

Maureen Hanson’s group at Cornel continues to pound out fascinating results. The latest one – a preprint, “Single-cell transcriptomics of the immune system in ME/CFS at baseline and following symptom provocation” – again demonstrates why the NIH-funded ME/CFS centers are so important – and why we want and need more of them. The study was large, complex, and innovative, and came up with some surprising results. Plus, as the Hanson group is wont to, it used an exercise stressor. Let’s hope the RECOVER Initiative is watching.
Preliminary results from this study showed up in an IACFS/ME conference presentation that Health Rising covered, but we’re covering the full paper as it’s chock full of potentially new and important insights into ME/CFS.
The Gist
- This NIH-funded ME/CFS research center study was complex and innovative, and it showed in its possibly ground-breaking immune results.
- Instead of assessing the gene expression of immune cells “in bulk” (that is – assessing the gene expression of all the immune cells at once), the Hanson research group – lead by Andrew Grimson and Jan Grenier – assessed them cell by cell – giving them much more fine-tuned results.
- The results were surprising. While the ME/CFS patients’ T-cells and NK cells were acting strangely, it was monocytes – immune cells ME/CFS researchers have paid little attention to over the years – that were the most dysregulated.
- Monocytes troll the bloodstream looking for signs that something is wrong. When they find it, they turn into macrophages which dive into the tissues and engulf pathogens and/or clean up the cellular debris left behind by an injury.
- The monocytes they found in ME/CFS (classical monocytes) were in an aggressive state, appeared to have been activated, and were ready to turn into macrophages. Macrophages tend to be active in environments where chronic inflammation is present.
- Some of the monocytes were “diseased”, or dysregulated, while others were normal. Those diseased monocytes seemed to be making an impact: whether it was functionality or post-exertional malaise or another symptom – the more diseased monocytes a person had, the worse off they were.
- The authors stated, “ME/CFS patients experience continual improper recruitment of monocytes to one or more tissues”. Given the monocytes’ tendency to turn into a prime mover of chronic inflammation (i.e., macrophages), the finding suggests inflammation could play a major role in this disease.
- Given the strengths of this study – a large, complex study employing ME/CFS patients from ME/CFS experts that used a more effective technique – it has the potential to alter how this disease is viewed immunologically.
- Further studies are needed – and the Hanson group suggested that the same type of study is now being done in long COVID – but one wonders if they’ve uncovered a prime immune driver of this disease.
- Another surprise came with platelets. Platelet activation has been found in long COVID, but this gene expression study suggested that platelets in ME/CFS were inactivated at baseline but – strangely enough – looked normal after strenuous exercise. No evidence of platelet activation was found.
- The authors suggested that exercise might have activated the inactivated platelets, which then got swept up in the microclots that exercise provoked, thus removing them from the circulation.
- The study also found a downregulation of genes associated with ribosomes – the seat of protein synthesis in the cell – in a wide variety of immune cells. That suggested that many of the immune cells in ME/CFS were in a quiescent state and underperforming.
Most gene expression studies use bulk samples: i.e., they assess gene expression patterns across thousands, and up to millions, of cells at a time. The problem with this approach is that it may obscure biological abnormalities in specific cell types – and, in fact, the gene expression results in ME/CFS have been underwhelming to say the least.
In this study, Andrew Grimson, the leader of the study, introduced a different method. He and Jan Grenier – two experts in single-cell sequencing – assessed the gene expression or transcriptomics cell by cell (scRNA-seq) – thus getting a much more fine-tuned picture – and that made all the difference.
The study used Workwell’s exercise protocol to compare the gene expression of immune cells before and after exercise. Jared Stevens (Workwell), Betsy Keller (Ithaca), and Susan Levine MD were amongst the co-authors, and John Chia MD provided patients as well.
The Study
The authors reported the study introduced a “new and important resource to investigate immune dysregulation” in ME/CFS. Only small differences were present in the proportions of immune cells: i.e., ME/CFS is not caused by some out-of-control immune cell that’s wreaking havoc.
Next, they analyzed the number of “significantly dysregulated genes per cell type”. Since which genes are turned on or off tells what the cells are or aren’t doing, they were looking for cells that were acting strangely. While most immune cells were acting perfectly normally, a few cell types exhibited “strong signals of dysregulation”: i.e., they were acting strangely, indeed. They included some big movers in the immune system: CD4+ T cells (naïve and effector/memory subsets), monocytes, and (as expected) cytotoxic NK cells.
