

In 1905 a Swiss patent officer named Albert Einstein published four groundbreaking papers – one of which was on special relativity. Eleven years later, he added gravitation to the mix in his general relativity hypothesis.
Could high muscle sodium levels be playing a core role in ME/CFS?
It wasn’t until 1919, though, that Einstein’s theory of general relativity was proved correct, and it wasn’t easy. British astronomers traveled halfway around the world to view an extended solar eclipse to determine if, as Einstein predicted, space-time gets curved by massive celestial bodies. When the position of stars appeared to move during the eclipse of the sun (it curved the light), an important part of Einstein’s theory was proved. (Other parts continued to be proved over the years).
Now, it’s hardly fair to compare anyone to Einstein, but a similar process recently took place in chronic fatigue syndrome (ME/CFS). In an April 2021 publication, “Pathophysiology of skeletal muscle disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)”, Klaus Wirth and Carmen Scheibenbogen put on their thinking caps and proposed that the dysfunctional B2AdR receptors’ inability to effectively stimulate the Na+/K+-ATPase enzyme resulted in sodium overload in the muscles of people with ME/CFS.
The consequences of that were potentially vast. The high sodium levels turned the tables on the calcium pump, causing calcium to be imported into the cell instead of getting exported out of it. That calcium overload then impaired the mitochondria and other metabolic parts of the cell from working properly and with that – bam! – a new hypothesis (calcium overload) explaining the fatigue, post-exertional malaise, and inability to exercise, think well, etc. was born.
It was all theory, though, until now. Thanks to funding from the Charite University Hospital in Berlin and researchers from Charite, the Center for Cardiovascular Research and the German Heart Center in Berlin teamed up together to have a go at Wirth and Scheibenbogen’s hypothesis.
The Study
In the introduction of the paper, the authors didn’t go through all findings regarding the muscles in ME/CFS, but they did enough to make one wonder once again why more researchers aren’t jumping at the opportunity to study this disease.
As has been noted many times in Health Rising blogs, in what is thus far a unique finding in all of medicine, repeat exercise studies have repeatedly shown that exercise one day impairs the ability to produce energy (e.g. exercise) the next in ME/CFS. Increased intramuscular acidosis, problems with muscle pH recovery, and reduced proton efflux (e.g. getting rid of the acids) from the muscles have been found as well. None of this is news at this point – some of those findings date back to 2010 – but they underscore how potentially impacted the muscles in ME/CFS are.
It’s not just ME/CFS. Something similar may be going on with the muscles in long COVID as well. A recent article from the Netherlands stated that the “similarities between skeletal muscle alterations in PASC and chronic fatigue syndrome deserve further study”, and invasive exercise tests in long COVID have produced similar results in that disease.
The Study

The study used a hand grip test to assess muscle fatigue. Fatigue was higher at all points in ME/CFS.
The Petter/Kleim study, “Muscle sodium content in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome”, used an MRI to assess the sodium content of the participants’ (6 ME/CFS and 6 healthy controls) calf muscles before and after exertion.
First, the participants rested – and got a baseline MRI scan – and then did heel raises to exhaustion (30 secs) – and had their calves scanned again. Handgrip strength was also assessed at different times to assess the degree of muscle fatigue. Five different muscle compartments were tested. Factors like BMI, age, gender and salt intake did not differ between the ME/CFS patients and the healthy controls.
Results
The study found significantly increased sodium levels in the ME/CFS patients’ muscles – both before and after exercise. With that one test, part of the Wirth/Scheibenbogen hypothesis was validated. Handgrip strength was also reduced and was associated with fatigue.
Importantly, the study also found a positive relationship between muscle fatigue (as demonstrated in the handgrip test) and muscle sodium levels; i.e. the higher the muscle sodium levels were, the greater the muscle fatigue. That made sense.
The sodium-proton exchanger subtype1 (NHE1) exchanges the acids (protons) produced during exercise for sodium ions. In low-energy states, though, proton production goes way up – causing sodium levels in the cell to climb. The sodium-potassium pump, Na+/K+- ATPase, is tasked with removing the sodium but – and here’s the clincher – note that ATPase – it takes a lot of ATP to make the pump work, and if ATP is scarce, it can’t function properly.
The B2 adrenergic receptors that Scheibenbogen and others have been studying in ME/CFS come into play as well. First of all, by hampering blood flows, they could be causing a multitude of problems including ATP production, but they also, interestingly enough, stimulate NA+/K+ ATPase to remove sodium.
