyond the quest lab.
Where do we get the hhvv a/b lab?
My 10 year experience of severe , bed ridden ME involves no pain other than regular migraine and occasional muscle spasms.
The Criteria to meet ME/CFS, offer pain as an option, not a must have. So I’m always uncomfortable when I see research focusing on pain. Can anyone enlighten me please.
I have the classic PEM, POTS/OI, dizziness, heaviness, weakness, brain fog.
So sorry, Jane, for al the limits this illness makes you endure. Your post puts its finger on something I have been grappling with–what is PEM? To me, it IS pain. But it is a unique kind of pain, a sickening feeling, as if I have ben poisoned. (Or, as I tell my docs, it’s as if each of my muscles has its own stomach and they are all nauseous.) I also feel weakness, but I tell my neuro that weakness IS pain. Weakness, for me, feels painful.
Thanks fo.r the comment. PEM for me is an exacerbation of all my symptoms. Plus my legs are so numb they can sarcely hold me up and I suffer extreme air hunger….as if I am suffocating and I can do nothing other than lie down for many hours longing for it to subside. I put it down to fatigue of my chest muscles and it’s by far my worst symptom.
Great explanation! I used to say I felt hungover and severely sleep deprived despite no alcohol and getting 8 hours of sleep. It’s so odd isn’t it. It’s not pain, or localized discomfort, i just feel like garbage.
It needs to be kept in mind what the Goebel team did and what they did not do 😉 They show that the *pain* experienced with LongCovid may be caused by autoimmune reactivity within the dorsal root ganglia. No more, no less.
They neither showed that Long Covid doesn´t affect the brain nor that Long Covid is only about autoimmunity. The pathobiology of Long Covid and ME/CFS has many interwoven layers, and while the pain experienced by some (not all) patients may be the result of specific autoimmunity within the DRGs (i.e. outside the CNS), other clinical features (like fatigue, or PEM, or sensory hypersensitivity) may be based on entirely different processes in different locations. Could glial reactivity within the CNS explain fatigue, PEM, etc.? Of course (we made a case for this in our paper). Could the glial activation within the CNS be triggered through autoimmunity? Of course, but it could also be caused by other processes. Like abnormal (“toxic”) metabolites (which could result from peroxisomal/mitochondrial dysfunction). Or, like tissue hypoxia (which could result from endothelial dysfunction/disturbed blood flow – both in the periphery and within the CNS). Or, like inflammatory mediators swirling around…
And all this does not even touch on the question what may be fueling the mitochondrial/peroxisomal dysfunction, the endothelial dysfunction, the inflammation… Reactivation of endogenous microbes? Which ones?
The many questionmarks in my comment may show that we still have a way to go.
What is important about this work is that it suggests methods that others can use and which can and should be expanded.
Here, for instance, we should expect that the team goes back to their original idea with which they studied FM: inject IgG from ME/CFS patients into mice and see what happens. This piece is still missing: what if the mice did or did not behave much differently? What if they did, and die glia in the CNS were shown to become reactive after the injection?
As I said: There is still a way to go, and indeed, Cort, hope that the NIH is reading all this!
I wondering if bc007 could help POTS patients too?
Thanks Herbert,
Just this morning I was struck by how many different potential pathways there are! Neuroinflammation and microglial activation in the brain, blood vessel problems, blood clotting problems and platelylet activation, mitochondrial issues (peroxisomes, calcium problems, oxidative stress), autonomic nervous system problems, autoimmune responses attacking the receptors on the blood vessels, the mitochondria or the dorsal root ganglia), persistent viruses, hypersensitive immune response, cortisol, etc. CLauw showed he could produce small fiber neuropathy in mice by giving them central sensitization! Littlejohn believes microglial activation in the brain and the sensory nerves is occurring. Griffiths is finding evidence of, if I remember correctly, a channelopathy.
Hopefully, long COVID is where the medical field gets enough resources to separate the wheat from the chaff and we get some real clarity. For now it’s encouraging how many different possibilities – many of which may have connections that we don’t know about now – have popped up. This has become a rich field indeed.
Herbert, what I’m understanding from t\the write-up
is that they proved an effect in a petri dish of isolated glial cells from mice.
Now, what is going on in a human with many moving parts – that was not tested.
Glial cells don’t exist in isolation.
Thank you Mr. Renz-Polster for the details: how much longer do we have to go? Patients are so sick, they are ending their lives. There is real urgency here. BC007 is moving along, but so slowly. How any years before there is real help? Thank you
Do you think bc007 could hep people with POTS too?
