

It was remarkable to see two studies and an NIH blog suggesting that a biomarker may have been found for chronic fatigue syndrome (ME/CFS) get published on the same day. The effort to promote and link these studies together worked and numerous media outlets have covered them. Part I of this series examined the large study coming out of Ian Lipkin’s ME/CFS research center.
Part II of this series checks out the Xiong/Oh paper, “Multi-‘omics of gut microbiome-host interactions in short- and long-term myalgic encephalomyelitis/chronic fatigue syndrome patients“, coming out of Derya Unutmaz’s NIH-funded research center at the Jackson Lab. This study was focused on the gut microbiome and the metabolome. In a twist, it compared short-duration patients (<4 years) with long-duration patients (>10 years) and in doing so, came up with a new hypothesis for how chronic fatigue syndrome (ME/CFS) begins.
The Unutmaz study piggybacked on an earlier Mady Hornig/Ian Lipkin study which suggested that shorter-duration ME/CFS patients had a dramatically different immune profile than longer-duration patients. In fact, the immune profiles of the two groups were reversed: the shorter-duration patients showed a highly upregulated immune system while the longer-duration patients showed possible evidence of immune exhaustion. Back in 2015, Mady Hornig suggested looking in the gut to find out why that had happened.
As Lipkin did in a past paper, the authors found that integrating all the variables – the microbiome and metabolite findings, as well as several models – helped them to best differentiate ME/CFS from the healthy controls. That suggests that large, multi-dimensional studies are the most effective at understanding this disorder.
When it came to distinguishing the ME/CFS patients from the age and sex-matched healthy controls it wasn’t the low butyrate but the low “evenness” found in the ME/CFS patients’ microbiome that really set them apart. Less common bacteria that produced compounds like tryptophan, butyrate, and propionic acid were less abundant. (The Williams Lipkin study also found a depletion of rarer microbes). That, of course, brings up the question of what could throw the microbiome off in that way. The authors noted that similar pattern has been found in the microbiome of “frail older adults”.
Cell Membranes – Again…
This study, too, suggests the cell membranes in ME/CFS may be getting hit hard.
With regard to microbial genes, people with ME/CFS displayed decreased genes involved in the production of betaine (an anti-inflammatory), sphingomyelin, serotonin, and cholesterol. Sphingomyelin was also found reduced in Lipkin’s peroxisome study as well as low cholesterol.
Both appear to fit with Lipkin’s findings suggesting that high levels of cellular membrane damage are present. The cell membrane theme popped up again in the metabolomics results when lipid and cholesterol metabolites were found to discriminate ME/CFS patients from the healthy controls.
More Gut Issues at First, More Metabolic Issues Later
Interestingly, the greatest dysbiosis (i.e. the greatest gut flora alterations), reduced plasma isobutyrate, and more gut symptoms were found in the shorter-term patients (<10 years). Over time, the gut flora of the longer-term patients (>10 years) appears to have rebounded to some extent. While media reports and the abstract emphasized that the pattern the microbiome in the older ME/CFS patients still displayed low levels of the less common bacteria, including several butyrate producers. F. prausnitzii – highlighted in the Lipkin group’s paper – was also found reduced.
With two cholesterol and several lipid metabolites discriminating the longer-duration ME/CFS patients from healthy controls, the older group displayed more metabolic abnormalities. (Again, the damaged cellular membrane theme (cholesterol, lipids) pops up). Plus, the longer-duration patients were more apt to pick up a fibromyalgia diagnosis, had a trend towards worsening sleep problems, and had more PEM; i.e. things had not gotten better after ten years.
In a finding that bodes well for the future, six out of the ten metabolic biomarkers the Unutmaz team identified in this paper were also highlighted by the Lipkin team and Hanson teams (Germain) in their large metabolomic studies. That suggests these large studies are also coming up with some core metabolic abnormalities.
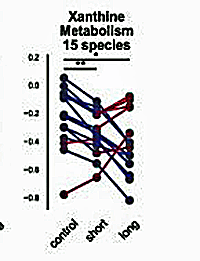
The Unutmaz team found a dramatic reduction in xanthine metabolites in ME/CFS.
