


Long COVID is getting a lot of attention. Is that translating into clinical trials?
The bottom line is always treatments. Long COVID has brought a ton of interest to post-infectious illnesses. Researchers are asking whether they are actually autoimmune diseases, how big of a role coagulation or gut microbiome problems play, if viral reactivation is driving symptoms, or if the glial cells have gone bananas or … it goes on and on.
The bottom line for patients, though, is treatments. Since we haven’t gotten a handle on the core problem or problems in long COVID, it’s too early to expect someone to be working on a new drug for it, but old drugs in new bottles (i.e. drugs repurposed to treat long COVID) – that’s something we would expect in spades – and that’s happening. The more eyes on a disease, the more possibilities that should crop up, and as you’ll see long COVID has gotten quite a few drug companies thinking that they have something to offer.
Another hope was that long COVID would change how the medical world sees fatigue, exertion intolerant, and pain-producing diseases like chronic fatigue syndrome (ME/CFS) in such a way as to allow the testing of more serious drugs. For a variety of reasons – a heterogeneous patient population, lack of a biomarker, a reliance on symptom assessments, the fact that ME/CFS usually debilitates in an invisible manner but does not kill – all these factors and probably more have left ME/CFS out in the cold regarding high potency immune drugs.
Despite the fact that studies have shown that people with ME/CFS are considerably more functionally impaired than people with very serious diseases like heart disease or multiple sclerosis, they’ve been cut off from the high-level immune and other drugs that serious diseases typically get.
As this survey shows, at least with long COVID, that’s changing. Despite the fact that we don’t know what’s causing long COVID, expensive, high powered drugs are being trialed in long COVID, and sometimes in quite large studies.
So are drugs that one wouldn’t ordinarily associate with long COVID or ME/CFS, like low-dose antipsychotics that may help with neuroinflammation, statins, a Japanese anti-inflammatory drug, a xanthine derivative, and a myasthenia gravis drug.
Lastly, several trials are large enough to actually give some solid answers about treatment efficacy – something that’s been lacking in ME/CFS. As the biological similarities between ME/CFS and long COVID pile up, one would think drug companies will be more and more willing to give ME/CFS a shot if their drugs or treatments work out in long COVID.
One thing we don’t see yet – new drugs trying to get at Epstein-Barr virus reactivation.
Immune Drugs
A Stem Cell Trial in Long COVID (!)
Treatment of Long COVID Utilizing Autologous Stem Cells
This was a shocker – at least, to me. The last I heard about stem cells in connection with ME/CFS was at least ten years ago when Dr. Cheney was trying them out. People in this small, 20-person study are going to get 150 million ATCell™ “autologous adipose-derived mesenchymal stem cells” (and they won’t have to go to Mexico to do it). In fact, the stem cells are going to be gathered via liposuction from each individual and then expanded in the lab.
This U.S. study from American CryoStem Corporation is going to assess a bunch of interesting factors (lactate dehydrogenase (LDH), prothrombin time/partial thromboplastin time (PT/PTT Coagulation factors II), Troponin, D-dimer, Fibrinogen (Coagulation factors II), estimated glomerular filtration rate (eGFR), urinalysis and spot protein creatinine).
It’s expected to start in April 2023. People in the placebo arm will be allowed to try the stem cells at the end of the trial (nice!).
- Contact: Anthony Dudzinski – 1-732-747-1007; tdudzinski@americancryostem.com
Bringing in the Big Guns
Impact of Monoclonal Antibody Treatment on Post-Acute COVID-19 Syndrome
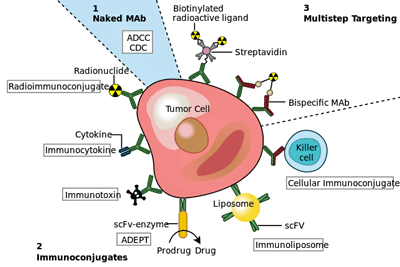
Antibodies against cancer. (Image fr. Ramujana-original-raster-Wikimedia-Commons.gi)
Now we’re talking. Monoclonal antibodies are all the rage in many diseases. These powerful and expensive drugs can target and turn off specific factors in the immune system. They’ve been talked about in ME/CFS, but except for a perhaps underpowered Rituximab trial, have never received a shot.
They’re going to get at least a preliminary shot here. This 260-person casirivimab-imdevimab trial at the Intermountain Medical Center in Murray, Utah used an antibody that worked well to both treat people infected with earlier variants and reduce the incidence of infection. This trial isn’t aiming to get rid of long COVID; instead, by giving it early in an infection, it’s trying to reduce the incidence of long COVID later.
If the trial succeeds, though, one might expect the RECOVER Initiative to do as it did with Paxlovid – and follow up with a trial in long COVID.
Autoimmunity Take II: The RSLV-132 Trial
Phase 2 Study of RSLV-132 in Subjects With Long COVID
RSLV-132 is a “fusion protein” that’s designed to remove RNA that’s apparently escaped from the cell. We think of RNA – ribonucleic acid – as an essential component (mRNA) of the cell – but in autoimmune diseases such as Sjogren’s Syndrome, it escapes outside of the cell and accumulates in the blood, where it triggers inflammation and ultimately the production of autoantibodies and autoimmune disease. RSLV-132 mops up the RNA in the blood and apparently did well in a lupus trial.
The 70-person study has been underway at centers across the U.S. since 2021 and is about to end soon (March 2023).
The “Mop Up” Trials
Plasmapheresis gets trialed … in long COVID in France…
I could see some hackles raising. Plasmapheresis … isn’t that like the next big thing that we’ve been talking about it in ME/CFS for several years? Yes, indeed. In fact, several very small studies suggest it may be helpful.
