
The VA brings big data and big studies to the hunt to understand long COVID.
Veteran’s Administration Joins the Long-COVID Fray
A new long-COVID preprint that recently came out just screams that times have changed. For one thing – the study is huge – 9,000 patients! It also shows long COVID has made it into the Veteran’s Administration (VA) database in the U.S. – which is a very good thing -as it’s a monster.
While we aren’t generally aware of it – because they’ve never been used in chronic fatigue syndrome (ME/CFS) – the VA has massive electronic databases that are often used in medical research. In fact, VA – which has 171 medical centers, 1,298 healthcare facilities, and 1,113 outpatient clinics – is easily the single, integrated healthcare system in the U.S.
Over the last month or so, VA researchers have published papers on e-cigarettes, sleep and brain trauma, gut microbiome enhancement and alcohol abuse, COVID-19 and neurologic disorders, and others. In the COVID-19 paper, researchers compared 150,000 VA patients who’d contracted COVID-19 with more than 11 million people who had not. (It concluded, a year later, that the people coming down with COVID-19 had a higher risk of stroke, memory disorders, nervous system disorders, episodic disorders like migraines and seizures, Guillain-Barré syndrome, and sensory disorders (ouch!). Findings like that should ensure that long COVID remains a subject of great interest for quite a while.)
Getting long COVID into the VA’s database, then, is a pretty big deal. (Similar studies are underway in the RECOVER Initiative.)
The VA Study

The protease that Paxlovid attaches to stops the coronavirus from replicating (from Vcpmartin Wikimedia Commons).
The title of the study, “Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19“, buries the lead for most of us. Nirmatrelvir is Paxlovid – the only antiviral drug (note the -vir) – being used in large amounts in COVID-19.
I learned about this study from Eric Topol’s recent blog post on it. Topol’s engagement with long COVID (he said it was the thing he feared most from COVID-19) is another nice sign of the condition’s emerging prominence. Topol is one of the top 10 most cited researchers in medicine and received a $207 million NIH grant to co-lead the Precision Medicine Initiative in 2016.
The “Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19” study followed 9,000 COVID-19 patients treated with Paxlovid from March-June 2022 and compared them to 47,000 COVID-19 patients who were not treated with Paxlovid or any other antivirals or antibodies.
The study used electronic records to assess 12 potential sequelae, as well as hospitalization and death. Besides things like cardiovascular, coagulation, kidney, etc. problems, the study assessed three symptoms (fatigue, musculoskeletal pain and cognitive issues) commonly found in ME/CFS and fibromyalgia.
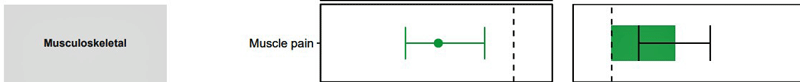
The study results show just how powerful these big studies can be – as well as the limitations they present. The study found that people given Paxlovid in the first five days of their infection were 26% less likely to come down with long COVID. Paxlovid significantly reduced 10 of the 12 sequelae assessed, including cardiovascular disease, coagulation disorders, kidney problems, etc. as well as fatigue, musculoskeletal pain, and cognitive problems. Interestingly, if I’m reading the graph right, the drug appeared to have one of its largest effects on fatigue.
The Gist
- A huge Veterans Administration study (50+ thousand) determines we are truly in a different world with long COVID. The biggest integrated health system in the US brings huge databases and lots of yummy data to medical research.
- A recent 11 million-person study (yes 11 million), for instance, found that having a COVID-19 infection produced a greater risk of coming down with a wide variety of neurological disorders.
- The study followed 9,000 COVID-19 patients treated with Paxlovid from March-June 2022 and compared them to 47,000 COVID-19 patients who were not treated with Paxlovid or any other antivirals or antibodies.
- People given Paxlovid in the first five days of their infection were 26% less likely to come down with long COVID. Paxlovid significantly reduced 10 of the 12 sequelae assessed, including fatigue, musculoskeletal pain, and cognitive problems. It also reduced the risk of death (48% reduction) and hospitalization (24% reduction). The results pertained whether a person was vaccinated or not.
- The study did not assess the incidence of ME/CFS or the effect of Paxlovid on post-exertional malaise, orthostatic intolerance, and sleep problems. Because it was also done on people with the ability to get Paxlovid (i.e. they had at least one risk factor for severe COVID-19) we don’t know if it applies to healthier people.