The similarities didn’t stop there, though. The overlap between the pathway abnormalities Naviaux found in his 2016 ground-breaking metabolomics paper and these more recent papers is rather remarkable as well. Naviaux’s study predicted many of the themes that are now popping up now including sphingolipids, phospholipids, cholesterol, purine, microbiome, branched-chain amino acid, peroxisomal (peroxisomes!), and mitochondrial metabolism.
Take purines. Back in 2017, Naviaux singled out purines. In 2023, the Unutmaz team highlighted a reduction in xanthines – a purine base.
Likewise, a small 2020 Japanese study had similar findings: an emphasis on microbiome and lipoproteins and cell membranes (all healthy lipoproteins reduced; all unhealthy lipoproteins increased), and reduced levels of Faecalibacterium.
Pentoxyifylline for Long COVID and ME/CFS?
The low levels of xanthines are interesting given that xanthines increase central nervous system alertness. Some people with ME/CFS/long COVID have used methylated xanthines such as caffeine, pentoxifylline and theobromine (found in dark chocolate) to increase alertness.
I have in my notes this, from a doctor, apparently. Unfortunately, I have no idea who it came from.
“I’ve had great success treating patients with LC with pentoxifylline. I also have them use nattokinase 4000fu and asa81mg. In 7-10 days the brain fog starts to lift and then the fatigue!! It is inexpensive and accessible; fairly safe. Just watch for bleeding when combining with other anticoagulants.”
The University Health Network, Toronto, has included pentoxifylline in its RECLAIM suite of treatment trials. Pentoxifylline is an old, reportedly safe, and inexpensive drug that has been found to do a bunch of things that could potentially be helpful in these diseases, including inhibiting the pro-inflammatory cytokine tumor necrosis factor, and improving blood vessel functioning by decreasing viscosity, lowering fibrinogen, and opening constricted blood vessels.
WebMD states pentoxifylline “increases the amount of oxygen that can be delivered by the blood when the muscles need more (such as during exercise) thereby increasing walking distance and duration.” Pentoxifylline – which I’ve never heard of until the last couple of months – sounds like a potentially good fit for long COVID and ME/CFS. It’s another new possibility that the long-COVID pandemic has uncovered.
A Gut Origin Hypothesis

Could poor gut flora kick off ME/CFS (and other diseases)?
Oh and the Unutmaz team providing an intriguing gut origin hypothesis for ME/CFS. They believe that the “modest but widespread” gut changes they’ve found occurring early in the disease could produce “cascading events” that have “cumulative and long-term effects”. They believe it may all begin with the loss of butyrate and tryptophan-producing bacteria, in particular. That loss ends up producing the “irreversible and unrecoverable” metabolite changes found later in the disease.
(They aren’t saying that the condition is irreversible; indeed, at the end of the paper they look forward to treatments. They appear to be saying even the partial recovery of the microbiome they saw in the longer-term patients isn’t enough to reverse the changes that end up occurring at the metabolic level. Indeed, the longer-term patients are sicker than ever.)
They were able to point to a study suggesting that an early depletion of butyrate-producing bacteria may precede ischemic heart disease and that the depletion occurs prior to that disease presenting itself. That suggests that determining how butyrate and tryptophan-producing bacteria get depleted in the first place (viruses? stomach flu? Giardia? pharmaceutical drugs?) should become a major theme. (Several years before I came down with ME/CFS, I experienced a Giardia infection while backpacking. Could that have been my trigger?).
Interestingly, the cardiovascular study showed that statins helped to restore the microbiome and improve the health of heart disease patients. Bruce Patterson is using them in long COVID and ME/CFS, and in 2020, Japanese researchers, noting that statins can reduce awakenings during sleep, suggested they be tried in ME/CFS. A recent fibromyalgia study suggested they might be helpful in that disease as well.
The Unutmaz team also pointed out the possibility that a similar microbiome soup (reduced butyrate, short-chain fatty acids, tryptophan) might precede other disorders as well. Given F. prausnitzii’s sensitivity to inflammatory environments, the Lipkin team questioned whether some sort of gut inflammation might spark its decline.
Still Homeless at the NIH

So long as ME/CFS is homeless at the NIH, it will struggle to get adequate funding.
Both studies came from the NIH-funded ME/CFS research centers (Ian Lipkin’s/Derya Unutmaz’s) and they underscore why these centers are so valuable and why more and bigger centers are needed. The NIH is one of the few places that routinely provides the funding necessary for big studies like these that are crucial for moving fields forward.