By filtering pro-inflammatory cytokines and/or autoimmune markers (such as adrenergic receptors) out of the plasma, plasmapheresis hold promise for ME/CFS. It’s long COVID, though, that’s going to get the first somewhat major plasmapheresis trial. Teeth gnashing aside, if this – and two other trials – are successful, it should boost interest in ME/CFS.
This 60-person French trial at the Hôpital Européen in Marseille, France should be starting soon.
- Contact: Myriam BENNANI 0413428351 ext +33 m.bennani@hopital-europeen.fr
And Spain…
Plasma Exchange Therapy for Post- COVID-19 Condition: A Pilot, Randomized Double-Blind Study
This prospective, randomized (1:1), double-blind, placebo-controlled study plasma exchange trial is going to include six two-hour sessions. It began last year and is expected to end in April 2024.
- Contact: Lourdes Mateu Pruñonosa, MD, Barcelona, Spain – Phd +34 93 497 29 64 lmateu.germanstrias@gencat.ca
As Well as Immunoadsorption
Repeat Immunoadsorption Post Covid ME/CFS
This is another “mop up” approach that’s being looked at in ME/CFS – and is getting a chance in long COVID. This small German study (n=20) is for people with long COVID who have high levels of the adrenergic autoantibodies Carmen Scheibenbogen’s been studying for quite some time. They know that people with long COVID have high levels of these autoantibodies (yet another similarity with ME/CFS) because they’ve been testing them (paper to be published). The immunoadsorption technique mops up these autoantibodies. This Berlin study begins in August of this year.
- Contact: Carmen Scheibenbogen, Prof. Dr. +49 30 450 524103 carmen.scheibenbogen@charite.de
None of these trials are going to be particularly big; these “mop-up” trials aren’t getting the big bucks that some of the monoclonal antibody trials are, but with 130 patients, the numbers are starting to add up. We should know a lot more about the efficacy of plasma exchange in long COVID over the next year or so – and if it works in long COVID, there’s no reason to think it wouldn’t in ME/CFS.
Good Drug … Good Company
Study to Evaluate the Efficacy and Safety of Ampligen in Patients With Post-COVID Conditions
They used to say about Ampligen and its manufacturer – good drug/bad company. The drug remains the same, but the company has been remade and what a difference that has made. After decades of derision and missed steps, Ampligen is now being trialed in several different cancers and is due to start an 80-person, randomized, double-blinded, placebo-controlled long-COVID trial in March.
- Contact: Diane Young 352-448-7797; diane.young@aimimmuo.com
Canada Goes Big in RECLAIM Long-COVID Study
RECLAIM: Recovering From COVID-19 Lingering Symptoms Adaptive Integrative Medicine Trial (RECLAIM)
There are trials, and then there are 1,000-person trials! In this case, Canada has done something very interesting. They’ve developed a Canada-wide clinical platform to methodically assess treatments for long COVID. The US has RECOVER; Canada has RECLAIM (Recovering From COVID-19 Lingering Symptoms Adaptive Integrative Medicine Trial).
My guess is that the two drugs named are only some of the treatments that will eventually be assessed. In any case, Canada ‘s testing two drugs no one else is.
- Ibudilast is a drug mainly used in Japan which has some intriguing properties: it’s an anti-inflammatory that vasodilates the blood vessels (check), has neuroprotective effects (check), inhibits platelet aggregation (check), and even suppresses microglial cell activation (check). Good for Canada to give this drug a try.
- Pentoxifylline is a xanthine derivative that’s used to treat muscle pain in people with blood vessel problems and peripheral neuropathy (interesting!). Inexpensive and readily available, pentoxifylline also inhibits the pro-inflammatory tumor necrosis factor (TNF-a) that Nancy Klimas is trying to beat down in Gulf War Illness and ME/CFS with etanercept.
The trials are beginning this month.
- Contact: Judy Scher, MSc, CCRC – 416-340-4841; judy.scher@uhnresearch.ca
- Contact: Jeevitha Srighanthan, BSc, MSc – jeevitha.srighanthan@uhn.ca
STRONGER! With Statins?
Statin TReatment for COVID-19 to Optimise NeuroloGical recovERy (STRONGER)
Atorvastatin or Lipitor – the most commonly prescribed drug in the U.S. – went generic long ago and is also readily available and affordable. It inhibits the production of cholesterol. So why devote a 400-person trial to it? The funders hope this 18-month (yes, 18-month) trial will help improve cognition and reduce neuroinflammation. Indeed, atorvastatin was recently shown to have neuroprotective effects and reduce microglial activation in mice. There’s more.
Atorvastatin also made good in the gut in some intriguing ways. By elevating butyrate levels (low in ME/CFS) and reducing leaky gut (present in ME/CFS), it may have found another way to reduce neuroinflammation.
This interesting study is going to use cognitive tests and brain MRIs to assess its effectiveness. Who knows? Statins might be a class of drugs that help a bit. Given their affordability, they might be a nice option.
The STRONGER Australian study has been underway and should be wrapping up in the spring.
A Biologic to the Rescue?
You can tell we’re (people with ME/CFS) in a different world when a 200-person biologic trial pops up in long COVID. Temelimab may have the coolest brand name (Imjudo – take that overactive immune system!) going. It’s a monoclonal antibody that targets a protein found on the human endogenous retrovirus.