That was all to the good: significant dysregulation across a few immune cell types provides a much easier target than widespread dysregulation.
Surprise Immune Cell Comes to the Fore
The strongest center of dysregulation occurred in monocytes – particularly classical monocytes. Monocytes are large, white blood cells that play a major role in inflammation. The classical monocytes that showed up in the ME/CFS patients aggressively target damaged areas. Elevated levels of genes associated with chemokine signaling, migration, and activation suggested the monocytes in ME/CFS are highly activated and on the move.
The high CCL4 expression suggested that the monocytes in ME/CFS were primed and ready to turn into macrophages. When monocytes find a damaged site, they leave the bloodstream and burrow into inflamed sites, where they turn into macrophages that then remove dead or dying cells, or cellular debris, and/or engulf (swallow) pathogens. That’s all good, but it also signifies inflammation. Macrophages, it should be noted, play a particularly prominent role in chronic inflammation.
Nath, it should be noted, found small blood vessel leaks in the brains of people who’d died from COVID-19. Macrophages had invaded the tissues found outside the blood vessels in an attempt to clean up the mess. One wonders, given the problems with blood flows, if something similar is happening in other tissues in ME/CFS.
(Bruce Patterson MD believes that the monocytes are attacking the endothelial cells in long COVID – and a major part of his protocol involves stopping them. While Patterson found low levels of CCL4 in his long-COVID patients, though, this study found high levels of it in ME/CFS patients.)
Next came an exercise where they labeled each monocyte as diseased or normal. They found that people with ME/CFS patients contained both normal and diseased monocytes, and in a finding they called “notable”, people with more diseased monocytes tended to be worse off – across a wide range of symptom scores (functionality, general health, SF-36 physical component, PEM severity). The more diseased monocytes a person has, the worse off they were in many ways.
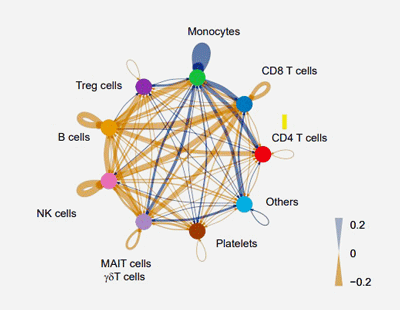
An analysis of signal strength (blue lines) suggested that monocytes were having an outsized effect on other immune cells.
Next, an “interactome” analysis found the monocytes were interacting a lot with the two other immune cells the study pulled up – T and NK cells. That was a nice package, as many ME/CFS studies have focused on NK cells and T-cells.
This study suggested, though, that when it comes to the immune system, monocytes may be “it” in ME/CFS. Nobody in the decades studying this disease has ever done a deep dive into monocytes/macrophages in ME/CFS. It looks like that is about to change.
In a Cornell ME/CFS Center Facebook post, Grimson – who is the co-PI on the Cornell Research Center grant – wrote that the aberrant monocyte migration
“could contribute to many of the symptoms of ME/CFS. This work sets up lots of questions that motivate our work now – where are the monocytes going in ME/CFS individuals, what is causing them to be dysregulated, and ultimately, can we reverse this dysregulation?”
and that
Beyond changes in gene expression in monocytes in ME/CFS, we also find changes in expression in other immune cells, which will be an invaluable resource for both us and other researchers as we build towards a comprehensive understanding of immune alterations in ME/CFS.
All in all, their findings suggest that “ME/CFS patients experience continual improper recruitment of monocytes to one or more tissues”.
This study checks all the boxes: it’s a large study of ME/CFS patients (derived, it should be noted, from ME/CFS experts), which used a more effective technique (single-cell gene expression), that ended up highlighting a factor (diseased monocytes) that impacted symptoms across the board. This finding clearly has the potential to redefine how we think about ME/CFS immunologically – and that’s good news.
The Platelet Paradox
Finally (and weirdly), the Hanson team found a factor that was abnormal at baseline but normal after exercise. Platelet gene expression was reduced at baseline but returned to normal 24 hours after exercise (!). This is the first factor I can remember that was abnormal at baseline but returned to normal after exercise.
