

This University of Colorado/ National Jewish Health Center study, “Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post-Acute Sequelae of COVID-19 (PASC)“, follows on the heels of another study published earlier this year that highlighted fatty oxidation and lactate.
That study found that all 50 of the long-COVID participants produced significantly lower levels of fatty acid oxidation. That meant that a key source of the electrons needed to produce energy in the mitochondria was impaired. It also happens to be the same problem that recent studies have found in ME/CFS.
Increased levels of lactate early in the exercise test, however, suggested that their anaerobic energy production system had kicked in early. They proposed that the lactate increase might result from a switch in the production of muscle fibers from slow-twitch muscle fibers to fast-twitch muscle fibers. A similar finding showed up in ME/CFS back in 2009.
Slow-twitch muscle fibers – which we use for things like walking – use aerobic metabolism to produce energy. Fast-twitch muscle fibers – which we use for short bursts of energy like weightlifting – use anaerobic metabolism.
Now they’ve returned with a rare (thus far) metabolomic study of long-COVID patients. The study – which was relatively small – contained 29 non-hospitalized long-COVID patients, 16 people who had recovered from COVID-19, and 30 healthy controls. The metabolomics analysis was “untargeted”; i.e., they looked at as many metabolites as possible and analyzed what popped up.
Their goal was to see if their metabolomic findings matched up with their earlier findings; i.e., did they indicate, on a molecular level, that problems with fatty acid metabolism and lactate production were present?
Results
“These results, which, to our knowledge, provide the first characterization of the plasma metabolome in individuals with PASC, indicate several major metabolic derangements.”
They did. They even called their findings “derangements” – a word that packs some punch. A look at some of the study graphs shows dramatic changes indeed.
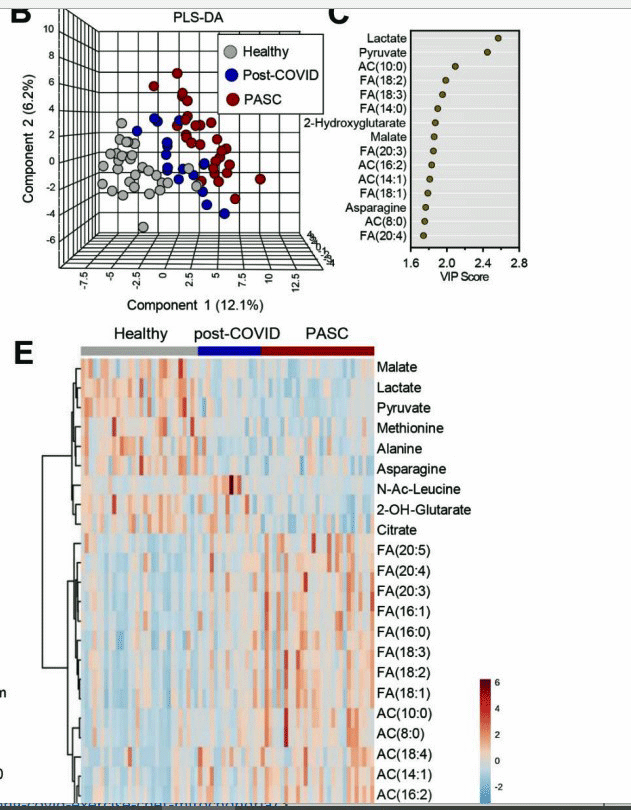
Check out at the top how highly segregated the long-COVID patients (PASC – red) are from the recovered long-COVID patients (blue) and the healthy controls (grey); then see the dramatic shift in fatty acid levels (bottom figure) in the long-COVID patients (bottom right- red) as well as lactate, pyruvate and others at the top.
They stated that the “results of this plasma metabolomics study… support the hypothesis that a dysfunction in substrate utilization in mitochondria underlies the metabolic manifestations of PASC.” The key phrase here is “substrate utilization”. We’ve seen similar phrases used again and again in ME/CFS as study after study has found that people with ME/CFS are not using high-energy “substrates” like glucose and fatty acids to power their mitochondria. Instead, they’ve turned more to inefficient substrates like amino acids to feed their mitochondria. Given that, it’s no wonder that energy production is impaired.