HERV proteins can trigger inflammation in the brain and damage the neurons, and have possibly been implicated in long COVID, ME/CFS, multiple sclerosis and other diseases. Elevated levels of this protein found in the white blood cells of COVID-19 patients have been correlated with T-cell exhaustion and other immune issues. Plus, the protein has shown up in the brains of people with COVID-19 who died.
This Swiss study began in August of last year and is expected to wrap up in August of this year.
- Contact: Karim KEDDAD, MD, PhD – +41 22 552 48 00; kk@geneuro.com
- Contact: Nathalie BERTHUY – +41 22 552 48 00; nab@geneuro.co
The POTS Contingent
Efficacy and Safety Study of Efgartigimod in Adults With Post-COVID-19 POTS
Efgartigimod (better known as Vyvgart) is used to treat myasthenia gravis – a disease people with ME/CFS have some acquaintance with via Mestinon (pyridostigmine bromide) which is also used to treat that disease. It’s also used in lupus, Sjogren’s Syndrome and … COVID-19-caused postural orthostatic tachycardia syndrome (POTS), which is common in ME/CFS as well. That’s the group this small 42-person Illinois study is targeting. It began in September of last year and is expected to wrap up in November of this year.
- Contact: LaKesha Legree, MD – +1 800 201 8725; Llegree@argenx.com
Something Very Different
Zofin to Treat COVID-19 Long Haulers
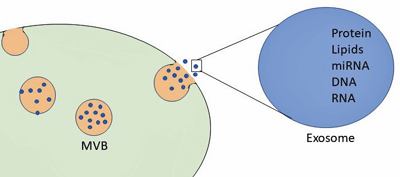
Zofin is using trying to use exosomes to alter immune functioning in long COVID. (From Kalamedits – Wikimedia Commons)
Zofin is a new drug derived from amniotic fluid collected at childbirth that contains extracellular vesicles that can attach to cells and deliver microRNAs to them. Zofin contains microRNAs (miRNAs) that target the ACE2 receptor that the coronavirus enters the cells through. If the ACE2 receptor – which is found in cells across the body – has been gotten messed up by the coronavirus, Zofin could help return it to health. The interesting thing about the ACE2 receptor is that small studies suggest it’s messed up in ME/CFS as well.
Organicell, the small Miami drug company that produces Zofin, jumped on the long-hauler pandemic quickly and by December 2020, reported it had treated 6 patients, all of whom reported significant benefits.
The 30-person, randomized, double-blinded, and placebo-controlled trial began in April 2022 and is expected to last until April 2024. It’s taking place in sites in southern California, Houston, and Miami.
- Contact: Mari Mitrani, MD, PhD – (888) 963-7881; clinicaltrials@organicell.com
Brain / Nervous System
Anesthetizing the Fight/Flight System Back to Normality?
Dual Sympathetic Blocks for Patients Experiencing Sympathetically-Mediated Symptoms From Long COVID
Stellate ganglion block sounds almost too good to be true… Simply anesthetize the area around the stellate ganglion and watch the sympathetic nervous system (fight or flight system) calm down – reducing pain levels, improving sleep, gut functioning, cognition, and definitely feelings of well-being.
The thing is that anecdotally it appears to have worked with some long COVID patients in Alaska. The really nice thing about this 40-person trial is that you don’t have to go to the far North to try it out. This trial is taking place in Hudson, New York and it just got started.
- Contact: Megan Nicklay, BS, MAT – mnicklay@hudsonmedical.com
Anti-depressant or Anti-inflammatory? The Lithium Study
Low-dose Abilify helps some. Another anti-psychotic is under trial. So are psychedelics. These are brain chemistry-altering drugs and studies suggest that brain chemistry is altered in ME/CFS/FM. If Jarred Younger and his neuroinflammation hypothesis is correct, it’s probably been very altered, so why not explore more brain-altering drugs? We don’t even know the mode of action of many of these drugs.
How about Lithium, for example? Lithium is prescribed for manic depression and comes loaded with an impressive array of potential side effects (diarrhea, vomiting, poor coordination, tremor, frequent urination, sleepiness, muscle rigidity, and hypothyroidism) if the dose is too high. This Buffalo study, though, is using low-dose Lithium and is giving it a shot for its anti-inflammatory effects.
This 40-person trial University of Buffalo trial started in November 2022 and is expected to last until June 2023.
- Contact: Rachel Shepherd, RN 716-932-6080 rlaporta@buffalo.edu
The Paxlovid Study
This “Phase 2, 1:1 randomized, double-blind, superiority, placebo-controlled study” (the more descriptors the better) RECOVER Initiative Paxlovid trial is looking for “100 highly symptomatic” long-COVID patients. This antiviral trial, which hopes to squash any remaining viruses in the body, comes on the heels of a study showing that people taking Paxlovid had a 40% reduced risk of coming down with long COVID.
Plus, you get to take part in Akiko Iwasaki’s study which will analyze the treatment effects.
The decentralized study begins in May and does not require site visits, and participants in Connecticut and New York who meet the entry criteria can enroll. It is designed to make it convenient to participate. The study drugs will be delivered to the participant’s designated address.
- Contact: Study Team – 203-497-1246; YalePaxStudy@yale.edu
A Stimulating Study
Addressing Cognitive Fog in Long-COVID-19 Patients
Feeling foggy? Less alert? How about some stimulants. How about an old-time stimulant? Dextroamphetamine is used to treat ADHD (common in ME/CFS/FM), and narcolepsy, to enhance cognition in college students worldwide, as an athletic performance enhancer, and recreationally as an aphrodisiac. The study leaders state the drug is “known to reduce cognitive impairment in other chronic medical conditions, such as Inflammatory Bowel Disease”. What’s not to like?