Platelet activation may be a big deal in long COVID, and a recent study suggested it was present in ME/CFS as well. This study suggested the same but flipped it on its head.
The genes expressed by the platelets at baseline didn’t suggest platelet activation at all. In fact, it made them look like “older” platelets that were hardly eager to become activated. (Were they exhausted?). All in all, platelet activation was reduced at baseline, returned to normal 24 hours after strenuous exercise, and never showed evidence of increased activation.
The authors had two possible explanations for this bizarre finding. Exercise induced both platelet activation and microclot formation in people with ME/CFS. The activated platelets then adhered to the clots – thus removing them from the circulation – leaving only normal platelets behind. Or, it’s possible that exercise, in combination with clot formation or removal of the older-looking platelets, might trigger an influx of normal platelets.
Immune Downregulation
A gene set enrichment analysis (GSEA) found a downregulation of ribosomal protein genes and core translational machinery across multiple cell types. Since ribosomes are the center of protein synthesis, this seems to imply that a breakdown in protein synthesis had occurred across the immune system and that the cells were likely underperforming. Interestingly, a recent study found a similar pattern – broad immune cell quiescence in ME/CFS – with the exception of the monocytes.
Conclusion
They haven’t gotten much press, but this study suggests that monocytes might be the real immune problem in ME/CFS. (Image from Blausen – Wikimedia Commons)
A more effective technique of measuring gene expression that hasn’t been used before in ME/CFS produced some surprising results. Instead of the usual suspects – NK, T, and B-cells – it was the monocytes – which have never received much attention in ME/CFS – that showed up in spades. Was a prime mover of immune dysregulation in ME/CFS missed all this time? Time will tell.
Another surprising result was platelet activation, or rather, the lack of it. Instead, the gene expression study suggested that the platelets in ME/CFS at baseline were snoozers – hardly activated at all. After exercise, things, strangely enough, returned to normal for reasons that are unclear.
The study also uncovered a reduction of ribosomal gene expression (e.g., protein production) across many immune cell types in ME/CFS, suggesting that many immune cells are in a kind of quiescent, sleepy state – and not likely to rise to the occasion when stressors occur.
All in all, this study showed why NIH-funded ME/CFS research centers are potentially so important and why we need more of them: they have the ability to redefine how we see this illness.






Thanks for this information…
I appreciate you keeping us updated. As always you give hope to us that somedays feel like there is no hope of getting better.
Thanks! I particularly love it when new technology opens up new potential new vistas and possibilities. How nice it would be if this study found something that tied together in some way all these immune findings. Time will tell…
I studied Biochemistry for over a decade at two of our best “research institutions”. ME/CFS has become one giant tornado!
We’ll all be gone by the time they understand this beast. I guess we can hope that generations behind us do not have to suffer from this “worse than death” disease.
Sorry to agree with you I feel the same way after suffering 51 years bed ridden over 30.
No money to research all of my young life.
Other diseases got celebrities to help and tons of research not us with CFS…..
Ot only are we I’ll some of us are so ill talking drains us.
I have had 3 types of cancer and what is suppose to be awful treatments and none compared to CFS! Never ending..,,..
Good new information. Thanks!
Amazing, thanks Cort. Definitely gives us a lot of hope (and makes me want to shove this study under the nose of that guy Wise who wrote that extremely problematic NY Magazine article recently).
I’ll be curious what conclusions are eventually reached about long haulers who are also diagnosed with ME (like me). I did Bruce Patterson’s protocol last year and I got about 80% better. Then I had a hellish and extremely activating week at work followed by a planned hiking weekend, and I had a complete crash. The protocol stopped working at that point. After 9 months off of it, I’ve been trying it again recently. No improvement. It’s clear to me (from my own experience and that of hundreds of other long haulers who did not get better on Patterson’s protocol, though many also did) that the monocyte factor is definitely at play, but it’s far from the full picture, at least with long COVID. Also, I fear for those who get better with Patterson but don’t keep pacing, like me. I’d do anything to get that 80% back.
The fact that you got some much better suggested that he’s onto something. Time will tell but people have so many different reactions to so many things that I would be surprised if any one thing was the answer. Finding just a piece of the puzzle would be a real step forward. Let’s hope this is a piece.