They found higher levels of plasma carnitine-conjugated and free fatty acids in the long-COVID patients. The carnitines and other fatty acids provide quick and efficient food for the mitochondria, but in this context, high levels are not good because it means they’re not being broken down and being used by the mitochondria. Interestingly, this pattern has shown up early in COVID-19 and seems to persist in the long haulers.
This went along with reductions in multiple amino acids, including branched-chain amino acids, suggesting that the people with long COVID were, as appears to be happening in ME/CFS, using amino acids to produce energy.
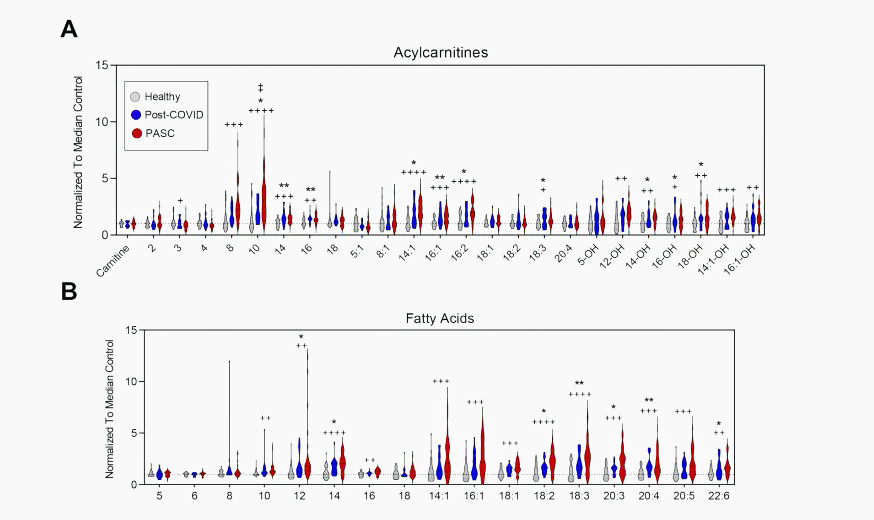
Again, look at the dramatic changes in the levels of the acylcarnitines and fatty acids in the long-COVID patients (red) compared to the other groups.
Not only is a valuable energy resource not being used but higher than normal levels of just about anything can have consequences, and so it is with these fatty acids. The authors speculated that they could be triggering red blood cell dysfunction – which could impair oxygen delivery.
The Gist
- A University of Colorado/Jewish Health Center team jumped on long COVID early and in mid-2020 began having their patients do exercise tests.
- That resulted in an earlier paper that found significantly lower levels of fatty acid oxidation in all 50 of the long-COVID participants. Because fatty acids provide an energy source for our mitochondria, problems with fatty acid oxidation or breakdown could help explain the fatigue and exercise problems in long COVID.
- Their goal in their latest study was to see if their metabolomic findings matched up with their earlier findings; i.e., did they indicate, on a molecular level, that problems with fatty acid metabolism and lactate production were present?
- The study did – to the extent that the authors referred to the “metabolic derangements” found. The study found evidence that fatty acid metabolism was impaired and the long-COVID patients were turning to less efficient amino acids to power their mitochondria.
- They also found evidence of problems with taurine and tryptophan metabolism.
- The authors stated that “compelling evidence of metabolic dysfunction in PASC (long COVID)… that should fuel future investigations of oxygen and lactate kinetics and mitochondria biology. Interventions aimed at restoring promoting mitochondrial activity and restoring fatty acid oxidation should be explored.“
- Then, ignoring the dozen or so ME/CFS studies that have found similar metabolic issues, they turned to sepsis and type II diabetes to explain how their findings could help explain exercise intolerance.
- Interestingly, similar findings in fibromyalgia (FM) have recently shown up.
- ME/CFS with its bigger, more complex metabolomic studies is ahead of the game and several causes (peroxisomal dysfunction, hypoxia, mitochondrial problems) have been proposed.