It’s also a “Schedule II controlled substance that has a risk for abuse, tolerance, and psychological dependence”. This lower dose (10 mg/day for 12 weeks) study also includes “a digital behavioral tool with an embedded health coach” arm that helps with relaxation and coping. Participants will be closely monitored for side effects.
This University of Pittsburg Medical Center study will begin in February.
- Contact: Kate Saucier – 412-353-3987; mcauliffk@upmc.edu
- Contact: Meredith Strassburger – 412-368-6485; strassburgermb2@upmc.edu
Tonix Tackles Long COVID
One thing you have to give Tonix – the maker of TNX-102 – they don’t lack confidence in their drug. After several setbacks (wrong doses, COVID-19) and numerous trials, they’re still hammering away at fibromyalgia, and, in the midst of all that, they’ve started a 470-person long-COVID trial.
My prediction – this pain-reducing and sleep-enhancing drug is going to succeed! If this company is willing to throw tons of money at a big long-COVID trial after all the problems with the FM trials, it must be very confident of its drug.
This phase 2, 14-week, double-blind, randomized, parallel, multicenter, placebo-controlled study wins the prize for the most descriptors (6) thus far. It began in August 2022. If you experience widespread pain, you’re eligible for this trial.
It’s underway in many sites across the U.S. (Check the website for locations).
- Contact: Clinical Program Manager- 212-980-9155; megha.tevar@tonixpharma.com
A Different Kind of Stimulation
Trial of Auricular Vagus Nerve Stimulation in Painful Covid Long
Lauren Stiles, the founder of Dysautonomia International, thinks vagus nerve stimulation is going to be big in POTS and ME/CFS – it just needs more study. It’s going to get more study – not a lot – but more – in this 30-person trial at the Service Centre d’Evaluation et de Traitement de la Douleur (eg. The Pain Assessment and Treatment Center Service) in Paris.
- Contact: Stéphanie Mauboussin-Carlos – 01 49 28 23 08; stephanie.mauboussincarlos@aphp.fr
A POTS Drug for Long COVID Patients with POTS
Ivabradine for Long-Term Effects of COVID-19 With POTS Cohort
There’s not much to say about Ivabradine except it makes sense to try this POTS drug in long-COVID patients with POTS. The authors must think so – they’ve opened a 250-person trial.
Find out more about this “comparative cohort, nested, randomized, controlled trial” – which forgot to give its location but which appears to be based in Bethesda, Maryland – here, and learn more about Ivabradine here.
- Contact: Roshila Mohammed, MBBS – (301) 318-6024; clinical.research.unit.53-ggg@usuhs.edu
Low-dose Naltrexone for Post-COVID Fatigue Syndrome
LDN was shown long ago to work in fibromyalgia, but it’s taken years to get an LDN trial underway in ME/CFS. What happens, though, when tens of millions of people come down with long COVID? Big LDN trials start popping up.
This 160-person trial is going to blow the small LDN fibromyalgia trials out of the water and that’s a good thing; it’s way past time for a nice hearty trial of this compounded drug to get underway.
This double-blinded, randomized trial is getting underway at the BC Women’s Hospital + Health Centre, Vancouver, British Columbia. Now, if we could just get a dextro-naltrexone trial underway, we’d really be sitting pretty.
- Contact: Travis Boulter 236-990-9519 LDNtrial@phsa.ca
Antidepressant or Cognitive Enhancer?
Vortioxetine for Post-COVID-19 Condition
Who knows what vortioxetine is doing or will do? Cymbalta – another “antidepressant” – was able to reduce pain in people who were not depressed. Could vortioxetine do the same for people with brain fog? This Toronto, Canada group apparently thinks it might. Another immune modulator, it does, after all, have anti-inflammatory effects.
Stating that vortioxetine is “an antidepressant with established pro-cognitive properties“, they hope it will improve cognition as well as general functioning, well-being, motivation, energy, and even sleep. They’re excited enough about its prospects that they’re going to try it out in 200 people.
This randomized, double-blinded, placebo-controlled study started in September 2021 and is expected to last until this month.
Stimulate Your Brain … From Home!
Home-based Brain Stimulation Treatment for Post-acute Sequelae of COVID-19 (PASC)
Will it be the next big thing? Currently, you have to go to a clinic or hospital and get the electrodes placed and set just right to get your brain gently stimulated to relieve your pain or improve your mood. Home kits are now coming available, and if they work … talk about a brave new world where you can potentially plug in for a while and emerge a healthier person.
This 40-person 1-month trial will attempt to improve the problems with executive functioning (ability to sustain attention, slowed information processing speed, etc.) via 30-minute sessions of low-intensity, home-based transcranial direct current stimulation (tDCS).
This Harvard study was due to end up in July of this year.
- Contact: Hamdi Eryilmaz, Ph.D – 6176437462; hamdi.eryilmaz@mgh.harvard.edu
- Contact: Alexandra O’Neill, B.S. – (617) 726-8753; agoneill@mgh.harvard.edu
A Beta Blocker for Long COVID?
Metropol succinate – now there’s something different. Stuffthatworks reports that metropol succinte (Tropol XL) has been rarely tried but has “overwhelmingly positive reports” from those who have tried it. Forty-five percent of people who tried it reported it worked “very well”. Metropol succinate is a beta blocker that’s often used to treat high blood pressure and chest pain.
This 20-person Hackensack University, New Jersey trial began in March of last year and is expected to last for several years.