The core problem – an essential cellular pump is not getting the stimulation it needs to work.
If Scheibenbogen and Wirth are correct, balky B2 adrenergic receptors are not only causing problems with the blood vessels but they’re also messing up the transport of sodium out of the cell. Two other studies which found low intracellular potassium levels suggest this may be happening in ME/CFS.
Plus, other factors could be in play: the small fiber neuropathy found in ME/CFS could lower the levels of another sodium-potassium pump stimulator – CGRP.
Plus, people with ME/CFS appear to have low levels of several other sodium-potassium pump stimulators (cortisol, aldosterone, and T3/triiodothyronine). Finally, the mitochondrial dysfunction found in ME/CFS could be affecting ATPase – a key feature of the sodium-potassium pump. All told, a multitude of factors could be affecting this critical intracellular pump in ME/CFS.
Because the authors believe those high pre- and post-exercise sodium cellular levels they found in ME/CFS are probably much lower than those which occur during the exercise itself, the current findings may be understating the problem. (It’s impossible to directly measure sodium levels during exercise.)
The authors believe the crux of the matter in the muscles is that high sodium levels can cause the cell to import instead of export calcium. The impaired blood flows are making the situation worse by reducing ATP production in the muscles, but it’s the calcium overload that really takes a hammer to the mitochondria and, as it’s doing so, it also damages (the already damaged) blood vessels. (Nice!)
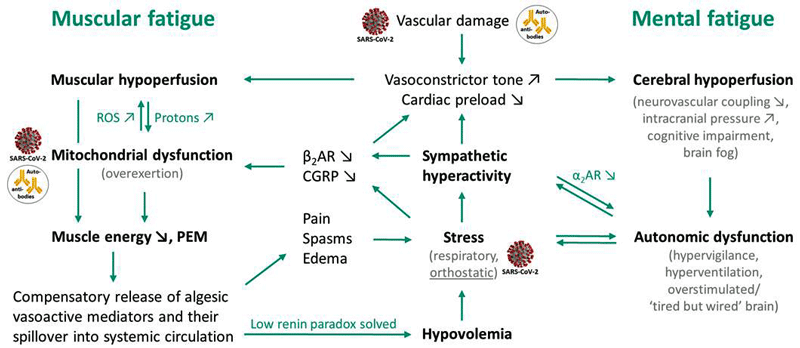
The model
The authors state:
“Changes in intracellular and mitochondrial calcium via NCX induced by the rise in intramuscular sodium are considered the key pathomechanism in the energetic and mitochondrial disturbance in ME/CFS”.
They believe this mix of mitochondrial and endothelial (blood vessel) damage produce a vicious circle in ME/CFS.
“This entire mitochondrial and blood vessel mix vicious circle … can explain post-exertional malaise, exercise intolerance and chronifcation (chronic illness state).”
The finding of high sodium levels in the muscles before and after exercise doesn’t prove that calcium loading is occurring – they weren’t able to measure intracellular calcium levels – but they do demonstrate that the conditions are ripe for that to occur. They’re also enough for Wirth to move forward (see below).
The Gist
- Wirth and Scheibenbogen have produced a series of hypothesis papers on chronic fatigue syndrome (ME/CFS). Like a Russian nested egg, each has built on the past. Their ability to keep churning out complicated hypothesis papers that build on and agree with each other has been unique in ME/CFS.
- Their latest hypothesis paper implicated the B2aDR receptors in the mitochondrial dysfunction in ME/CFS. They proposed that balky B2aDR receptors in ME/CFS are causing the sodium-potassium pump to fail to remove sodium out of the cell – resulting in high intracellular sodium levels.
- This, in turn, causes calcium levels to rise and disrupt mitochondrial output. It’s this calcium overload that they believe is predominantly responsible for low energy levels in ME/CFS.
- This small German study (12 people) put their sodium loading hypothesis to the test. During rest and after exercise (heel raises to exhaustion), an MRI assessed muscle sodium levels. A separate handgrip test assessed muscle fatigue.
- The results vindicated their hypothesis: muscle sodium levels were greatly increased both at rest and after exercise. Plus, the handgrip test found that muscle fatigue was correlated with muscle sodium levels as well.
- They believe this may be happening for a number of reasons. For one, poorly functioning B2aDR receptors are failing to stimulate the sodium-potassium pump to remove sodium from the cell. At the same time they’re doing that, by constricting blood flows, they’re reducing ATP production and the sodium-potassium pump (Na+K+ATPase) requires a lot of ATP.