Bc007 has become the new XMRV lol. Will be a sad awakening in a few years when they finally have to deliver real data and not just wishful twitter posts.
We should all really be cautious about anything in the early stages. There was Ritxumab as well – there were two small studies that produced excellent results and then it failed miserably in the big trial.
I’m a bit confused by this:
“He and his team took purified IgG antibodies from-long COVID patients experiencing widespread pain, from people who’d recovered from COVID-19, and from an FM patient, and then planted them in cultures of glial cells from the dorsal root ganglia of mice.
They then did an inflorescence study to see if the antibodies from the patients or healthy controls were binding to or attacking those glial cells – thus activating them and causing them to begin spewing out pro-inflammatory factors that were tweaking the nerves and causing increased pain.
It turned out that the antibodies from the long-COVID patients in pain and from the fibromyalgia patients were attacking the glial cells. In fact, the scans of the long-COVID patients and fibromyalgia were identical. That did not happen in the recovered (and pain-free long-COVID) patients.”
the study was all done on cultured glial cells in vitro. Yet when you mention “that did not happen in the recovered patients” – it makes it sound like scans of the insides of live people – in vivo.
The study is all in vitro with mice glial cells – correct?
why can’t they use human glial cells, i wonder. i’m ignorant
All in vitro – yes – that’s my understanding. It may be hard to harvest human glial cell from the dorsal root ganglia. I imagine although I don’t know that that’s an issue.
Can you tell me which journal your finds have been published in please?
Research gate has it under Goebel
The dysautonomia and Mast cell activation communities are both aware that FM, CRPS, POTS and long covid are related in a neurologic-autoimmune way. They are also eager for more research to tease out the details of the process. I experienced a kind of ‘long covid’ from a coronavirus I had back in 1994. That led to development of CRPS, FM, polyneuropathy and POTS. But it is my belief that those who are predisposed to develop these conditions have the underlying condition of mast cell activation syndrome as well as early childhood trauma. That might sound strange to some but there is a lot of evidence for this combination. I just heard a number of international researchers speak to this connection in the recent Mast Cell Conference.
Pas de douleurs non plus.
EM/SFC depuis 18 ans. Fatigue invalidante, Malaise post- effort. Au moment de la digestion, j’ai des spasmes digestifs, musculaires, cardiaques et bouffées de chaleurs . Nez bouché en permanence.
Mon osthéopathe me dit que j’ai un disfonctionnement entre le système nerveux central et le système nerveux autonome , c’est pourquoi j’ai un problème de flux sanguin et d’oxygène…
NEW Study says COVID Attacks DNA in Heart, Unlike Flu > https://www.webmd.com/lung/news/20220930/covid-attacks-dna-in-heart-unlike-flu
Hi Another Anne,
Coppe Laboratories has developed testing.
https://hhv-6foundation.org/cihhv-6/coppe-labs-to-offer-mrna-cihhv-6-testing-and-immunohistochemistry-for-hhv-6
Our daughter was positive through a uterine biopsy.
Could RSLV-132 be a potential treatment? It is being investigated for long covid:
https://ard.bmj.com/content/78/Suppl_2/177.1
Because of the ways that fasting can reset the immune system and cells, it might prove valuable here. Or perhaps rapamycin.
Thanks, Cort, for posting this. I’ve suffered with myofascial pain syndrome/FM throughout most of my 75-year life. The pain is truly a 9.5 out of 10 when it happens. I receive trigger point injections regularly which have lengthened the interval between episodes, but I live in constant fear of the next time it happens. This article makes me feel somewhat hopeful that an effective treatment may be available in my lifetime.
The whole problem with fibromyalgia lies in reaching a diagnosis in the first place.The patient would just suffer and suffer and seek the advice of multiple doctors, but it only takes one smart doctor who is paying attention to give the right diagnosis.I personally know someone, would stay in bed for weeks and wouldn’t be able to perform any kind of daily activates. And neither her nor her family would understand what is the problem with her.Untill she found an online medical service named Docspert Health.She got connected with a will know rheumatologist in the UK whom to schedule an appointment with would take months,but via docspert the whole procedure took only days and she met him via zoom and finally got a diagnosis and a detailed treatment plan.
In regards to “Something in the blood” – Remove the nano tech with 72% min pure chocolate 1-2 cups daily until the nano is gone, & try taking out pain spots with a terrahertz wand would be a good start. I would love to know how many people this helped after 1 year.
People need to be educated of the nanotech via food etc.