- Still, the results suggested that an antiviral drug can reduce the incidence of long COVID. Eric Topol suggested that because Paxlovid stops replication of the virus it may reduce the viral reservoir left behind by it resulting in less immune activation or autoimmunity directed at the virus.
- The study results came not long after the RECOVER Initiative named their first clinical trial: a 1700-person trial of Paxlovid to start in January of next year. It also comes not long after the Long COVID Research Initiative announced it was spending $15 million (and hopefully up to $100 million) to understand the role viral persistence plays in long COVID.
- Long COVID gave antiviral research a big boost and Paxlovid is just the first and almost certainly not the last or the best antiviral that is being tried out in COVID and now in long COVID.
- With EBV reactivation being found in long COVID EBV antivirals are getting more attention as well. That’s a good thing as the current drugs used are not specific for EBV or particularly effective.
- Once again, long COVID is doing what we hoped it would – force the research community to take a deep look at some prominent research themes in ME/CFS such as viral persistence and antivirals that were never fully explored.
- It’s also recruiting many allies to our and long COVID’s cause. The senior author of the VA study has published 9 papers on long COVID. In the latest he wrote: “Given the scale and the chronic nature of several of its sequelae, long COVID will reverberate with us for decades, and will have broad and deep social, economic, political and global security implications, long after the COVID-19 pandemic abates. This pandemic provides a historic opportunity not only to understand long COVID but also other post-viral conditions and infection-associated chronic illnesses…”
Whatever a person’s state of health, Paxlovid proved to be a powerful drug that prevented long COVID from appearing in about 25% of those who had taken it. Interestingly, the more risk factors a person had, the more effective it was.
Studies that rely on large electronic databases are going to have limitations, and this one did. Chronic fatigue syndrome (ME/CFS) does not appear to be in the VA’s database, as the study did not assess the incidence of ME/CFS. Nor did the study assess the effect of Paxlovid on some symptoms commonly found in ME/CFS such as post-exertional malaise, orthostatic intolerance, and sleep problems. These factors will hopefully work their way into the VA’s databases over time as it comes to grip with long COVID.
While the study focused on non-hospitalized patients (a good thing for ME/CFS), because it also focused on people who had at least one risk factor for COVID-19, we also don’t have data on how effective Paxlovid is in preventing long COVID in people without risk factors. (Paxlovid is approved for people with a risk factor for severe COVID-19).
Still, the study showed that an antiviral drug taken early in the course of a coronavirus infection was able to cut down long COVID by a quarter. So, what the heck is going on?
In his blog, Topol noted that because Paxlovid stops the replication of the virus, it likely reduces its ability to penetrate deeper into the body and leave behind a viral reservoir, or remnants of the virus, behind. Less virus in the body possibly means less immune activation or autoimmunity directed at the virus. It also suggests some sort of immune hole may be preventing people who come down with long COVID from rapidly dispatching the virus.
Of course, the findings also point a finger at the possible impact of antivirals. We should remember that as good as Paxlovid is, it isn’t the be-all and end-all of coronavirus antivirals. It’s simply the first one to break through, and others that are in development/testing may be more effective at reducing the incidence of long COVID. Plus as Dr. Peluso pointed out – it may take several different kinds of treatments to fully get at long COVID.
Dr. Michael Peluso, an assistant professor of medicine at the University of California, San Francisco, told the NY Times “The results are quite provocative and suggest that further investigation of antiviral agents and their effects on long Covid is urgently needed.”.
The VA study results were timely given the recent RECOVER Initiative announcement that its first long-COVID clinical trial will feature none other than Paxlovid.
The Paxlovid RECOVER Initiative Long-COVID Trial
At the end of October, the RECOVER Initiative announced its first long COVID clinical trial – Paxlovid (300 mg w/100 mg ritonavir). The 1,700 people participating in the trial provided yet another reminder for people with ME/CFS that “we’re not in Kansas, anymore”, and that long-COVID research can, at times, be taking place on a scale that we’re not used to.
The trial is clearly being informed by ME/CFS research, as it’s using a modified DePaul post-exertional questionnaire developed by Lenny Jason, as well as questionnaires assessing cognition and autonomic nervous system symptoms using an “active stand test” which sounds a whole heck of a lot like the NASA Lean Test the Bateman Horne Center has been promoting.
All Together Now
The VA study results suggest that the RECOVER Initiative is on the right track in trying out Paxlovid. Three major efforts – it, the RECOVER Initiative clinical trial result, and the Long COVID Research Initiative effort, all appear to be on the same page.