Getting results like this proves that these Centers work, demonstrates that ME/CFS is not simply some sort of “wastebasket” disease that is not amenable to study, and shows that this field is missing precisely what we’ve been arguing it’s missing all along – large, rigorously put together studies.
The Gist
- This is the 2nd part of a series of blogs exploring the recent NIH-funded gut studies that may have produced a biomarker for ME/CFS. This study from the Unutmaz research center largely replicated the low butyrate and short-chain fatty acid problems found in the Lipkin center study as well as others.
- Inspired by an earlier study that found dramatic differences in immune functioning in shorter vs longer-duration patients, this study compared a shorter-duration (< 4 years) and a long-duration group (>10 years).
- When it came to distinguishing the ME/CFS patients from the age and sex-matched healthy controls it wasn’t the low butyrate but the low “evenness” found in the ME/CFS patients’ microbiome that really set them apart. Less common bacteria that produced compounds like tryptophan, butyrate, and propionic acid were less abundant.
- Possible cell membrane damage reared its head again when a microbial gene analysis found reduced expression of genes associated with cholesterol and lipids as well as serotonin.
- The greatest dysbiosis (i.e. the greatest gut flora alteration, reduced butyrate levels) and more gut symptoms were found in the shorter-term patients (<10 years).
- With two cholesterol and several lipid metabolites discriminating the longer-duration ME/CFS patients from healthy controls, the older group displayed more metabolic abnormalities, were more likely to pick up a fibromyalgia diagnosis, had a trend towards worsening sleep problems, and had more PEM; i.e. things had not gotten better after ten years.
- Bob Naviaux’s 2016 paper also predicted many of the findings researchers are coming up with today. In a finding that bodes well for the future, six out of the ten metabolic biomarkers the Unutmaz team identified in this paper were also highlighted by the Lipkin team and Hanson teams (Germain) in their large metabolomic studies. That suggests these large studies are also coming up with some core metabolic abnormalities.
- The low xanthine levels suggest that pentoxifylline, an old, reportedly safe, and inexpensive drug that increases the amount of oxygen that can be delivered by the blood to the muscles might be helpful. Canada’s RECLAIM study is assessing this drug in long COVID.
- The Unutmaz team proposed that the depletion of butyrate early in the disease could pave the way for the metabolic abnormalities found later and pointed to a heart disease which suggested a similar scenario exists in that disease.
- These studies from two of the NIH-funded research centers highlight how important centers like these that can produce large and complex studies are. That showed up in spades when the leaders of both studies used their similar results to vigorously push for treatment trials – something the NIH has shied away from in the past in ME/CFS.
- Despite the attention given to ME/CFS by long COVID, and the research centers’ results, many Institutes that provided funding during the first NIH centers’ contract refused to provide even minimal funding this time around. Two NIH Institutes (NAIAD, NINDS) stepped forward to make up the gap but the ME/CFS research centers remain woefully underfunded for a disease of this prevalence and scope.
- An archaic and inefficient funding mechanism that relies on Institutes which bear no responsibility for ME/CFS to provide funding for it is at the root of the problem. ME/CFS is still homeless at the NIH and until it finds a home; i.e until it finds an Institute that is willing to give it its due, it’s hard to see how – short of a Congressional authorization – funding will grow at a suitable pace for those suffering from it.
- We’ll see what happens with the Nath studies. Nath’s intramural project was designed to give the NIH results it could use for future ME/CFS studies. Nath has said the project was successful. Time will tell if Nath’s project will prompt the NIH to do what it’s never done before: fully fund ME/CFS research.
With no Institute willing to take ME/CFS on, ME/CFS has been dependent for about 20 years upon funding from a variety of Institutes – none of which have responsibility for it. That approach was predicted to be a failure at the time and it has been. ME/CFS funding actually declined dramatically during that period and only began to rise again when Francis Collins committed the NIH to reinvigorate ME/CFS research. This time around, many of the contributing Centers refused to provide even minimal funding for ME/CFS.
Something obviously changed between the awarding of the first Center’s 5-year contract and this one to have those Centers drop out. It certainly wasn’t poor results – the Centers have proven themselves. It’s pure speculation, but one wonders if Francis Collins’s retirement had something to do with it. Perhaps Collins – who did reinvigorate ME/CFS research – made it known that he wanted to see more support from the Institutes.