- With the exception of some ME/CFS studies, most of the studies have been small and these findings need to be validated. Still, they suggest that core metabolic abnormalities may be present in long COVID, ME/CFS, and FM.
After noting several limitations – small sample size, and a lack of stratification on gender and age – the authors stated that their data offer:
“compelling evidence of metabolic dysfunction in PASC… that should fuel future investigations of oxygen and lactate kinetics and mitochondria biology. Interventions aimed at restoring promoting mitochondrial activity and restoring fatty acid oxidation should be explored.”
The Chinese Study
The Colorado study came right on the heels of a Chinese long-COVID study that produced similar findings. Its findings (increased levels of fatty acid metabolites) and its outcome, “Our findings clearly revealed that their general metabolism is still in a state of disorder and exhibited abnormal fatty acid and amino acid metabolism”, could have come right out of an ME/CFS paper. The Chinese study also found evidence of suppressed tryptophan metabolism (low levels of kynurenine, arginine, and tryptophan).
Frustrating
It was frustrating to see the Colorado authors ignore the now substantial metabolomic literature in ME/CFS that’s shown similarly impaired fatty acid metabolism and carnitine issues, and turn to sickle cell anemia, type II diabetes and sepsis as examples of diseases with similar findings that exhibit exercise intolerance.
A quick review found ME/CFS studies dating back ten years have used different techniques in multiple compartments (cultured cells, the mitochondria, metabolomics (Che, Nagy-Syzkal, Fluge, Hoel, Germain, Germain, Germain, McGregor, Armstrong)) to come to essentially the same conclusion: a substrate shift away from using fatty acids/glucose toward amino acids has occurred that is affecting energy production.
While fatty acid metabolism isn’t the end of the story for ME/CFS the conclusions, particularly from the more recent studies, are refreshingly consistent.
“Peroxisomal β-oxidation of very long-chain fatty acids leads to their breakdown into short-chain products that serve as substrates for mitochondrial β-oxidation. We posit that (peroxisomal) dysregulation contributes to the fatigue and cognitive dysfunction that are hallmarks of ME/CFS.” Che
“Some changes were common in the patient group, and these were compatible with … altered utilization of fatty acids and amino acids as catabolic fuels.” Fluge
“Some changes were common in the patient group, and these were compatible with effects of elevated energy strain and altered utilization of fatty acids and amino acids as catabolic fuels.” Hoel
“Carnitine is an ammonium compound essential for fatty acid oxidation, and both “oxidation of branched-chain fatty acids” and “carnitine synthesis” were affected in ME/CFS patient pathway analysis.” Germain
“In our study compounds in the choline-carnitine pathway were decreased in ME/CFS patients. Our results are consistent with earlier reports that suggest that metabolites linked to lipid and energy metabolism are affected in ME/CFS. Nagy-Szakal
Noting that this metabolomic study was done at rest, the Colorado authors asserted that future studies should examine the effects of exercise on fatty acid metabolism. The authors missed a metabolomic ME/CFS exercise study that did just that and came to a conclusion – “The recovery period of participants with ME/CFS is highly disrupted compared with healthy controls” – that would have supported their call for something similar in long COVID.
Fibromyalgia as Well?
A similar signature (including problems with carnitine synthesis, lipid oxidation, and tryptophan metabolism) has shown up in several small metabolomic fibromyalgia studies. The latest one highlighted “lipid and amino acid metabolism networks“. A mitochondrial study suggested that problems metabolizing fatty acids are present as well in FM.
Exciting
Still, these studies – many of which are admittedly rather small – provide preliminary evidence that some core abnormalities may be present at the molecular level in long COVID, ME/CFS, and fibromyalgia. That would be a remarkable thing given their different triggers and one wonders if other post-infectious illnesses will join the crowd. What we now need are bigger studies that can solidify these findings (if they turn out to be accurate) in long COVID, ME/CFS, and FM.
Metabolically speaking, the ME/CFS field, at this point, is way ahead of the long-COVID and FM fields, with recent studies even fingering a possible culprit in the cell – the peroxisomes. The authors of the Colorado study did not propose a cause, but several have been proposed in ME/CFS including peroxisome dysfunction, tissue hypoxia, and mitochondrial problems.