The Long COVID Research Initiative is starting off with $15 million to search for evidence of coronavirus persistence in long COVID and hopes to dedicate $100 million to it. Rather quickly, virus persistence – a subject which, with the exception of Epstein-Barr Virus, was basically dead in the water research-wise in ME/CFS, has become a major theme in long COVID.
And what about the Epstein-Barr virus? Several good studies have found EBV reactivation in long COVID, and the EBV field in ME/CFS is nothing if not fascinating. From a putative immune hole in ME/CFS, to an active infection, to smoldering broken forms of the virus, to EBV-triggered pathogenic genes, to EBV-triggered neuroinflammation in ME/CFS, to EBV as a kind of an autoimmune inoculator – the list of EBV-associated possibilities in ME/CFS and elsewhere seems to go on and on.
The pandemic has reportedly vastly increased interest in antivirals. Just yesterday, three possible new antivirals for COVID were announced. The coronavirus isn’t the only virus getting attention: Epstein-Barr virus and other herpes viruses are as well. EBV-specific monoclonal antibodies, for instance, are being explored.
Antivirals have worked in ME/CFS for some people, but the antivirals we have now are not specific to EBV nor very effective at knocking it down. Given that, it’s no surprise then that the antiviral recovery stories on Health Rising indicate that when antivirals work, they often take up to a year to do so. A new crop of COVID-19-inspired antivirals might do far better…
Once again, long COVID is doing what we hoped it would – force the research community to take a deep look at some prominent research themes in ME/CFS that were never fully explored. It’s also recruiting many allies to our and long COVID’s cause that we couldn’t have anticipated.
The VA paper’s senior author, Ziyad Al-Aly, for instance, has published at least 9 studies and papers on long COVID thus far. Just last week, he published “Long COVID: long-term health outcomes and implications for policy and research“, in which he documented the enormous range of other illnesses and effects (kidney disease, dysrhythmias, ischaemic heart disease, heart failure, pericarditis, myocarditis and thromboembolic disease, new-onset diabetes mellitus, structural changes in the brain, hemorrhagic stroke, seizure disorders and disruptions in cognition and memory) a COVID-19 infection is increasing the risk of getting.
Al-Aly wrote
“Given the scale and the chronic nature of several of its sequelae, long COVID will reverberate with us for decades, and will have broad and deep social, economic, political and global security implications, long after the COVID-19 pandemic abates. This pandemic provides a historic opportunity not only to understand long COVID but also other post-viral conditions and infection-associated chronic illnesses…“








Cort,
Those risk reduction charts are a bit unfamiliar to me. So when it says “Absolute risk reduction in percentage at 90 days (95% CI)” it is saying that between ~0.2% and ~0.85% (for CI = 95%) of the “general population” would have no fatigue at 90 days (associated with taking the drug)?
I ask because the same absolute risk reduction for PASC is ~1.7% to ~2.9% (CI = 95%) associated with taking the drug. So the percentage of people (of the “general population) who take the drug and subsequently are not expected to go on to develop PASC is greater than the percentage of people (again of the “general population”) who take the drug and subsequently are not expected to go on to experience fatigue as a symptom.
So does that mean that PASC didn’t include fatigue as a mandatory criterion, or does it mean that a lot of subjects are left with fatigue but don’t qualify as having PASC, or both?
Sorry, I realise this all sounds like gibberish but I’m trying to understand how relevant the PASC absolute risk reduction is to ME/CFS if it appears that PASC doesn’t have to involve or resolve fatigue. Antivirals are expensive!
I’m afraid we are going to need someone who knows more about statistics to answer this! I’m not sure how PASC was decided. It may have been defined, though, as having a significant increase in any of the sequelae that were assessed.
This week >
https://www.omf.ngo/does-me-cfs-change-molecularly-throughout-a-day-bergquist-and-armstrongs-new-study/
https://www.omf.ngo/does-me-cfs-have-a-biomolecular-signature/
I’ve heard that Paxlovid has been used successfully at a SF clinic to cure CFS but I’ll have to check this out more fully.
I managed to get my hands on Paxlovid via stealth!
As I realised it was unknown that when I eventually become infected with Covid my doctor may or may not be able to prescribe it for me.
My doctor said she “would try” which meant the fear of not getting Paxlovid. So I became fully isolated.
I also wanted to know if I would even tolerate Paxlovid . As I’m hypersensitive to many medications.
So a couple weeks later I craftily broke the rules and emailed my doctor saying that I had caught Covid and sent a photo of (a friend’s) positive Rapid Antigen Test. (Basically I faked I had Covid lol)
She wrote the script and it was accepted.