I’m sorry that you experienced major improvement only to lose it again. I had a similar experience with Acyclovir several years ago. I improved to an almost normal state, then 3 1/2 months later, it just stopped working. No warning. No explanations. As you, I have tried to duplicate the experience, but have not been able to. I have concluded that my body has learned to recognize anything that moves the needle toward improvement and negates it…maintaining the state IT (whatever the heck “IT” is) wants me to be in. The fact that we are able to get back to some level of pre-illness functioning at all is very encouraging. Many with ME/CFS have had the same experience. Also, what works for some won’t work for others. Several researchers seem so close to finding pieces to this elusive puzzle. Prayers said and everything crossed we have effective treatment options very soon.
Thank you for your kind reply and insights. There is indeed much to be hopeful about!
I so get you! I also had the crash of my life one year ago overdoing it for 7 weeks.
i dont know the patterson method, but i have imoproved noticably with High dose liposomal Vit. C, and antihistamins h1 AND h2(h2 crosses blood brain barrier).
Also tried REISHI MUSHROOM -way too potent for me so I stopped, for now).
my brainfog is GONE sinxe I started Vit C (and reisgi for a few weeks) and I take long walks already in the morning and am productive most of the day.
these supplements ALL deal with inflammation
I do yoga nidra every day to get the NS into complete relaxation AND do IFS trauma therapy – great benefit.
Wish you and all a good hour, minute, breath, heartbeat. Just one…that is how healing starts.🧡💛🧡
So, would Patterson’s maraviroc protocol act to correct this disruption in the monocytes?
Yes it stops the pro-inflammatory state of the cells and keeps the inflammatory cells from migrated all over the body
So if this is it, is there any plausible treatment?
yes, i want to know this to…so many decades of research, findings, hypothesis, etc The only thing that interests me is when am i/we are going to be (a bit) better? when are there tests and treatments?
Cort, all what is said in your article about the role of monocytes (cleaning up inflammatory sequels) makes you wonder if these findings may be a reflection of the other major biopathological feature of ME/CFS – viral reactivation (now again brought to the fore by Rosen´s supherb work: https://www.frontiersin.org/articles/10.3389/fimmu.2022.949787/full). Indeed, monocytes are geared to detect viral genomes by their TLR9 receptors and seem to participate in the innate immune control of herpesviruses like EBV ((https://pubmed.ncbi.nlm.nih.gov/26428378/)). This may be the reason why monocytes were shown to be activated in several autoimmune diseases also associated with viral reactivation, including systemic sclerosis ((https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-017-1237-9)). So why shouldn´t monocytes be central to the immune matrix in ME/CFS, too?
Great reply to a fascinating study. This is of particular interest to me, as I’ve had consistently high Monocytes for years, not high enough for GP’s to show any interest, but always at the top of, or slightly above range. This gives me renewed hope of a link, and i’ll be following with interest. Thanks for your interpretation, summary & write-up Cort, as always – very much appreciated!
Do you just ask your doctor if he/she can do a monocyte blood test?
Tracey E – Monocytes are generally part of a first-line blood test, or “general workup” where standard markers are looked at. I’m not medical, so only have personal experience on this, and have had many such tests over the years. I’m guessing there are are more in-depth Monocytes tests which could be undertaken by specialist labs, but within the NHS the scope is limited as to what GPS can request.
Very good thank you. Changing innate immune control of herpesviruses
TLR9 rs115440379
Build 37 52256621 Variants C or T my Genotype C / C
Build 38 52222605 Variants C or T my Genotype C / C
would de-methylation (crispr cas9) work?
@SWA – I have no idea about manipulation these pathways, sorry (I am not an immunologist). What appears plausible to me would be boosting the innate immune system the evolved way, i.e. I suggest “trained immunity” approaches. I have no idea if they work but they work in other immune disorders, and we therefore should try:
https://www.kinder-verstehen.de/wp-content/uploads/BCG_hypothesis_short_251222.pdf
very interesting thanks Herbert
Because Bruce Patterson opened his Long-Covid protocol to ME/CFS and post-Lyme, I decided to try it. Based on my cytokine blood lab result, Dr Patterson instructed me to take maraviroc, pravastatin and fluvoximine. I took it for 5 months but unfortunately did not have any improvement. He said my cytokine levels showed elevated cytokines due in part by “long term engaged macrophages” so this study does seem to line up. It seems there must be a drug already out there that can be used, such as is the case in what Patterson uses in his protocol, albeit not perfected yet.