BIG (Little) Donation Drive Update







The tryptophan metabolite 5-HTP has worked very well for me. It gives me severe stomach pain that precludes me from taking it orally, but if I put the powder under my tongue for 10 minutes or so, the energy and mood lift I get is significant.
500mg of 5-HTP, white powder, under the tongue once in the morning and once in the afternoon. If I do it after 3 pm I won’t be able to sleep.
Buy the pure white powder from Bulk Supplements or Pure Bulk. Put 1/4 tsp under your tongue and leave it there for 10 to 15 minutes. Then spit it out and rinse your mouth. Again at 2 pm.
Did anybody try Taurine supplementation ?
Absolutely. 1,5 mg gives energy boost after 10 minutes. My wife has cfs for 25 years and has the best results with Taurine.
Sorry, I just went to put some in my mouth and looked at the measuring spoon. It is one-eighth of a teaspoon, not one quarter of a teaspoon. No side effects either way, but I settled on 1/8 after some experimentation.
thank you!!!
is it for ME/cfs or long covid? what did it do for you if i may ask? thanks!
Yes, konijn, it’s for ME/cfs and long covid. It gives me energy and better mood. Go to Bulk Supplements and Pure Bulk and compare their prices for a small amount of pure powder and give it a try. It’s cheap and easy to find. You can get it at any health food store, but it will be more expensive than the bulk sources online.
Dr. Tate in New Zealand is planning on administering MitoQ to Me/CFS patients. Has anyone ever taken this supplement, and how does it function biochemically?
1 Have taken Mito Q every day for 2 years and have not found any improvement
OK, so we have two different Ann’s here. I am the one at the top of this comment thread. The second Ann begins with the Mito Q post. I’ve never tried Mito Q. Has anyone had any success?
I tried MitoQ some years ago and it made me feel worse so I stopped taking it. Its very expensive in NZ. I have had moderate ME/CFS now for 34 years. At the moment I’m house bound but not bedridden.
I always wonder how much of these changes in the metabolome of LongCovid, ME/CFS and fibromyalgia may have to do with the other “known” of these disorders, namely viral reactivation. Viruses are known to hijack the metabolism of the infected cells so that they can replicate. This reprogramming process of host cells (and their counter response) especially affects how fatty acids and amino acids are being utilized (https://mediatum.ub.tum.de/doc/1519207/1519207.pdf). So could the metabolic changes described possibly be a reflection of the ongoing metabolic battle between host and endogenous viruses?
Interesting. This makes sense and provides the connection we’re looking for. Thanks for sharing.
This isn’t the first time we’ve heard that we’ve heard that long chain fatty acids are underutilized with CFS, but it continues to be a distressing concept. For most people, foods like salmon, olive oil, and nuts are emphasized as mainstays. The entire Mediterranean diet rests on long chain fatty acids. I’ve stopped taking fish oil due to studies like these, but I really have no idea how to eat. Fruit and veggies are still okay, but in terms of healthy proteins and oils I’m confused.
I’m going to wait. I’ve tried so many variations of diets none of which has really done anything significant that I would be shocked if diet was a major answer.
I’d say, listen to your body on what foods you do well with. I’ve seen some patients say that keto diet with lots of fats helps them, I on the other hand do not tolerate fats well. (I might still try if digestive enzymes help, but usually just gravitate towards proteins & veggies.) There’s nothing wrong with trying out diets als long as you have the energy to prepare them. But everyone’s an individual and may not react the same. I don’t think we sufficiently understand the complex processes in the body that determine what food agrees with whom (probably including the composition of gut bacteria). I believe your own body should have the last word more than the theory behind a diet.
Stuck Keto out for 6 weeks in that time I lost all my energy and was couch bound. Within 48hrs of reverting to a diet with a lot more carbohydrates in it I was back to my normal level of energy for me.
:). Isn’t that something. Keto didn’t do that for me – but it also didn’t help me; I was surprised!