Because I didn’t have Covid this was my chance to test my tolerance. I sliced off a tiny piece the equivalent to a grain of salt.
There’s two drugs in separate tablets in Paxlovid, so I had to try minute amounts of each separately on different days.
Slowly I increased the amounts to 2 and then 3 grain of salt sized amounts each day. Up until I got to a 3rd of a tablet.
Then I combined the two meds as it’s intended to be taken together and thank god tolerated it!! So I’m satisfied I will tolerate a course of Paxlovid.
When I actually catch covid I’ll order another script so I’ll have more than enough, as I see some researchers think the dose is too short anyway. So it’s a win win.
For those that think what I did is ethically wrong, I say in response, that holding back on ME/CFS research for decades, that ends up driving patients to go to such lengths to get adequate medical resources, is far more unethical than getting a preemptive prescription for an infection we are all 100% going to get anyway. i.e. no harm done
Sometimes you have to break a finger nail to get the job done.
Thank you for testing this out! I have MCAS and the same issues so even though everyone is different it’s very helpful to know that you tolerated it ok! 🙂
Cort, I was surprised to see you say that viruses and antivirals have not gotten much attention in ME/CFS until Long Covid came along. .
If anyone takes a serious review of Dr. John Chia’s decades of work looking at enteroviruses, we would see that he has been on the quest for antivirals that could help. And yet his work doesn’t seem to get the serious attention that it deserves. It’s not just re-activated EBV that is on the radar. Antivirals for enteroviruses should also be given serious attention.
I’ve heard him publicly ask for his research findings to be challenged. Why haven’t we heard anyone answering his challenge?
This reminds me of the outbreak at Incline Village. According to court records, there was a major sewage spill there, with perfect timing for the outbreak. Doesn’t that sound like a possible enterovirus?
Actually, to me the Chia saga demonstrates how little attention viruses, particularly enteroviruses, have received from the field at large. Despite his decade-plus and very public efforts to give enteroviruses more prominence with the exception of a small CDC study that was never published no one has tried to replicate his results. That’s been really surprising to me.
EBV, of course, has gotten attention in ME/CFS and Prusty has been studying HHV-6 but these are small efforts – nothing like we’re likely to see in long COVID.
Thank you for mentioning Dr. Chia’s decades long work. My daughter is his patient and her blood is among those he has sampled and used in his lab studies. It’s frustrating, from as a patient’s advocate to see little, if any, interest in validating/invalidating his hypotheses. Is there anything that can be done to help support and bring more visibility to Dr. Chia’s work?
We need to try to get Washington Post or NY Times science reporter to interview Dr. Chia to bring more attention to his research from the general public.
Limited relevance to ME/CFS. Because ME/CFS can be caused by many different types of viruses, or none at all. Unless a broad spectrum antiviral is found, it has limited application. Also, I don’t think all viral triggers of ME/CFS are severe, so how would people with a mild or moderate viral symptomology who might contract ME/CFS know that they should take an anti viral?
I guess it does have potential relevance in so far as it talks to the impact of a virus and it’s ability to trigger long covid or ME/CFS.
There’s no reason to think Paxlovid – an antiviral developed to attack the coronavirus – will be helpful in ME/CFS – that wasn’t the point of the blog. It’s the idea that’s taking hold that viral persistence could be causing long COVID and therefore could also do so in ME/CFS – and that better antivirals could be helpful – it’s that idea was the point of the blog.
With ME/CFS the viral rreservoir or virus that is causing the problem would have to be identified. That’s where the Long COVID Research Initiative would come in. If it can show that the coronavirus is causing problems in long COVID it should be able to use the same techniques in ME/CFS to find the pathogens there.
But you would need to get to that viral reservoir EARLY. I assume that paxlovid was given following infection.
The question still remains – given that there is no evidence that ME/CFS is caused by ONE virus – unlike long covid which is caused by covid – I fail to see how you could ever potentially prevent ME/CFS in the same way that you might with long covid.
And by the way, there is absolutely no definitive evidence that ME/CFS is caused by a virus. A significant number of people do not link their illness to a specific viral illness. I think it far more likely that ME/CFS is triggered by several different things, with viruses being one trigger (as it was for me)
Matthias, Dr Hyde says ME is caused only by an Enterovirus. He says that CFS is not a disease but a cluster of symptoms that could be anything but ME is distinctly caused by an Enterovirus infection of the CNS which causes a vasculitis injury in the brain. I am with you though, I have heard that ME can be caused by many different things, eg organophosphate poisoning causes ME. The Sheep dip farmers got it when they used Organophosphates in the UK.