In any case, the departure of many of the Institutes left NIAID (National Institute of Allergy and Infectious Diseases) and NINDS (National Institute of Neurological Diseases and Stroke) to pick up the slack. Thankfully, they did – but only up to the level of funding provided during the pre-COVID contract. While that’s appreciated, it’s also clear that neither Institute is willing to step forward and commit to supporting ME/CFS at the level its seriousness and prevalence indicate it should be supported.
So here we are with a bunch of successful studies that are clearly moving this disease forward in ways that it has not before – and in ways that the NIH has stated it’s wanted to see – and the NIH’s reward has been … more of the same.
Avindra Nath has stated that the intramural study – which Collins designed to produce results the NIH could follow up on – was successful. That’s good news – but with ME/CFS still dependent on this archaic and ineffective funding base – we’ll see what happens.
Conclusion
The most important outcome of this study was that it largely replicated the results of past NIH studies as well as others – thus appearing to provide a solid base of microbiome and metabolic results that focus on a deficiency of butyrate and other short-chain fatty acids and metabolites associated with cell membrane damage.
The senior authors of both papers clearly viewed the studies as stepping stones to more studies and treatment trials. Now they have a target – F. prausnitzii and butyrate-producing bacteria – it’s time to go after it.
Julia Oh stated she viewed the studies as “the prelude to many other mechanistic experiments that we hope to do to understand more about ME/CFS and its underlying causes.” Brent Williams echoed her assessment, stating, “these microbiome-symptom relationships present potentially actionable, manipulatable targets for future therapeutic trials… These trials could perhaps focus on dietary, probiotic, prebiotic, or synbiotic interventions and could provide direct evidence that gut bacteria influence chronic symptom presentation.”
It appears that animal models – a key tool in disease research that the Simmaron team is developing – are now on the table as well. Williams noted that “A tractable mouse model to study the gut microbiome disturbances found in ME/CFS would provide an important tool to evaluate causal hypotheses, mechanisms, and treatments”.
Despite the excellent results, ME/CFS is still clearly hampered by an inefficient funding mechanism at the NIH that relies on the goodwill of Institutes that have no responsibility for the disease. The inadequacy of that approach showed up in spades this year, when, despite the attention given to ME/CFS by long COVID, and the research centers’ results, many Institutes that provided funding during the first NIH centers contract refused to provide even minimal funding this time around.
Short of a Congressional mandate that the NIH fund ME/CFS more, it’s hard to see how major increases in funding for ME/CFS will occur given the current funding situation. We’ll see what happens when the Nath studies come out but if a long COVID pandemic didn’t do it and a successful research centers program didn’t either, one wonders what will.
- Up next – The Gut Pt. III – Two ME/CFS patients seek to dramatically alter their microbiome and achieve health






Thanks Cort! I think it’s high time we organize and start demanding more funding. It’s simply not going to happen without somehow amplifying our incredibly vital need.
All I can ever say is Thank you, once again, Cort! Very interesting for this ol’ gal who has had CFS since 1978 to see that there are differences seen in long duration and short. I sure hope there is treatment coming down the road so that those just getting CFS won’t live the such a long and difficult life as us long haulers have. And I sure hope that a treatment becomes available so that I can leave the insufferable insomnia behind.
Cort are you referring to reduced E.coli in the gut for tryptophan synthesis?
I don’t believe they referred to the specific tryptophan producing bacteria that was in low abundance, actually.
hi Cort, thanks and great effect that you took the effort to promote and link these studies together and that it worked and numerous media outlets have covered them!
we need tons of money indeed for huge researchtrials, mousemodels, treatment trials, etc worldwide.
i really hope that somehow, even if it would make us only a little better, something happens for treatment with what you did and wrote! And the effect on the media outletts.