Thanks for your reply. May I add that when I am more unwell, I also don’t do well with carbohydrates 😀
I have finally digested Dr. Systron’s work. Much of it makes a great deal of Physiological sense. I was never able to make the connection between POTS and mitochondrial dysfunction. Dr. Tittlebaum often asks his patients what one drug they would bring with them to a desert island. The response is usually “Klonopin”. That may be because ME/CFS patients are hyperventilating. The autoimmune small fiber neuropathy of the those nerves responsible for vasoconstriction is causing blood to pool in the lower extremities. ME/CFS patients hyperventilate to compensate. This causes the pH of the blood to rise. Blood is already alkaline to begin with. An increase in alkalinity will inhibit oxygen intended for the mitochondria from properly entering the cell. I know that in the past many ME/CFS were dismissed by Neurologists, because their blood gas data came back as showing “hyperventilation”. Therefore, most Neurologists dismissed ME/CFS patients as having a “Psych problem”. When in fact, they have an autoimmune condition preventing adequate blood quantities of blood from filling their atria.
The ‘only’ drug that has ever given me any relief of my symptoms in a major crash is Ativan, just like Whitney Dafoe. I think Klonopin is in the same class of drug…
I thought it was Abilify that gave Dafoe some relief? Maybe he took Ativan as well, which is also a benzodiazepine like Klonopin, but Abilify is an antipsychotic drug.
It’s important to remember how devastating long term benzodiazepine use can be for so many people. There are countless stories of lives wrecked by this class of drugs, including some on this blog. Calling Klonopin a “desert island drug” implies that it more common and safe than it really is in reality.
Whitney does take Abilify but he also takes Ativan once a month, especially when he needs to go to the hospital for a procedure.
Are we any closer to TREATMENT? Money spent on reinventing the wheel a tiny bit more precisely is infuriating, not frustrating.
Science is supposed to BUILD on prior science – or PROVE it wrong, say why, and fix it.
And ignoring ME/CFS longstanding results is arrogance. I’m really tired of that arrogance.
It is frustrating to see studies not taking note of MECFS findings. (grrrr! I’m going to email them by the way.) At least the findings seemed to match up and that’s potentially really good news in part because we’ve made real progress in that area.
With regards to treatment – the authors did point a finger at some types of treatments – but what we really need are big studies that can say with some finality – yes (or no) fatty acid metabolism and/or peroxisomes and/or lipid metabolism is a major player – resulting in a major focus on that and treatments to help with it.
The ME/CFS field has certainly cleared a path for something like that to happen more quickly than it would otherwise.
I hope the RECOVER Initiative is taking a close look at metabolism. That initiative is designed so they don’t have a bunch of findings that don’t get followed up on.
Oh, duh. I just suggested that you email them and here it says you plan to do so. So much for being able to even READ tonight.
thanks for writing them!!!indeed over and over inventing the wheel does not help us further. also not only studying long covid and not looking at ME/cfs, FM, …
I was and am still afraid, long covid has the money… we do not… so even if they find same things in long covid, what do we have from it without money for really big studys?
and yes, i/we want treatments!
Very glad to hear Cort, that you’ll email the authors pointing out the ME/CFS research they’ve ignored! It shouldn’t be up to us (tired) patients, to point these things out to researchers, but I have a feeling that its the only way the folk jumping on the LC band wagon, will take notice of it. Thanks for your work on this!!
I just contacted the lab -said how frustrating it was and then laid out our findings. It was actually encouraging – they have quite a lab there! Good to see them get engaged in this. If they answer I’ll report back.
I can’t tell you how much I agree with you! Yes, long covid got some new studies done but we deserve better! We deserve more!!
It isn’t necessarily arrogance. It could just be that they didn’t look for it or have there grad students look for it. I used to miss things too back when I did science and published things. There is so much out there that it is impossible to find everything, even with the internet. Granted, it seems that a cursory search would have turned up the ME/CFS connection, but maybe not.
I’m planning to see Dr Systrom. After seeing him explain POTS and the cause being either nerve or mitochondrial, I finally realized he sees the two sides of the same coin. Dysautonomia International and OMF should merge haha!