- Contact: Jana Tancredi, RN – 5519962353; Jana.tancredi@hmhn.org
The HOT LOCo Trial: Flooding the Brain with Oxygen
Given how much attention hyperbaric oxygen treatment (HBOT) has garnered in fibromyalgia and ME/CFS it was no surprise to see a HBOT trial show up in long COVID. The Isreali’s have led the way – now we need outside groups to take HBOT on and that’s what this study will do.
This 80-person, randomized, placebo-controlled, double-blind, phase II trial from the Karolinska Institute in Stockholm, Sweden began in Sept. 2021 and is slated to finish in Sept. 2023.
- Contact: Hyperbaric unit +46812394680 ; Anders Kjellberg, MD – +46851775212 anders.kjellberg@ki.se
Supplements
A few of the supplement trials are shown below.
The Cannabis and Hemp Trials

Finally, a few cannabanoid/hemp studies. We could use many more…(Image from elsa olofsson – Wikimedia Commons)
Chronic fatigue syndrome (ME/CFS) and fibromyalgia should simply be awash in Cannabis trials that aim to find a cheap and simple help for these diseases. There’s little doubt that Cannabis can help with pain and sleep and may even help with cognition, yet Cannabis trials are rare, if they’ve happened at all.
Two long-COVID Cannabis/hemp trials are underway – a cannabidiol trial in New York and a 111-person hemp flower extract trial at the Bateman Horne Center in Salt Lake City, Utah.
- Cannabidiol trial: Contact: Michael Lynskey, Ph.D.- 07385613429; michael.lynskey@drugscience.org.uk or Hannah Thurgur, Ph.D. – 07385613429; 0738561342
- Hemp Flower Extract – Suzanne Vernon 801-893-6229; sdvernon@batemanhornecenter.org
Sea Urchin Eggs For Better Health?
Passing over the question of how one harvests sea urchin eggs, this 60-person placebo-controlled, randomized Argentinian study notes that they are anti-inflammatory, have antioxidant properties, and have been used in eastern medicine for a long time.
- Contact: Fernando Saldarini, MD – fernando.saldarini@gmail.com
- Contact: Valeria Brichetti, MD – neumobrichetti@gmail.com
Supplement Trial Digs Deep
Immulina Supplements on Inflammatory Biomarkers Correlated With Clinical Symptoms in Patients With Long COVID (PASC)
This University of Mississippi Immulina trial is notable for its size (120), its scope (blood inflammatory biomarkers, memory T cell, memory B cell, and antiviral antibody titers), and the fact that its principal investigator, Gailen Marshall, has long been associated with ME/CFS and is one of the ME/CFS experts involved with the RECOVER Initiative. The trial is just beginning.
University of Mississippi Medical Center
- Contact: Donielle D Drakes, MBA 601-496-7821 ddrakes@umc.edu
- Contact: Denise Montgomery, MT(ASCP) 6018155374 dmontgomery@umc.edu
- Principal Investigator: Gailen D Marshall Jr., MD, PhD
Energy Enhancement with an NAD+ Booster
Clinical Trial of Niagen to Examine Recovery in People With Persistent Cognitive and Physical Symptoms After COVID19 Illness
Another 100-person trial, this double-blinded, randomized, parallel-group, placebo-controlled Harvard trial will examine whether Niagen, an NAD+ booster, helps with long COVID. It’s currently underway.
- Contact: Jessica A Gerber 617-724-1992 jgerber2@mgh.harvard.edu
The Poo Study
Fecal Matter Transplant for Post-acute COVID-19 Syndrome (FMT-PACS)
Of course, there was going to be a poo study, In fact, I was surprised not to see more fecal matter trials. This 60-person Hong Kong, open-label, fecal transplant study is now underway.
- Contact: Raphaela Iris Lau – lauhiulamiris@link.cuhk.edu.hk; Jessica Ching Ph.D. – jessicaching@cuhk.edu.hk
Coming Up – the RECOVER Trials
Finally, we should see trials that will attempt to improve autonomic nervous system functioning, exercise intolerance, sleep, and cognition from the RECOVER Initiative.
Last BIG (Little) Donation Update!

The drive is ending on a high note – looking at the many different clinical trials ahead.
Yes, this is (finally) the last blog of the donation drive. We thought we’d go out on a high note – looking at all the clinical trials coming up. Thanks to everybody who has supported us during this long drive.
This, too, is a long and rather exhausting kind of blog to write and undoubtedly to read, but how rich it is in all its details and how much I learned doing it. Hopefully, you got something out of it – I certainly did writing it – and if you did please support us if it’s appropriate for you to do so.






Great article and one that makes me very hopeful that something that is at least reasonably effective at treating long covid will come out of this, and ME/CFS patients will also benefit!!!
I reckon we’ll have something within two years.
Also came across this link to a study regarding brain fog… https://www.sciencealert.com/one-of-long-covids-worst-symptoms-may-have-a-potential-readily-available-treatment
Good to see studies with drugs. Let’s hope something is found that works.
Yet in my opinion there will never be found 1 medicine for a disease that helps or cures all patients.
Even with well-known diseases and medication, the effectiveness is often sadly low.
(…)Allen Roses, of GlaxoSmithKline, is quoted in a national newspaper as saying more than 90% of drugs only work in 30-50% of people (…)
http://news.bbc.co.uk/2/hi/health/3299945.stm
https://www.independent.co.uk/news/science/glaxo-chief-our-drugs-do-not-work-on-most-patients-5508670.html
Just listen to Professor Robert Clancy an immunologist. He flawlessly explains how the immune system works and what the effects of an mRNA vaccine can have.
https://www.youtube.com/watch?v=yMyERFBdB4E
Anyone with reservations about MRNA vaccines should opt to get Novavax, a traditional vaccine with fewer side effects and more staying power. It is also cheaper and does not require the extreme low storage temperature of the other vaccines.