There is a lot going on. We recently heard about guanifacine (which I’m going to try), pentoxifylline is a possibility, stem cells, monoclonal antibodies, plasmapheresis, Paxlovid, immunoadsorption, Ampligen, ibudilast, statins, a myasthenia drug, Zofin, vagus nerve stimulation, oxaloacetate, hyperbaric oxygen, fecal matter transplant, Axcella’s product, and others – are ALL being trialed in long COVID. Imagine since I wrote that blog others have been added to the list.
https://www.healthrising.org/blog/2023/01/13/long-covid-clinical-trials-big-drugs-big-studies-and-much-more/
Some of these are going to provide benefit.
i wish i was better. i wish my gp stood open for things. I am 98% bedridden, can not go to hospital… have “normal” issues on my age and being over decades verry severe and can not even go for that. can not even go to dentist, neck or back issues, etc I should in fact be turned inside out with all i have on top of my ME but it would be to hard, impossible, they do not do that here, It costs society to much. And I am labeled with CFS… that says here enough…it is still only cfs and rot in hell or CBT. .
The things you name, what i could try on my own, I would not know if they would match with what i take. the rest, I must be shure it helps because long time ago, if i went somewhere, I declined over the effort. I feel really stuck. Need help at my bedside. Or knowing something will really help instead of decline. But here is only de meirleir and oh boy, i declined!!! Sothings can really permanent backfire/damage you.And i need someone watching me… I do not know if you understand?
When you get really ill it becomes harder to experiment and you’re in hostile country to boot. It will take longer for you but I can only think that things will filter down, attitudes will change and things will open up.
I actually don’t see how that could not be – even for the more fragile severely ill patients. Things will be found that will improve you a bit – then you’ll have more resilience – then you’ll be able to try more. I’m not saying a complete return to health but improvement – yes.
The key is having nice big studies that make a splash.
thank you Cort for your kind words! i really hope for a miracle because getting worse and worse. I hope i somehow will survive but at the same time, realistic, i will be in x months in a oldmanshome where it will even be worse then here and decline faster and will be treated awfull. (not knowledgeble, not my needs). I wish there where ME homes like for MS, i would have been long gone here… but no one speaks about it world wide. Even Ron Davis not…And they are really needed for so many of us…
Ampligen?
I don’t get your fixation with it Cort, I really don’t!
I wouldn’t call it a “fixation” (lol). I’d love to see it get a decent trial, though 🙂
How about Cortene? Not looking good?
Still looking for funding 🙁
Interesting indeed – my functional MD tested my gut through Viome and found low butyrate last year. I’m coming up on my three year long COVID-versary in March, with a largely ME/CFS presentation (I have both diagnoses). I’m curious about potential treatments or supplementation. I tried tributyrin supplements last year, but to no effect and perhaps not for long enough. Now I’m taking probiotics, digestive enzymes, and I make this veggie mash each week in an attempt to increase the diversity in my gut biome. No real changes thus far, but I think this is a long-game as far as the gut goes.
Here’s the veggie mash page is anyone’s interested. It takes a lot of spoons to do 12 or more veggies and herbs, so I do 8 or so and just change up the veggies the following week: http://rebeccasnow.com/veggie-mash-up-medicine-for-your-gut/
Dr Datis Kharrazian is great Amy. I have his book, ‘Why isn’t my brain working?’ I think that’s what it’s called anyway. Terry Wahls is also into eating lots of vegetables. I’m intolerant to many vegetables but I eat kale & spinach, take probiotics, digestive enzymes, apple cider vinegar & tip some psyllium husk on my food too (apparently its good food for the good bugs in the gut.) I’ve been doing this for years. I used to take Citricidal and caprylic acid (comes from coconut) which are apparently good for combatting the less helpful bacteria in our gut. I’ve been trying to improve my gut for over 10 years 🙂
Thanks Tracey Anne…I guess it really is a long game :(. I’ve been taking apple cider vinegar in my water as my first drink of the day. It’s so hard to know if this gut stuff can be fixed and if so, how.
I just wrote a really long reply Amy & it didn’t seem to go through & then said it was a duplicate comment & now I think I’ve lost it…
I like the veggie mash. Not to be too blunt but nothing makes me poop like veggies. In fact, nothing makes me poop but veggies. They also often provide small increases in energy. Lately, I’ve been making all sorts of different kinds of cole slaw – just delicious…
Cort, I’m so envious! Ever since COVID, my poops are…let’s just say small and not ideal. I WISH the veggie mash made me go!
Have you tried magnesium supplements? Some are highly laxative and magnesium deficiency is common. You could also try figs when you need to get things moving. Fig rolls were the cure for my child’s issues when lactulose had no impact – and were a lot easier to get into them.