Chronically activated macrophages – that’s what this study seemed to find. It’s nice to see two research groups coming up with similar answers.
Hi Jill,
I have both M.E. and type I diabetes and the M.E. was playing havoc with the diabetes. When test were done, I found raised levels of multiple stress hormones, along with Thyroid and Mitochondrial abnormalities. In addition to that I noticed that I was never 100% shifting infections even with normal courses of antibiotics. They just kept recurring.
I knew from experience that infections pushed up blood sugars, and this is well known to diabetic nurses. I formed the theory that failure to fully fight infection was causing this lingering stress hormone production.
The mechanism used by the immune system to trigger the Adrenaline production is a collection of five interleukins. And guess what? Those interleukins are produced by Macrophages.
They don’t only eat ‘bad stuff’ they are a key co-ordinator of other immune response. They trigger other immune cells locally via cytokines, and manage both stress hormones and febrile response via interleukins.
Their over activity however could just be a side effect of the immune system in general being ‘somewhat lazy’. They are supposed to stay active until any infection is 100% eliminated.
I would be interested to know how many of us have ‘insignificant’ levels of residual infection that drive this response.
This question is I think especially relevant for those of use with persistently high rather than low stress hormone levels.
I formed a theory some years ago that the levels were high earlier in the disease when it was milder and lower later with increased disease severity. I created an objective measure of symptom severity (rather than a 1-100 scale) and then tested the theory. I found people had a phenotype irrespective of severity.
I would generally expect more stress hormones to correspond with greater macrophage activation/
I watch with baited breath.
Further to the technical info above.
Those stress hormones affect ‘background insulin’ that is increased in illness. They do not affect the insulin needed with food. I.e. if you eat more of less food, the change in Insulin remains the same.
With I.V. antibiotics for severe infection two things happen. My insulin returns to pre-ME levels. The M.E. symptoms disappear with no diet, nor supplements, minimal rest.
This I think speaks volumes.
I hope and pray some treatment will come from this! In 2015, my doctor started me on a preparation called NK Immune — now called Ai/E10 — an immune REGULATOR (not a booster). It made a big difference for me; after just two weeks, I could see a difference in my functionality. Although I am still prone to relapse, I am still fairly functional, as long as I take this preparation every day. I still wear out ‘way faster than others, and must pace myself to quite a degree, never scheduling much on any given day. Notably, if I cannot obtain a new bottle of Ai/E10 before I’ve completely run out, I relapse within days. So I’m really living on the edge, absolutely dependent on this preparation to keep me functional. If an immune regulator for this monocytic abnormality were available, I might gain even better functionality — or (dare I say) experience wellness for the first time in two decades! You can bet I’ll be passing this information on to my practitioner so that he can share it with the makers of Ai/E10!
Hi Chris- Would you mind sharing what brand of Ai/E1 you’re using/source/dosage? (I see more than one on google).
Also is your doctor an ME specialist or naturopath or ?
Wondering where she/he got the idea to try this product so I could share with my doctor.
Thanks so much!
Hi Chris, I too would love to see the information Birdie requests. Maybe you will see this somehow.
TY for sharing this. I have been looking into the BodyWise version from David Bergsma, co-developer of Ai/E10. I understand this to be an immune modulator (up when you need it and down when you don’t.) I am very worried to herx, have an allergic reaction, and be bedbound again. Based on your experience, do you find it to be well tolerated? Did you get an overactive response or not?
Where did you get it?
Good article and interesting study. Monocytes are know to sustain chronic inflammatory conditions, of which ME/CFS is one. This might be a significant study.
Now what is causing this, and how is it resolved (or at least mitigated significantly)? Do the authors hypothesise on causative factors?
They really don”t. Instead, they’re just going to dig deeper. I added this part after I published the blog. It’s from Grimson – the senior author of the study.