Totally agree 🙂
I absolutely agree !
A bit of anecdotal – I’ve had two AZ, then a Moderna then a Novavax. I had side effect reactions to AZ and Moderna (just the standard stuff for a day or two afterwards, luckily). I had absolutely zero reaction to Novavax. I’ve no idea what that means, if anything, but there you go.
Despite what the US media and Government claims, Ivermectin is a very safe and effective tool to both prevent and treat Covid. Many other countries promote it to save their citizens, but not here.
Ask yourself why?
See the evidence for yourself:
https://c19ivm.org/meta.html
Totally agree, Bridget
https://covid19criticalcare.com/
Wow Cort … you have been very busy ! Thank you ! So much activity !
All I can say is that something “ gotta” give !!
Cort, thank you for this excellent, hope-inspiring write-up. As you wrote, the bottom line is always treatments. And for 35+ years I’ve watched as researchers have tried to understand what the drivers of ME/CFS are, i.e., the biomechanics of the disease. And it seems like the more they have learned, the more devilishly complicated the picture becomes.
These researchers’ intentions are surely noble, but I’ve always wanted to shout out to them, “Hey, it doesn’t matter to me one whit what’s causing my illness! I just want a treatment that will stop the day-to-day agony of this illness!”
Finally, the focus is where it should be if we patients are to stand a chance of relief from the daily torture of this disease: TREATMENTS.
HIV cost the insurance industry too much for multi drug cocktails.
Unfortunately it scared the crap out of them.
They will fight these treatments tooth and nail.
30 years of pain have made me very pessimistic.
Thymosin Alpha 1 worked for my loved one..wish they would investigate
Cort, thank you for the well written article.
I hate to be Debbie Downer, but I believe the insurance industry is going to push all these treatments into the experimental (not covered) category.
HIV aids scared the crap out of them when they had to start covering extensive multi-drug cocktails.
Ultimately, I believe that it will take several of these drugs taken together to alleviate symptoms.
There is no cure, just bandaids that could give us back our lives.
In the end, money will kill all this hard being done by these researchers.
30 years of this has made me quite pessimistic.
Did it helped their fatigue? They had long covid Or ME/CFS?
She has had ME/CFS -post viral disease for close to 20 years. Was mistreated with overdose of antivirals and had big setback several years ago. The only things that has helped is Thymosin Alpha one. Before she could not clean the house, barely leave the house. On TMA1 she was able to shop- go out- not 100% for sure, but definite improvement. The issue is that the recommended compounding pharmacy in another state does not ship to California- where we are. Oxaloacetate and other treatments did not bring an improvement.
Oxaloacetate didn’t worked for me either. I am severe 100% bedbound
Can u link the Thymosin alpha 1 which she used??
can you friend me on facebook beverly eitingin weiss and will message you… if that is an issue i will post me email here
JThymosin Alpha one ? Was she bedbound?
Before you take read this on dangers of this stuff and history of complaints:
https://www.npr.org/2020/10/01/914433778/web-of-wellness-doctors-promote-injections-of-unproven-coronavirus-treatment
homebound.
No, she was not bedbound. But could not do any cleaning- mostly was on the couch,, could cook her meals for the week on one day, but needed to recuperate for 3 days after. She also has MCAS and orthostatic intolerance.
Hi Beverly, I did some research on thymosin alpha 1 and was impressed. My doctor has agreed to let me try it. Do you know the dose and frequency that your loved one used? Also, how long before improvements were noticed. Thanks, Betty
Initially Dr.Kaufman prescribed the standard dose for a male, my daughter weighs much less and they had to reduce the dose, it varied over time, i think she began feeling the benefits shortly after the dose was titrated for her weight. Initial dose caused some jitteriness. Good luck, i hope this brings you some relief.
What symptoms did she/he have?
Cort, can you confirm that the temelimab trial is supposed to end in August? I believe the ClinicalTrials.gov page says it’s scheduled to wrap up by the end of March: https://clinicaltrials.gov/ct2/show/NCT05497089
Totally agree that we need dextro-naltrexone trials! I was glad to see it mentioned again in your interview with Younger, though disappointing that he still can’t get funding for it.
Thanks for this thorough roundup. Has anyone met with FDA to find out if they would extend an indication for ME/CFS to an agent studied in a Long COVID-focused trial? Not many insurance companies are going to pay for off label use of something like an expensive monoclonal antibody, otherwise …
Great blog, Cort! Thank you. Very hopeful!
Do you know the status of this trial of suramin for long Covid? Naviaux is quoted:
https://www.prnewswire.com/news-releases/paxmedica-plans-to-initiate-phase-1b-study-for-pax-101-in-patients-with-long-covid-19-syndrome-301456006.html
There is a mistake in the text. Unfortunately, severe forms of MECFS can kill. Just a few days ago Kara Jane, a famous young singer from the UK died of it. She wanted her body to be released for post mortem examination. Because the main factors what happens in the body are still unknown. 40% of Long Covid sufferers get MECFS, so it can kill.
The blog does not say ME/CFS cannot kill. We’ve done several autopsy blogs – and I’ve personally done too many memorial blogs to not know that it can kill. Not on the scale, though, of diseases like heart disease, multiple sclerosis, etc.