Thank you for the link 😀
My thinking is that trying to improve the gut is sensible. But just because the gut microbiome is altered in pwME doesn’t necessarily mean it’s the cause of the illness. Too much stress, too little sleep, foods we eat/don’t eat etc., can all effect the gut. But having an unhealthy gut isn’t going to help us either. I’ve found that if I irritate my gut, then this can profoundly affect my brain, as in my brain becomes irritated too. I call it Irritable Brain Syndrome, another IBS. So best avoided.
I’ve followed the work of many of the functional medicine people for years and though what they propose has helped to improve my health in general, there was always something missing for me, in terms of energy. But I do think focusing on an anti-inflammatory diet, supplements, attempting to improve the gut, calming the sympathetic nervous system & enhancing the parasympathetic system & good sleep has been vital. It’s been the groundwork for me, I think.
What has made a massive difference to my baseline energy levels, was something that occurred last year. A sort of reconfiguring. It’s hard to describe but it felt like I was finally on the mend from an illness. And then, after months of sleeping really deeply whenever I could, for as long as I could, the feeling of dragging myself around drifted away. I feel more normal now, which is taking a bit of getting used to. I still have food intolerances & brain issues & my life is still stressful but I’m on a different/better physical level of functioning now. So it’s taken me about fifteen years of floundering around, with many very alarming episodes, to get here.
Hi Tracey Anne
do you put the reconfiguring down to all the things you mention in the paragraph before?
thanks
jo
Hi Jo, yes I think so. My thinking about it all, from what I’d read & watched & from what was happening within myself, was that everything needed to align for the possibility of a positive change to occur. I didn’t really know that anything would/could change, I just had an idea in my mind of what I was aiming for.
As in, if my system was under chronic stress then I thought that was more likely to tighten the grip and if I could reach a sort of neutral postion, like the gears in a car, then maybe something could shift. Does that make sense to you?
ps i wonder in all the studys, also on the gut and centers of excellence, how many 25%ME folks are involved? the home and/or bedbound. I get “depressed” thinking about how few. I know the decode ME study is also for verry severelly ill ones. OMF does probebly a bit. There are so many home and/or bedbound, me included.
Probably not many but the results I would hope apply. Since the symptoms of the severely ill can be found in the less severely ill – they’re just worse in the severely ill – my guess and hope is that some of the treatments that help the less severely ill will help in the more severely ill.
The closer we get to the heart of this – and we certainly seem to be getting closer (how close we are we don’t know) – we’ll find treatments that fit and work better.
Thank you Cort, you allways give me a glimmer of hope! I hope i will see these treatments, also for the severelly ill ones and in other countrys.
If ME starts in the gut, what would be the connection between all those different viruses or infections which is mostley the starting point of this disease.
One of the more startling facts I’ve learned from COVID is that viruses don’t need to cause the stomach flu to have a profound effect on the gut – so I think a viral/gut connection is entirely possible.
I’ve had the theory for a while now that immune activation can cause all sorts of changes to the gut. With the raised levels of cytokines, and immune cells scavenging everywhere, if one was say low on certain bacteria it could well be eradicated or allow for another to take over. Especially since F. prausnitzii was said to be fragile, this seems highly possible. Given that for many of us every immune insult seems to cause more disturbance, or at least to the point of disability and a new normal, it seems to reinforce this.
As the researchers demonstrate, normalizing the gut is only one part of the equation since longer patients don’t improve by that alone. If one can normalize the inflammatory response which seems to persist (as shown by Patterson, et al.) with an improved gut, maybe better bacteria will thrive and could re-establish homeostasis. It remains to be seen if Mast Cells or other activated innate immune cells could be causing a downstream cascade.
I had GI problems after food poisoning and I would not doubt that likely set the stage for the onset of ME/POTS/Autoimmunity or whatever it is I have. IVIg makes a massive difference, but even then the gut issues persist with a vengeance.
In July 2021 I had an ILI influenza like illness, with even more:- weakness & brain fog , headache, balance problems, gut problems, D&V, pain , etc I reestablished my protocol after a few days and slowly clawed back over months to a new weakened baseline, at the beginning of February I had another ILI and am still crawling back from that. I initiated as much of the FLCCC protocol[incl IVM] for clearing spike protein as possible, because that is the toxic commonality however you were ‘infected’ .It has helped with clearing the brain fog/headache to a degree, that it is mostly better than pre infxn. The Chinese not that CoV hit the gut 12 days post infxn, It was immediate for me.