“This work sets up lots of questions that motivate our work now – where are the monocytes going in ME/CFS individuals, what is causing them to be dysregulated, and ultimately, can we reverse this dysregulation?”
Thanks. Well let’s hope they / other researchers move quickly. As said above, we need answers sooner not later. Surely not too much to expect in our scientifically advanced times?
Monocytes are linked to food allergies, interesting. I had lots of food allergies early in my illness, as I got better the food allergies subsided. I always thought that this was not a coincidence.
https://www.science.org/content/article/blood-cells-could-determine-whether-kids-get-allergies
Has any one else experienced this? Bad allergies early in the illness, that then subsided in parallel to overall improvements in condition?
I’ve experienced it in a way, but since I’ve only gotten worse I started out without food sensitivities and now as ME symptoms are worse I have developed significant histamine sensitivity.
My frustration with all of these studies is that they keep finding new things that are messed up or a new potential “culprit” in ME/CFS. This means that they are not asking WHY things are messed up or testing potential solutions. Instead, they find an issue and then ask more questions and look for a bandaid to fix the results of the problem they have found.
Yes, they are really in the discovery phase. The difference with this study and others like it is that they are large, rigorously done, and are using new better technologies – so hopefully we and they will get followed up, and we’ll really get somewhere.
I’m not convinced by that statement made in the article. I was in an nk function study over 10years ago and it had from memory I think 100 mecfs patients and 50 or so healthy controls, done over 18 months. Similar size groups also done, which was by the Griffith University group. It was obvious in the study I was in that low nk function could be a biomarker used to make a diagnosis. But nothing comes of it, also no real attempt at trialling a drug to improve nk function and cd8 t cell dysfunction they also found. So to say they are only small studies done in this area is to discount the work of Griffith university in Australia.
I guess theoretically a finding like this can help lead back to ‘the why?’ But I agree it’s frustrating. We have had a lot of it over the past 20 years…
I sympathise Chris. I often feel the same.
I find it interesting that they have found a link with inflammation.
So my mind goes to “What is causing the inflammation? What is the solution?”
I recall Dr Jared Younger talking about inflammation in the brain and saying that he didn’t know what is causing the inflammation.
I follow Doctors trained in functional medicine who say that 2 major causes (there are others) are stress and nutrition.
When I had ME/CFS, I was super stressed and I don’t doubt that was driving all sorts of biochemical changes.
As Cort says, much research is in the discovery phase.
I believe that there is a need for multidisciplinary teams, where scientists of different disciplines talk to each other, so that they can join up the dots.
The solutions may exist outside one scientist’s area of expertise.
This is promising, as I have always had sky high monocytes since getting ME. Let’s hope this paper leads to more research and a fast discovery of the causes/solutions.
Why are monocytes so aggressive, hyped? Have you looked at chronic stressors patients were exposed to?
We showed our poster session paper at the IACFS/ME conference with our Biomarker research.
Someone will figure ME/CFS out. This is the new theory of the week – ask me in a year if it panned out.
Can’t afford the adrenaline surge that comes with each new ‘hope.’
Hope my tax dollars supporting research eventually benefit me, preferably PRE-mortem.
🙂 The hopeful thing for me is that the study was large, contained ME/CFS patients from ME/CFS experts, and used a better technique: on the basis of that I hope it really got somewhere! Time, of course, will tell!
Those are hopeful points, Cort. I agree.
I hope they continue. I hope they find something usable, especially for treating those of use who have been ill for decades (never a given), but time will tell is the only way to look at these things – because ‘truth’ whatever it is is the only solution, and likable features in a study prove nothing (except that those features were now not ignored, if they have been in the past.
I tell other people that we now have hope, all the while trying not to expend ANY of my extremely limited energy on hoping. I just can’t afford what it costs.
Surprise me, instead, with a treatment and cure, one that works for haulers far longer than long-covid people infected no longer ago than 2020. Prove it. Make it available to ME. (Not the stupid name – me as a person). Make me better – so I can have the other treatments which require energy to survive, such as me surviving surgery to walk again.
Sometimes I have to turn my back on it just to be able to live through each day. Then I might have a bit of my life back.
Meanwhile, I take care of myself by writing fiction with an ME/CFS main character – it takes forever, but it is the very best quality I can produce. AND I have some control over my own life.