I keep hoping for a trial that explains and treats post exertional malaise. Maybe one of the mop-up trials will help, but the rest all look like treating symptoms rather than the root cause. Feeling discouraged as I’m trapped in my recliner or bed. Thanks for your work Cort.
there just came “no end” on all the trials/treatments for long covid… but it was painfull when you think about ME/cfs, FM, other deseases like chronic lyme…
where are we with our ME/cfs, i think everyone wants those trials to…
last time i chequed, nancy klimas was not bussy on ME/cfs. probebly no money…
and it seems some researchers swtch sides to the money from long covid or so… and get their ideas from us…
i wish a list of treatments like this for ME/cfs, FM, chrinic lyme, etc
WOW! A very long list. Thank you, Cort, for writing it up! Hopefully they find something and some of it spill over to CFS and FM.
Unfortunately I took Atorvastatin for 3 years but my FM symptoms did not improve during that period.
Sorry this is posted to the wrong place!
Any research on antiretrovirals?
Here is why ME/CFS will never receive appropriate treatment until the cause is identified. I remember many years ago when a relative was told that his ulcer was caused by stress and should be treated with lots of milk and relaxation. Now we know that ulcers are caused by h-pylori and can be successfully treated with antibiotics.
Throwing all kinds of drugs at an illness without an identified cause (and this includes long Covid) is a waste of precious research funds and may actually make the patients worse.
Lithium is a psychiatric drug and me/cfs is not a psychiatric condition. Why are we wasting research dollars on psychiatric medication? None have, or will, deliver significant long-lasting effects.
All of these studies are a stab in the dark, and band aid solutions at best. You might be able to reduce inflammation temporarily with a drug, but until you work out what’s causing the inflammation and treat it there will not be an effective treatment for me/cfs or long Covid.
We need more studies looking for the biological cause of me/cfs, and the drugs can then be developed/found.
Err.. there is one person that dramatically improved on low dose lithium and documented it on a popular me/cfs forum. You can look it up.
Lithium shares many of the same functions/effects as sodium in the body (including getting glucose in cells, you can look up the science). So you can try sodium instead.
Oh wait, there is that fear-mongering campaign against consuming salt…
Though Cort has written up about the mineral water mix and doctors recommend it for POTS. Confused yet?
One anecdote does not mean it works. What is the mechanism by which this is supposed to help with me/cfs? I have personally been on lithium for years (for other reasons) with no improvement to my me/cfs. And lithium is extremely different from sodium. One is a powerful psychiatric drug, one is not. Again, me/cfs is not a psychiatric disorder so psychiatric drugs will never solve it.
WOW! A very long list. Thank you, Cort, for writing it up! Hopefully they find something and some of it spill over to CFS and FM.
Unfortunately I took Atorvastatin for 3 years but my FM symptoms did not improve during that period.
I was diagnosed with ME/CFS in 1984. There are some treatments that cannot be patented but do work for many patients including me. One is Nexavir, a newer version of Kutapressin, which has been around for more than 30 years. Nexavir now comes in cream form and can be ordered directly from the manufacturer NEXCO. It is not inexpensive but one lady was able to petition Medicare to pay for her treatment with it. Nexavir is an antiviral and an immune booster and according to the manufacturer is working for ME/CFS and Long Covid.
Second on the list is whey based protein, Immunopro. This is an immune booster and can be ordered on Amazon. I never get a cold or the flu as long as I remember to take two scoops a day.
Third is a cutting-edge treatment pioneered by Dr. Paul Cheney who first identified ME/CFS in Incline Village. These are small bottles of cell signaling factors, one for brain, one for heart and one, MTF, that he used to say was like stem cells in a bottle. These can be purchased from the Cheney Clinic without a prescription. Although Dr. Cheney passed away in 2021, he obtained the US patent for these treatments which are used by some doctors in Europe. The MTF is very powerful so a tiny amount can help bring you out of a decline.
Fourth, I have only good things to say about the supplement quercetin which can be purchased on Amazon. It is great if you have mast cell disorder and seems to have some protective effect against Covid.
Next is Bulouke, a supplement that acts as a blood thinner to deal with the coagulation problems found in both ME/CFS and Long Covid. This can be purchased on Amazon.
These treatments have kept me going and out of bed. I have been able to travel, even play a little golf and run our nonprofit, Birth Defect Research for Children. They are not a cure and all are out-of-pocket so the cost of treatment is not inexpensive, but they are the best treatments I have found in nearly 40 years.
One extra note, if you are a patient with ME/CFS or Long Covid and have trouble with gluten, try making baked goods with 00 Flour from Italy. My doctor told me about it. It is a different kind of wheat and I have no problems with it. 00 Flour can also be ordered on Amazon.
Finally, Dr. Paul Cheney found some interesting physical markers in the thousands of patients he treated from all over the world. One third are losing or have lost their fingerprints and they have bright red crimson crescents in the sides of their throats when they are at their worst. I don’t know if this is true for Long Covid, but I doubt if anyone has looked at it.
Betty Mekdeci
Thanks for this, Betty. I also take quercetin and it helps a lot. The problem with quercetin is that it is very poorly absorbed in the intestine. There are a couple of ways around this. One is liposomal quercetin. It works, but there is peppermint in it so I can’t take it at all.
https://www.amazon.com/Natural-Factors-Quercetin-LipoMicel-Softgels/dp/B08GZGXQK9/ref=sr_1_4?crid=I5IXWZBUEWSS&keywords=Natural+Factors+quercetin&qid=1673829269&sprefix=natural+factors+quercetin%2Caps%2C1642&sr=8-4
The other way is enzymatically treated quercetin.
https://www.amazon.com/Bioclinic-Naturals-Activated-Quercetin-vcaps/dp/B00GSPCQPS/ref=sr_1_2?crid=33ZBCQMH46QZ&keywords=natural+factors+bioactive+quercetin+emiq+50+mg&qid=1673829354&sprefix=Natural+Factors+quercetin+EMIQ%2Caps%2C1552&sr=8-2
I take three of these four times a day and it helps.