- Other factors in ME/CFS (low CGRP, cortisol, aldosterone, and T3/triiodothyronine levels) that stimulate the sodium-potassium pump may also come into play.
- Note that there’s a back-up sodium pump that can function when calcium levels are high and should jump in to prevent this situation. That may not be happening in ME/CFS, though, because of another factor – the Epstein-Barr virus (EBV). Not only does EBV shares a common genetic sequence with this pump, but a recent study found elevated levels of an EBV antibody that could be causing the body to attack this back-up pump – leaving the cells of people with ME/CFS possibly helpless against sodium and calcium influxes.
- The crux of the matter, though, is that high intracellular sodium levels can cause the cell to import instead of export calcium, and it’s this calcium overload that they believe is causing the energy production problems in this disease:
- “Changes in intracellular and mitochondrial calcium via NCX induced by the rise in intramuscular sodium are considered the key pathomechanism in the energetic and mitochondrial disturbance in ME/CFS”.
- They believe that the situation found in ME/CFS – reduced blood flows (B2ADr issues) and calcium overload – produces a vicious circle that is hard to break out of:
- “This entire mitochondrial and blood vessel mix vicious circle … can explain post-exertional malaise, exercise intolerance and chronification (chronic illness state).”
- Note that the way to fix this problem is to go to the source – and get the sodium-potassium pump working again.
- When asked if the high sodium dietary intake often seen in ME/CFS could be exacerbating this problem, the answer was an emphatic “no”. In fact, if the high sodium intake was helping with orthostatic intolerance (problems sitting up or standing), then it’s probably helping the situation.
- Wirth wrote to me that the hypothesis and the study results are getting us closer to a good “therapeutic strategy” for ME/CFS. When I asked him about that, he reported that he believes that drugs are available that could get at the core of this situation and has formed a company to facilitate that. That company is now looking for seed funding.
An Epstein-Barr Virus Connection?
Wirth reported that another possible mechanism may be in play involving something called the NCKX3 (potassium-dependent sodium-calcium exchanger). NCKX3 is a kind of back-up or reserve sodium-calcium exchanger that jumps into action when intracellular sodium levels are high. Unlike the sodium-calcium exchanger the authors focused on in the paper, though, the NCKX3 still removes calcium from the cell during times of high sodium levels. Thus it should be protecting ME/CFS patients’ muscle cells from calcium overload
People with ME/CFS, though, may have a complicating factor – the Epstein-Barr virus. It turns out that genetic sequences found in NCKX3 share a close resemblance to those found in an EBV antigen called EBNA6_0070. (An antigen is anything – a part of a pathogen, a toxin, etc. that provokes an immune response in the body. In this case, the antigen is found on an EBV protein.)
This protein is produced during one of EBV’s latency stages. Latency means that the virus is not replicating – it does not mean that the virus is not active. The virus can actually be quite active during latency.
The really interesting thing about this EBV antigen and ME/CFS is that just last year, Sepulveda et. Al found high levels of antibodies in two EBV antibodies – one of which just happened to be EBNA6_007. Sepulveda proposed that molecular mimicry – a state in which the immune system mistakenly directs an attack against human tissues that have similar genetic sequences as a foreign substance or antigen – might be occurring in ME/CFS and urged further study.
Wirth pointed out that if these antibodies were indeed harming NCXK3’s functioning, “one of the last protective mechanisms against calcium overload would be abolished and would fail.” He warned that this is still speculative and wrote that cellular studies are now needed to determine if this antibody is actually affecting the functioning of this sodium-calcium exchanger. He noted, though, that is just one of the possible scenarios that could be producing calcium overload in the muscle cells of ME/CFS patients.
Questions
Given that this is about sodium, and that many people with ME/CFS have high sodium intake, I thought it made sense to if high salt intake could have an effect on the high intracellular sodium levels he saw in this study?
It turned out it couldn’t. This is partly because the orthostatic stress (eg. difficulties sitting up or standing) issues may be making the calcium loading problems in ME/CFS worse. This is because chronic stress reduces (blood) perfusion to the brain and muscles and can desensitize ß2AdR and alpha2-adrenergic receptors. Since the “ß2AdR receptors are the most potent activators of the Na-K-ATPase in skeletal muscle”, any desensitization of these receptors can make the situation worse. Thus anything that helps with orthostatic intolerance such as increased salt and fluid intake could be helpful.