Is fixing monocytes now the thing to cure ME? Is this what this study means?
What it means to me is that they have a new immune factor to dig into. Given the lack of help that the past immune factors have been, that’s pretty good news to me. Maybe this one will make more of a difference.
can anyone tell me if this “Macrophage inflammatory reaction is the same pathway that Rapamycin down regulates?
I have a very proactive Dr who has started me on a 12 week course of low does Rapamaycin….. its been 6 weeks and am starting to actually exercise !!! .. 3 years ago I was med/severe CFS, bed bound and house bound on the good days. with a huge amount of trial and error with the usual supplements and everything else we are all very familiar with I got my self way less bed bound and can work for a couple of hours most days. but since this Rapamycin course I have been getting some very nice energy days, I try not to push and crash but have surprised myself by slowing adding some exercise into my day… with no PEM (so far) … I’m interested to know if the Rapamycin is actually helping via the same pathways as this article talks about..
Congratulations, Danny and good luck! if I’m reading this study right it seems that it might.
https://pubmed.ncbi.nlm.nih.gov/28489580/
“our results suggest that rapamycin negatively regulates macrophage activation by restricting a feedback loop of NLRP3 inflammasome-p38 MAPK-NFκB pathways in autophagy- and p62/SQSTM1-dependent manners.”
It appears that Rapamycin may be able to tamp down macrophage activity. Interesting!
Of course we have Jeremy’s Rapamycin story
https://www.healthrising.org/blog/2022/07/06/apamycin-resurgence-doctor-chronic-fatigue-syndrome/
Very interesting.
STUDIES PLEASE.
Danny, could you tell us more about the scheme (doses) of Rapamycin? Do you and the doctor monitor the concentration of Rapamycin in the blood with the help of analysis and so on? I will be very grateful for your answer
This is great to hear, Danny. Dr. Levine has prescribed rapamycin for me, but I am waiting until after the holidays to start it because it’s immuno-suppressing.
metformin also inhibits macrophage activation (https://diabetesjournals.org/diabetes/article/64/6/2028/34894/Metformin-Inhibits-Monocyte-to-Macrophage), as do ketogenic diet and fasting (https://www.cell.com/cell/fulltext/S0092-8674(19)30850-5)… these findings by Hanson et al. could explain why these interventions help improve symptoms for some people…very exciting news. Thanks for sharing Cort.
Interesting. Metformin has certainly come up in fibromyalgia
https://www.healthrising.org/blog/2019/05/11/fibromyalgia-diabetes-metformin-chronic-fatigue/
Matthias, in response to your question, I had food allergies early on in my disease. Over 37 years both my symptom severity and my allergies to foods has gotten worse. I may be a “universal reactor”. I wonder if monocytes could be causal or implicated? I have a strong suspicion that if my reactions to foods decreased, so would the severity of my CFS/Fibro symptoms. However, it is difficult to impossible to not eat offending foods when I tend to have negative reactions to nearly all foods. TY again Cort – for the great article summary.
Hello from France, and thank you Cort once again for this great research , which seems highly relevant, in my view .Like Matthias and Dave I have had periods of food and medication allergies/ sensitivities , bad initially , improved for some years when taking various antibiotics ( macrolides mainly ), but since covid vaccinations + actual Covid much worse again, and like Dave seem to have negative reactions to all foods.
Interesting that Rapamycin seems to damp down macrophage activity , and I wondered if anyone had tried alternative supps ? For example : EGCG, green tea or anything else ? I am getting desperate as need surgeries and I am considered ” high risk ” here as cardiac issues cannot be treated.
Any suggestions welcomed , MCAS queried , but I even have problems with anti-histamines , can take infrequent low doses. Thank you in advance ….
Just a thought ; does anyone take metformin , as I know it can help some people at low doses ? Whilst researching equivalents to Rapamycin ,I found some research titled ” Towards natural mimetics of metformin and rapamycin ” PubMed 2017 , and EGCG seemed to come out strongly so I am tempted to try it . I do drink green tea as good for the heart so perhaps stronger doses ( if tolerated ) might help & seems a no-brainer !