I’m anxiously awaiting his study results on the drug trial for the mito patients
may i ask, was it a webinar that you sawfrom Dr Syström? can you please give me the link? thanks!!!
https://www.youtube.com/watch?v=iL-B6mbEs9w&t=2756s
thank you!!!
Hi Cort, This is very exciting! Maybe you could educate the researchers a bit and let them know there there is some other info out there they can build on. I used to do research (before CFS) and it is hard to find everything about any given topic, even with the internet. And most of the big wigs aren’t looking – they leave that to their grad students. I know always appreciated it when someone brought a journal article to my attention if I had missed it. There are so many journals now that no one researcher can keep up with everything in their field.
I don’t quite understand all of the science, but I’m wondering after reading this if I should stop taking the l-acetyl-carnitine I started taking after reading Dr. Teitelbaum’s book?
Also, in working with Lorrie Rivers, she talks a lot about how a key part to her recovery from ME/CFS and later long COVID involved major dietary changes, and in particular, eating high quality animal protein (which I believe is high in taurine). I’m a pescatarian, been off red meat and pork for 30 years and poultry for 20. Tried eating turkey at thanksgiving and had to spit it out; it tasted metallic and unpleasant to me. I am so confused about the right diet here. I have both long COVID and ME.
How does it feel for ME patients who now also have long covid? Are the symptoms the same or is there a worsening of the ME disease?
How do you recognize the difference? How do you know for sure that you have these 2 diseases?
I had long COVID first. My ME was diagnosed at the six-month mark, because my manifestation of LC presents as pretty classic ME, but with some additional symptoms that are not characteristic of ME. That is why my doctors consider me to have both diagnoses. I know many COVID long haulers who have awful, debilitating symptoms that are not lined up with ME, so I do still believe these are two diagnoses.
But for those I’ve spoken to who have ME first and then develop LC, it seems that their ME symptoms get worse and they gain a host of new, bizarre symptoms that are characteristic of LC.
I don’t think it will hurt to continue but my son got a lot better when he started to supplement with animo acids and Recovery Factors (pig peptides ie: animos) and started eating meat again. I suggest you start with some bone broth and add in small amounts of free range chicken, eggs and grass fed meat as often as you can tolerate. Turkey has less whole protein. We stopped using carnitine and fish oil after a while as they didn’t seem to make any difference and found turmeric was more useful. As for short chain fatty acids, you have to produce these in your own gut so perhaps look up what foods encourage the production of butyrate via gut bacteria. If you want a healthy medium chain fatty acid suggestion, coconut oil is wonderful to cook with, it’s also very popular among those on a keto diet.
Thanks so much @Christobel; this was very helpful. Funny you should say start with bone broth. I said the same thing to my husband a few nights ago. And I do have low butyrate, so I’ve been focusing on foods that increase butyrate. Good suggestion about the coconut oil as well. Thanks again.
I still think we should very carefully look at every step of the chain of custody of both oxygen molecules and glucose molecules, from transport in the bloodstream until both are in the cell.
My heretical hypothesis suggests an autoimmune attack against a protein responsible for carrying one of the two, with an unrelated hapten on the binding site, in the bloodstream.
The paper from that group at a medical center down the street from Yale, back in February 2022, which said the oxygen is in the bloodstream but not being taken up into the cells continues to nag at the back of my mind as a key piece of data, however anecdotal. The paper itself was behind a paywall I couldn’t get past, so all I have to work on is what was reported in the news at the time that paper was released.
Diabetes is a disease of metabolic ‘derangement’.
Cancer metabolism = high lactic acid.
So metabolic ‘derangement’
Mental health/disease = a metabolic derangement .
What state of ill health isn’t a metabolic/mitochondrial problem?
Thyroid has a very direct function in the body’s metabolism. So does vitamin D. So does eating sufficiently [calories, proteins, nutrients and carbs]. Etc, etc, etc. Understanding these points provides a way towards treatment and health.
It may require one to investigate what does eating for health really looks like.
Does anyone know how the theories about blood microclots might fit with the fatty acid oxidation and peroxisomal dysfunction?