Hi,
The article says “ The coronavirus isn’t the only virus getting attention: Epstein-Barr virus and other herpes viruses are as well.” Can you point me towards any current research involving herpes viruses and me/cfs or long COVID?
Thank you
Here are some EBV long COVID papers. There are links to EBV ME/CFS papers in the blog
Distinguishing features of Long COVID identified through immune profiling. – https://pubmed.ncbi.nlm.nih.gov/35982667/
Klein J, Wood J, Jaycox J, Lu P, Dhodapkar RM, Gehlhausen JR, Tabachnikova A, Tabacof
Association between Epstein-Barr-Virus reactivation and development of Long-COVID fatigue – https://pubmed.ncbi.nlm.nih.gov/35950630/
Impact of Pre-Existing Chronic Viral Infection and Reactivation on the Development of Long COVID – https://pubmed.ncbi.nlm.nih.gov/35898346/
Multiple early factors anticipate post-acute COVID-19 sequelae. – https://pubmed.ncbi.nlm.nih.gov/35216672/
SARS-CoV-2 and EBV; the cost of a second mitochondrial “whammy”? – https://pubmed.ncbi.nlm.nih.gov/34717676/
I was specifically asking about herpes viruses and me/cfs or long Covid, given that your article states that they are getting more attention lately. Do you have any links to that research?
hi Cort, great article and sleuthing,
i am wondering if there is a link to a description of the “active stand test”?
thanks in advance
Nice to see paxlovid could help a subgroep of long covid patients but it is not for ME/CFS in general.
Another interesting and very big study from Israel. Unvaccinated people who gets infected with covid-19 do not have higher risk of pericarditis or myocarditis. These days we see al lot of young people especially male with these problems much more then normal. Makes me think if it is not due to a covid infection, how come? Especially because you see it after vaccination. Also many young people died by heartproblems. Elephant in the room?
(…)We did not observe an increased incidence of neither pericarditis nor myocarditis in adult patients recovering from COVID-19 infection….
https://pubmed.ncbi.nlm.nih.gov/35456309/
Right – the idea is that if Paxlovid works for long COVID another antiviral might work for ME/CFS.
Sorry Cort that’s simply nonsense.
Long covid might help to be prevented when an antiviral is taken for covid. There is a direct viral link between the two, right? Ie. the same virus.
As I have said there is NO definitive study that shows ME/CFS is caused only by viruses, let alone by one virus. There appears to be a strong likelihood that viruses are a common trigger for ME/CFS, but that’s a quite different thing.
I don’t get the obsession with ME/CFS being caused by a virus. A similar and linked obsession seems to be at play with an unjustified belief in the potential of ampligen. Why?
I realise it’s a ‘neat’ explanation that would be really nice to have ie. virus x causes ME/CFS, and antiviral y can treat it. I just think there’s little chance the cause of this illness will be that neat or simple. I wish it was.
I think you are misinterpreting the study. It is about the preventive effect of Paxlovid, not curative one. It didn’t give any boost to the antiviral therapy on CFS as far as I can see. To do that, they’ll have to show that Paxlovid can help long COVID patients recover.
The significant of the study to me is that it showed the longer the duration of the COVID infection the more likely you are to develop long COVID. That makes sense since it’s been long suspected that prolonged inflammation is the cause of post viral syndrome. Taken together with the fact that the COVID symptom severity and the vaccination status does not strongly predict who developes long COVID, we could surmise that the duration is more important than the severity in determining who would develop long COVID. And that the severity has an effect only insofar as it correlates to the duration.
I have had FM for thirty-five years. I recently got the pneumonia vaccine. While I had no bad side effects, the following day and every day since, I have experienced much less morning stiffness. I know the pneumonia vaccine contains both anti-viral and bacterial properties, but I thought this was interesting and relevant to this discussion. I wonder if anyone else has had anything similar happen to them.
I’ve had ME/CFS and FM for 35 plus years. I received both Pfizer COVID vaccines in Feb. 2021 but did not take any boosters because of long-lasting reactions to the vaccines. I tested positive for COVID in August 2022 and started taking Paxlovid on the second day. I tolerated the Paxlovid well. It’s difficult to say whether or not I now have long COVID or just typical relapse with some worsening of the ME/CFS and FM because so many of the symptoms are exactly the same. The bottom line is that while I normally have reactions to most medications, I did not have a reaction to the Paxlovid and would have no hesitation in taking it again if I contract COVID again.