Thanks, I will try the liposomal brand since peppermint doesn’t bother me. I forgot one other supplement that is excellent…the best chewable enzyme I have ever found.
https://www.amazon.com/dp/B0025PW4U2?psc=1&ref=ppx_yo2ov_dt_b_product_details
Betty, I can’t seem to find where to buy Dr. Cheney’s supplements. Can you provide a link? Thanks.
Hi Betty
Sorry to be such a pest, but I see that the Thymosin alpha 1 is an injection, is that right?
yes, and i think no banned in the us. trying to get compassionate care approval by fda
Thank you, Betty! And thank you for another great article, Cort!
Here is the email to order cell signaling factors.
cheneyinstitutemail@gmail.com
If you go to the Remembering Dr. Cheney Facebook, you can ask other patients about their experience with the cell signaling factors.
I have not used the Thymosin Alpha 1, but I looked it up and it also seems to be available in a nasal spray.
Thank you very much, Betty. I appreciate it.
blood thinners are used in Nutcracker Syndrome compression 81 to 100mg of baby aspirin. That could be why Ron Davis claims on thick blood nano needle, it is not thick blood it is blocked veins
Thanks for all the valuable information, Cort. I really appreciate all your efforts on behalf of the fibromyalgia community.
There are also trials scheduled for Maraviroc + Statin combo (Patterson protocol) and another trial in Germany for BC007, an autoantibody neutralizer. The German trial will be very interesting because the 4-person compassionate use trial resulted in fast, dramatic (and seemingly lasting) effects for the long covid patients.
Hi Cort, how about running an article where patients share what has helped them the most especially products that can be purchased without a prescription.
I’ve had great success treating patients with LC with pentoxifylline. I also have them use nattokinase 4000fu and asa81mg. In 7-10 days the brain fog starts to life and then the fatigue!! It is inexpensive and accessible; fairly safe. Just watch for bleeding when combining with other anticoagulants.
Great to hear. Thanks for sharing that Dr. Bowers.
What’s the dosage used of pentoxifylline??
400mg TID
How can i consult you mam?
I am 24 y male. Got CFS from dengue virus in 2018.i am very severe right now(bedbound currently)
I live in India. Is there any way to consult you
“They also found that in many cases, the symptoms of long COVID become nearly indistinguishable from several other conditions, such as chronic fatigue syndrome, mast cell activation syndrome and postural orthostatic tachycardia syndrome. Notably, they point, out, many such symptoms are consistent with autonomic dysfunction.”
https://medicalxpress.com/news/2023-01-covid-indefinitely-people-mimic-ailments.html
I will second an article where patients share what has helped them (and yes, I seem to remember Cort did something similar at one time).
And Betty, about your comment of people losing their finger prints, I do wonder if Dr. Cheney differentiated between those with ME/CFS and those who had ME/CFS AND Ehlers-Danlos. On an EDS blog, there were dozens of people complaining that they couldn’t use finger print I.D. or similar. Personally I can’t open those flimsy grocery bags and I have trouble using a smart phone because it doesn’t recognize my swiping. (Could be cold fingers as well). I’ve this issue since birth.
I’m frequently wondering if all my fatigue problems are ME/CFS or EDS? I have pronounced PEM and it did seem to significantly worsen after an illness. I also wonder why EDS is not listed as an exclusion for ME/CFS diagnosis.
Curcumin has helped me, but with dramatically shrinking a synovial cyst that was crushing my spinal cord. I was booked for back surgery as everything had gone numb and I was fast heading for cauda equina. As a Hail Mary I tried high dose curcumin and within 2 weeks I got my feeling back–and happily canceled the surgery! I still take it today.
The next thing on my experimental list is Metformin. I just learned that I am pre-diabetic!!!? I’m a skinny girl who eats a very healthy diet so I’m not sure where this came from. The only thing I can think of is that with ME/CFS I don’t get as much exercise as I would like. I also have some type of PCOS which is associated with the development of diabetes. Do hope I can convince my doctor to let me try it. At present, I’m using berberine as a substitute. I sent her your article, Cort and found some other research hypothesizing that it might be helpful.
Nancy, hEDS ‘looks’ like EDS but it is not. There is no gene involved, unlike the other types of EDS.
It’s just hypermobility + low metabolism/hormones/nutrition The moment one understands this, worlds open up on how to go on about healing. It did for me.
For example, the easy bruising can be a low vitamin C problem / high estrogen / low progesterone / high cortisol. The thin skin that tears easily = high cortisol. The ‘MCAS’ = low vitamin D / hypoglycemia. Mitral valve prolapse = hypothyroidism, etc.
Getting out of the stress response stabilized my joints.
Collagen gets wonky when B6 and/or B2 are low, when DHEA is low. They go together with hypothyroidism. Address the low metabolism, the low thyroid, the high stress hormones.
I recommend reading Broda Barnes and Ray Peat for starters.
Good luck!
Just a gentle reminder you have an international audience. When you say a study concludes in the spring… it doesn’t mean anything to me here in Australia. Thanks.
Ha! Didn’t think of that! I will try to remember to use months in the future. Thanks
Amazing list, thank you for compiling.
What strikes me as a Brit is not a single one in the UK. As our NHS falls apart, and as the home of the PACE trial, I’m ashamed of our care for MECFS and Long Covid. Although the new NICE guidelines are a welcome move in the right direction at last.