Interesting what Herbert Renz-Polster said about viral reactivation being the possible cause of the metabolic dysfunction in an earlier comment. Could that also cause blood clots?
Thanks for another informative and hopeful article Cort!
Thanks Cort for always keeping tabs on the latest science on LC and ME/CFS.
To all who benefit from this blog by cort…please donate to cort so he can feel comfortable and continue to help all of us with information. He has CFS/ME also. I donate to cort monthly which is great. Not so much as one time. He’s our information Angel. Sincerely, hipjaven
I think? it was Dr. Komaroff that said recently that the hypothalamus is ground zero for me/cfs. I looked in google amino acids and hypothalamus and it said that the hypothalamus decides how the amino acids should be distributed to the different parts of the brain. If the hypothalamus is not functioning that well it makes sense extra amino acids could possibly help. My daughter has found the Dr. Brooke Goldner’s plant based diet (75% kale, spinach, swiss chard) helpful for her symptoms. She has stuck to this diet when she did not stick with others because it helps her pain.
In going through documents (actually looking for something else) I found this Review on Chronic Fatigue Syndrome written by Dr. Paul Cheney in 2013.
Since he saw 12,000 patients, I believe he knew more about his than anyone.
Chronic Fatigue Syndrome – A Review
Paul R. Cheney MD, PhD
February 2013
Prevalence estimates of Chronic Fatigue Syndrome (CFS) range from 0.5% to 3% of the US population and the exact number is very sensitive to how one defines it, especially severity. That is 2-9 million patients in the US alone and three times that number in the first world. This is far higher than HIV or type I AODM combined. The illness tends to relatively spare the young and the old which is very odd for any illness that can present as a point epidemic. The average age of CFS patients has been steadily climbing as we have been following these patients over time and consistent with an epidemic of CFS beginning around 1980 or slightly before. Two studies (N > 500 each) of case production curves over time done at two points in time five years apart yielded the same initial rise in cases around 1980 and the same peak production year of 1987-88 for CFS, just ahead of the first Gulf War in 1991. Of added interest is that the first Gulf War appeared to produce an illness among veterans of that period indistinguishable from CFS (Gulf War Syndrome) whereas the second Gulf War did not. In 1987, the average age for CFS was 38. By 2005, it was 49 suggesting we were following a point epidemic moving over time. Two-thirds of adult CFS cases are women but among pre-pubescent CFS cases, males and females are equal. New cases of CFS still appear, especially in the younger cohorts from puberty to age 40 but not nearly as many as were being produced in 1987-88. During its peak production point, in the late 1980’s, 10% of families with a CFS case in the published Lake Tahoe cohort of 256 cases had more than one member sick. 90% of those who got sick and were disabled with CFS in the Lake Tahoe epidemic had returned to work or school 10 years later while 10% remained disabled.
My practice tends to collect those CFS cases that do not improve and I have patients who have been in my current NC practice for over twenty-five years since the late 1980’s. 90% of my patients are disabled and cannot work full time. My sense is that while many can and do improve, sometimes with little or no intervention, they are never the same as they once were and are at risk for severe relapses if put under significant stressors. About 10% of my practice are really quite ill and largely homebound and/or bed bound. About 50% need assistance from others for activities of daily living. CFS patients compare well using validated disability scores with the most disabling of all illnesses, namely chronic heart failure, to which they are actually related by cardiac output measurements and readily demonstrated by echocardiography (Ave CFS Cardiac Index or CI = 2.2, Normal CI > 3.0, Cardiogenic shock CI < 1.8). One third of my patients have a CI below 1.8 but nearly all have normal LV systolic function which distinguishes them from cases of more classic heart failure with systolic dysfunction.