Looking back on my history, EBV , tosillectomy, 2 bouts of flu HN , 2 bouts of viral D&V ,possible HPV, before finally sucumbing to ME/CFS/FM with gut issues dysbiosis irritable badder etc, I was set up for this diease.
niacin B1 methyl B12, quercetin plus a mass of other supplements, all keep me afloat. I believe the nigella seeds FLCCC were instrumental in helping brain fog 🙂
Pendulum (probiotics) now has a product called Butyricum which is supposed to “unlock butyrate production). Maybe worth a try?
We don’t know yet the gut microbiome anomaly is a symptom or the cause, and they admitted as much. They are simply proposing a possibility that it could be the cause. It would still be of value if it can serve as a biomarker, but that is iffy too IMO since we don’t know yet the specific anomaly only implies MECFS and nothing else. All we know is that it differs from the healthy controls. If other conditions, like aging or chronic infection for example, also leads to similar anomaly, then it can’t serve as a biomarker for MECFS.
Yes. Cort didn’t mention a key caveat of the researchers, that there is evidence that inactive people without ME/CFS can show some of these micro biome characteristics. So perhaps they show up in ME/CFS because we are inactive or far less active?
So as many questions as there are answers.
My own experience is that I had many more gastro issues earlier in my illness, which aligns with what the researchers have found. How about other people?
I have also said before that my allergies were much worse earlier in my illness. Which could relate to the gut and overactive immune system in the short haulers. Has any one else found this?
Other way round for me – more gut issues over time but probably wouldnt show up in a study like this since I’ve learnt how to partially deal with them. I wonder if they checked what supplements the long haulers were on.
I also started with gastro issues. Some kind of stomach virus with vomiting led me to lose 40 pounds over 3 1/2 months before I (not any of my doctors) discovered that I was sensitive to gluten which I stopped eating and was able to stabilize. I later had another stomach virus episode that resulted in full-blown ME. Now six years into it and, while my other ME symptoms have not improved, my gastro issues are considerably less severe. I’m able to eat many things I could not before, including gluten, as long as I don’t overdo it.
Hi Cort,
Are you aware that hurricane Hazel as she is known , a gastroenterologist turned global researcher is pioneering microbiome and faecal transplant research ? she has found the signatures and effective treatments for such conditions as dementia and Crohn’s disease she also found the microbiome signature for long covid … surely if somebody could touch base with her and explain the ME has been proven to be a precursor for Amazon and other neurodegenerative diseases she would be able to gain funding from the neurological institutes to run a trial on ME patients.
She is collaborating with Global researchers and can tap into multiple funding pots if you YouTube search Dr mobeen interview with hurricane Hazel on his medical seminars you will see all about it it she has even reversed autism through fecal transplant.
I’m going through courts now and as soon as I’ve been to my chemotherapy immunotherapies and your surgeries I’ll be taken on my medical Legal case and I’ll be checking my my own research alongside my biomedical Scientist sister who is now left the NHS and is working in the private sector.
All five of my hypotheses towards the pathophysiological mechanisms contributing to my all proven in the past year ear she specialises in immunology renal transplant cross matching serology electrophoresis and bone marrow transplants so I will be pushing for the corrupt Bristol hospitals to host my trials on plasmapheresis and ivig for the most severely affected.
It’s about time they redeemed themselves .. xoxo
I still think an overactive immune system early in the illness leads all these things. That the microbiome issues are a result, rather than the cause.
That kinda agrees with earlier Columbia study that found the immune system in short term patients overactive while in long term patients underactive. I was thinking about that study when I read the second microbiome study that found the discrepancy between short term and long term patients. Maybe the immune over/underactivity causes the discrepancy in microbiome in short/long term patients? Perhaps the immune system switching to underactivity allowed the gut microbiome to recover in long term patients.
I just saw my immunologist. He has specialized in ME/CFS for over 20 years. He has written several books (in French) on the subject. According to him, you can’t cure ME/CFS if you don’t take care of the microbiota. He advises;
1-PROBIOTICS with as many strains as possible.