I understand that Paxlovid is a combo of two antivirals. I know that one of them is not NEW or developed specifically for COVID. It was repurposed as it is used for HIV treatment. It interrupts the replication of the virus. I do not know about the second one in Paxlovid.
The virus in ME/CFS has GONE that’s why it’s called ME/CFS. Plus, you can get ME/CFS from organophosphate poisoning and Sarin gas poisoning. Antivirals have been tried by Lerner and others. If an antiviral could cure ME/CFS then we would know by now. The virus has GONE
Well, the long COVID Initiative – filled with scientists – would beg to differ. At least they would say the answer is not clear – hence the decision to spend tens of millions of dollars apparently, to find out if its present or not in long COVID. If it is they say they’ll move onto ME/CFS.
Antivirals actually have cured people with ME/CFS – check out HR’s Recovery stories section for a bunch of them.
I have searched and searched, but I can’t find evidence of any antiviral curing any virus…treating yes, but curing no. One of the great antiviral success stories is the newer treatment regimens for HIV. They can make the virus undetectable but if you stop taking the antivirals, HIV comes roaring back. The caution is that these antivirals have “very” serious adverse effects in some patients or lose their effectiveness in others. In 2021, 650,000 people died of AIDS-related illnesses worldwide. This is compared to 2 million deaths in 2004 so a qualified success story, but not a cure. Unfortunately most of your cure stories, Cort, are from Dr. Lerner who has sadly passed away. I would be interested in follow-up on all these cases to see if the treatment was in fact a cure or just a pause.
Once you have been infected with one or more of the herpes family viruses (Chickenpox, EBV, CMV, Herpes 1 and 2, HHV6 & 7), they live in your body forever. The goal is to keep them at bay, but at this time, there is no “cure”.
Hi Cort – where can I read studies that have shown antivirals have cured ME/CFS? I have had both most of my life, officially diagnosed in 1992 when doctors were telling me it was all in my head. I would love to read about this.
Please ask anyone involved with ME/CFS research and treatment to listen to Dr, Chia. I have had CFS for over 30 yrs and have seen too many health practitioners to count. Dr Chia is the only one who is not in it for the money or fame, but in it for helping all of us who are suffering. He is currently trying to get a study approved for use of Paxlovid and a pill form of Remdisiver to treat ME/CFS. I have been hopeless for so many years and I have confidence in Dr Chia.
Some of Erik’s people in Incline Village went to see Dr Chia and took his Equilibrant protocol against enterovirus, but they were not cured.
But Equilibrant is an immune modulator, not an antiviral.
Jannio, no Equilibrant is a Chinese herb formulation. I have a box of it here with me now. It has Astralagus Root extract, Shrubby Sophora Root extract, Oilve leaf extract and Shitake Mushroom extract. It is basically a nutritional supplement. As I said, Erik’s people went to see Dr Chia and they tried this and it did not cure them. This is what Erik told me anyway.
Was looking for an easy way to tell you about another drug trial https://www.theguardian.com/society/2021/nov/03/uk-launches-drug-trial-tackle-fatigue-long-covid-patients
If this works maybe someone will trial it for ME!
Thanks for posting @tatt. I have just read the article from the link you posted and the study looks for interesting and surely the drug they are testing would be a great candidate to test in ME/CFS if it shows promise in Long Covid.
It seems to me that the mitochondrial issue and high lactic acid in the cells exactly fits what has been found previously in the cells of ME/CFS patients?
The VA denies chronic Lyme and ME/CFS exist. They are only doing research on Long Covid for the money. The “massive” database is made on the backs of the military and veterans who they refuse proper care to but expect that these people will keep donating blood and other samples to for their database. The Drs they hire are paid minimal salaries and are shuffled around from state to state when they mess up. It’s very sad and I hope the situation changes eventually.
I can’t seem to find anything about Paxlovid and if it might help people like me who had Covid previously (2 years ago) and have had Long Covid with ME/CFS since then. I have had all of the vaccines and boosters, but now have Covid again. I’ve got a few days to decide whether or not to take Paxlovid, and am weighing the pros and cons. I wouldn’t expect it to help with the Long Covid I’ve had for a few years, but could it help to not make it worse?
I would definitely give it a shot. Since Paxlovid is now being trialed IN long COVID it’s possible it might help with that and/or reduce the risk of a worse flareup.