I have seen patients from 48 states and 27 foreign countries, over 12,000 cases since 1984 when I saw my first case in Lake Tahoe. The illness is striking with probably 99% exhibiting an abnormal physical exam and especially soft neurological findings (clonus and vestibular dysfunction), 90% are losing their fingerprints with abnormal fingerprint punch biopsies, 30% cannot be fingerprinted, 70% have tender lymph nodes and 90% respond abnormally to low levels of oxygen by Nasal Prongs. About 3% narcose on oxygen and pass out while 30% hypoventilate on oxygen and 30% hyperventilate on oxygen. 90% resist attempts to provoke desaturation by breath holding. 97% have diastolic dysfunction (DD) by echocardiographic criteria, a condition of cardiac muscle dysfunction driven by low cellular energy levels. As a result of DD, over 90% have a low cardiac output (CI 500) are toxic to oxygen by echo determined IVRT response criteria measured in msec and in real time and not seen in healthy controls. The oxygen toxicity is driven, I believe, by failure in CFS cases to buffer reactive oxygen species (ROS). Since energy generation is the biggest driver of ROS production, I believe the reduced cellular energy at the level of the mitochondria is caused by a compensatory feedback mechanism, perhaps through oxidative injury to the mitochondrial membranes for which evidence exists, and explains the relative stability of this illness over many decades and the low death rates observed.
Fatigue is therefore a solution to a deeper problem involving redox buffering though not without its complications. Reduced mitochondrial function in the mitochondrial rich myocardial cells will produced Diastolic Dysfunction (DD) and may lower the cardiac stroke volume as well as cardiac output resulting in significant disabilities and complications arising from this fact alone. Certain viruses (published repeatedly for HIV) are known to impair redox buffer control as it favors their ability to survive and replicate so a viral etiology has been a central theme as a possible trigger for this illness and CFS fits well into the larger framework of post-infectious syndromes described as far back as Hippocrates. It is one of the reasons CFS and Chronic Lyme cannot be clinically separated.
I personally think CFS and Chronic Lyme are the same illness because “Chronic Lyme” did not appear until after 1980 while acute Lyme was recognized and successfully treated for a decade before 1980 by Lyme literate physicians on Long Island, NY. Placebo controlled trials of antibiotics do not work very well in Chronic Lyme or CFS and anecdotal improvement on antibiotics proves nothing about the underlying cause of Chronic Lyme or CFS. Indeed, antibiotics can make things worse as they can further injure mitochondrial membranes and can also distort the gut microbiome.
Thanks so much Betty, that makes for really interesting reading!
Has anyone else lost their fingerprints? I have. Also, my fingernail beds are red one-quarter of the way from the tip towards the hand. My toenails are worse, they are red halfway from the tips towards the foot. Anyone else?
Have you been looking at my hands? 100% as you described!
I have no idea why this happens, Anneke. Reduced circulation? Clots? Inflammation? At least now we don’t have to wear gloves to commit crimes 😉
It would be interesting to know the prevalence of missing fingerprints in the readers of Health Rising. It is easy to figure out. Just get an ink stamp pad and press you finger on it and then on a piece of paper. If you have this symptom, it will be easy to see when you look at the print.
Dr. Paul Cheney on MEpedia said 10% no prints at all, another 30% more or less. New stripes horizontal and vertical across the fingertip. Those will be a giveaway in crime too, sorry Ann1.
Would be a good idea to check this out Betty.
I’m also very interested in how many ME/CFS/FM and maybe LC too had their blood volumes tested, with what method and by how much their blood volume was lowered.
Could you help out Cort?
When scientists can’t afford blood volume testing maybe this can help all of us.
Systrom mentioned this in his recent CDC talk – first time I’d heard of someone other than Cheney mention it.
I think there are many symptoms that ME/CFS patients may have in common. The missing fingerprints, crimson crescents, low blood volume, dehydration, frequent urination, stomach bloating, mast cell disorder, etc. For me, the first and continuing symptom for 38 years has been a sense of brain inflammation with constant tinnitus. In the early days, this was accompanied by horrendous headaches that could go on for days. Mercifully, these have stopped. Oddly, a shot of heparin would knock these headaches out.
I have never understood the obsession with mitochondrial dysfunction in Long COVID and ME as its found in loads of other diseases, and is never clearly linked to symtpoms I have like coat hanger pain and POTS.
Plus the recent mitochondrial supplement/medication trials in Long COVID patients have been a complete failure, as expected.
1,5 g of Taurine gives an energy boost after 10 minutes (25 years of CFS).