I think berberine is often mentioned as an alternative to metformin. Dr. Brad Stanfield on youtube does a lot on anti-aging supplementation and digs into a lot of research on these topics, you might want to check out his channel. GL
I started metformin about a month ago because long COVID made me pre-diabetic. It’s definitely too soon to say, I think, and I don’t feel any better physically, so I’m not sure.
Thanks Rick , I will check out Dr Stanfield on YouTube. I do have some Berberine having taken it before, so probably worth trying again .
A pre-print study from the Netherlands has found evidence of activated monocytes in long covid patients with severe fatigue. And the study mentions ME/CFS.
It’s still a big ‘so what’ for me. We have been aware of immune activation in the disease for a long time. What causes it and more importantly what can calm it down? What has happened to Klimas’s trials?
Also is the result of more monocyte activation that startling? It occurs in many inflammatory and autoimmune conditions.
It seems to me pretty clear by now that ME/CFS is a chronic inflammatory condition. Surely existing anti inflammatory treatments should be trialed more?
Nice! At least 3 studies – ha!
Je suis perdue entre les immunosuppresseurs et les immunomodulateurs. C’est peut être une question idiote, mais , par exemple, dans mon cas, je n’ai ,pas assez de cellules T, et celles-ci ne sont pas assez performantes. Est-ce que le système immunitaire ne vas pas s’affaiblir encore davantage avec des immunosuppresseurs?
Ne vaudrait t’il pas mieux de s’orienter vers des immunomodulateurs?
Dites moi si je dis n’importe quoi…
Translation
“I am lost between immunosuppressants and immunomodulators. It may be a silly question, but, for example, in my case, I don’t have enough T cells, and they don’t perform well enough. Won’t the immune system weaken even more with immunosuppressants?
Wouldn’t it be better to move towards immunomodulators?
Tell me if I say anything…”
This – whether to boost the immune system or suppress it or boost parts of it and suppress other parts – is a very tricky question…and I would guess depends on the patient.
Merci Cort d’avoir pris le temps de me répondre.
Thanks for your input Amy , just hope you do improve very soon , so much trial and error involved ..
Agree withMatthias that we are fundamentally dealing with a chronic inflammatory , probably autoimmune condition & there must be some appropriate anti-inflammatories out there , ready to be trialed. LDN helps some people I know , now being trialed for long Covid although it has been around for ages . I keep trying at miniscule doses , helps over a short period then a big reaction and return of old symptoms so I am trying again and pulsing more this time . I believe Jarred Younger has talked about a new improved LDN , does anyone know about this ? He really does good work , trying to help with treatments .
In the 1980,s I had IVGG with spectacular results but not maintained over the years alas . I believe that is also being trialed for Long Covid now .
Claudine , je vais essayer de te répondre prochainement , mais entretemps je te souhaite Bon Courage , surtout si tu es en France , pas évident ou facile ici !!
Yes a chronic inflammatory condition, possibly autoimmune. I am thinking more and more that other abnormalities, eg. Mitochondria, are secondary to the chronic inflammatory disease state.
I am also firmly of the view, and have been for a long time, that a chronic virus is not at play, hence antivirals are a waste of time and research resource. I would like to know from Cort about Nancy Kilmas’s trials, I though she was trialling some sort of anti- inflammatory treatment regimen?
I suspect that we will be like other inflammatory / autoimmune conditions in that a very specific causative factor and factor that perpetuates the disease is elusive. Hence the focus should be on anti inflammatory treatments that significantly improve symptomology, rather than ‘cure’.
Yes ! “The subclinical presence of mitochondrial diseases and metabolic disorders could be a critical factor to take into consideration when prognosis and therapies for cancer, infections, and autoimmune diseases are clinically discussed or vaccine efficacy is evaluated. ”
Mitochondrial damage from toxins of all type
Agree 100% and definitely relief of symptoms is a priority , ie reducing the chronic inflammatory state . I know I have mito dysfunction plus other secondary systemic problems, and finding the cause(s) may remain elusive for some time but I do feel that infections were the trigger in my case , but that is not universal .
Would also love to learn about Nancy Klimas’s trials ?
Yes! Excerpt : “The subclinical presence of mitochondrial diseases and
‘
metabolic disorders could be a critical factor to take into
consideration when prognosis and therapies for cancer, infections,
and autoimmune diseases are clinically discussed or vaccine efficacy
is evaluated.”