2- FUGIZONE to reduce the load of intestinal yeasts, a source of lymphocyte stimulation.
3- MILK THISTLE which decreases the production of interferon and improves 50% of its patients.
4- EPP or ALOE VERA or LAPACHO against viruses, bacteria…
5-PSYLLIUM if constipation.
6- hygieno-dietetic rules, too long to note everything, but basically it is to avoid bringing yeasts (candida and geotrichum candidum), such as foods rich in sugar and wheat flour.
Above all, do not stop taking probiotics and follow a Cretan diet.
Bonjour Claudine,
What’s the doctor called?
Merci
Bonjour niccon, c’est le Dr Cozon au CHU de Lyon. Attention, il prend sa retraite l’ année prochaine. Ci -joint une vidéo où il explique sa théorie sur EM/SFC. https://www.youtube.com/watch?v=rXEJFk341h0.
Hello niccon, this is Dr Cozon at the Lyon University Hospital. Watch out, he’s retiring next year. Attached is a video where he explains his theory on ME/CFS. https://www.youtube.com/watch?v=rXEJFk341h0.
Merci
I was delighted to see two large funded studies looking at the same parameters in ME/CFS. This is what should be required of all funded studies so we learn something important and don’t end with the “but, more study is needed” final statement.
That said, gut dysbiosis is certainly nothing new in this field. I had a study showing severe gut dysbiosis nearly 30 years ago. Treatment with a form of anticoagulant is nothing new either. That was the first medicine I was put on and maybe why I haven’t been as severe as others.
The nature of research doesn’t encourage collaboration and “going back to the well” to see what has been done before. And we all suffer from it.
This statement puzzled me “a new hypothesis for how chronic fatigue syndrome (ME/CFS) begins”. Did I miss something? I didn’t see any explanation for what causes this gut dysbiosis. Maybe viruses, maybe drugs, maybe infections? Where is the “ah-ha” in that statement.
And yet, the VA clearly linked Gulf War Syndrome to exposure to toxins. The same gut dysbiosis reported in these studies is found in GW Syndrome and the symptoms are almost identical to ME/CFS.
(Rather than appeal to NIH for funding, go to the VA and DOD. They have a lot of funding and are serious about figuring this out.)
Why haven’t we explored toxic exposures as the instigating factor in ME/CFS? Has a single study even asked the question?
In a support group we had long ago, one patient had been a pest control operator; another had been working in a building while it was being renovated; another had retrieved and sat in an uphostered chair from her home which had just been tented for termites and I had been exposed to Dursban for flea control in our home. (Dursban was also widely used in the Gulf.)
You may think you haven’t had any toxic exposures, but think back. Before you became ill, did you have your lawn or home sprayed? Did do you have a hobby like woodworking or stained glass that exposed you to toxins? Did you paint your home? Did you drink well water? Live near a dump site or factory spewing out unfiltered wastes? Live near a lake or golf course where they spray with herbicides. I could go on and on.
Today, we live in a toxic soup and to ignore it as a possible instigating factor in ME/CFS is irresponsible.
The WebMD link doesn’t work. Searching manually I did find the entry for pentoxifylline on their website, so there must be something wrong with the link.
Also, the RECLAIM suite of trials is being conducted by the University Health Network in Toronto Canada, the lead investigator is Dr. Angela Cheung. It is specifically for Long Covid patients, you can’t get into the trial if you have ME/CFS or if you have never had a positive Covid-19 test. I didn’t see anything about Health Canada being involved.
Thanks for intensive discussion of the two gut biome studies!
Big article on long covid in Scientific American, with lots of references to ME/CFS. Nath quoted a few times.
https://www.scientificamerican.com/article/long-covid-now-looks-like-a-neurological-disease-helping-doctors-to-focus-treatments/
Excellent article Matthias! Thanks 🙂
Well I can say that in my gradual onset ME/CFS gut issues were not the main problem at first (as long as I did not eat everything), but first real gut problems started last year at around 10yrs of ME/CFS (possibly caused by a range of triggers).
The doctor who you quoted about treating patients successfully came from Dr.Angela Bower as a comment on this other healthrising article.
https://www.healthrising.org/blog/2023/01/13/long-covid-clinical-trials-big-drugs-big-studies-and-much-more/