Plus, desensitization of the alpha2-adrenergic receptors – which inhibit norepinephrine release – allows the noradrenergic neurons to release more and more norepinephrine, resulting in hypervigilance (a “wired” state), blood vessel constriction, and reduced blood flows to the muscles.
In other words, reducing sodium uptake could make the situation worse. Plus, Wirth explained that:
“sodium uptake in the hypovolemic situation of ME/CFS causes water retention and volume expansion of the blood, but does not lead to a rise in plasma sodium.” In fact, “you cannot lower intracellular sodium in skeletal muscle by lowering sodium intake because high intramuscular sodium is due to insufficient Na-K-ATPase mainly.”
The way to reduce the high intramuscular sodium in ME/CFS is to find a drug that stimulates Na-K-ATPase to get sodium out of the cell.
Given the important role the B2AdR receptors play in their hypothesis I asked if problems with the receptors were showing up in long COVID?
“Autoantibodies against B2AdR have also been found in long Covid (Szewczykowski et al, Wallukat et Al.). Autoantibodies as well as desensitization by chronic stress are the main presumed disturbances in our hypothesis. In long COVID, orthostatic intolerance occurs early on. Orthostatic stress is the biggest stressor in our hypothesis.”
Wirth has created a startup company to bring a drug to market that he believes could help resolve the core issues in ME/CFS.
Finally, I asked about next steps.
“You told me that the hypothesis and the study results are getting us closer to a good “therapeutic strategy” for ME/CFS. Are there drugs or treatments that could help? What are your next steps?”
“ME/CFS is one of the most complicated diseases. Although according to our unifying hypothesis, the pathophysiology may be understood, it remains complex. Such complex disease hypotheses are usually not proven or refuted by a single biomarker, patient or other experimental findings. The final proof of a disease hypothesis comes from the efficacy of a drug principle that is derived out of the disease hypothesis.”
“With the demonstration of efficacy of a drug principle, the hypothesis would be proven and a drug treatment would be available, supposed it would be sufficiently tolerated. We are at that point now and have derived pharmacological mechanisms necessary to address the core of the pathophysiological mechanisms, the vicious circles, that keep the disease process running. Key disturbances are the ionic disturbances in skeletal muscle and disturbed skeletal muscle and cerebral blood flow. To realize this drug project, a company has been recently founded and is looking for seed funding to finance the project.”
Keep the Information Coming!’
Support Health Rising






It would be great if some of these treatments helped the immune system combat EBV.
It seems to be a contributing factor in so many cases of M.E. & Long Covid.
Very interesting and hope providing! Thank you all researchers and Cort!
Where does the oxygen craving come in to this one wonders?
I know that periods I must work harder with singing practice (calmly at home, for the choir I still try to hang on to) I feel generally better. Even if the practice itself is tiring and energy consuming.
I feel that the extra oxygen intake during this practice of singing is helping otherwise in life too.
I deteriorate at choir holidays, summer and Christmas when I do no singing practice.
But one can never be sure of when you are overdoing things.
It seems like there is always a shortage of oxygen. As if the breathing muscles themselves can not work properly to get the air and have to be “forced”= nudged to do more. And after a while they themself, dispite protests, have had the extra air they can work better and start providing the rest of the body too. (Night CPAP is not enough; one would need it at daytime too).
And this day’s one hour singing practise muscle improvement is holding over to the next day. Singing and working these muscles is usually easier the next day.
Also just 5 mg of an old unspecific “beta blocker” that calms the “nerves” and heart palpitations at performances, also seems to calm and aid the breathing singing muscles, taken hours before. But not often, tolerance seems to build up.
Even if otherwise all this is somehow also tiring extra air seems important.
This balancing, pacing is very hard to understand and do. I never know when or IF I am harming myself for the future.
But extra oxygen must be crucial if small blood vessels are damaged.
That’s a great way to get more oxygen in, Susan! I think that’s a great practice :). I’ve heard its quite health inducing.
Wirth actually produced another hypothesis blog focusing on orthostatic intolerance and explaining it using blood vessel problems – https://pubmed.ncbi.nlm.nih.gov/36557009/ – a blog on that is coming up.
Yes, on the oxygen issue. I’ve been watching documentaries about people, climbing Mount Everest. It seems that the hypoxia (high altitude sickness) symptoms they experience is very similar to me/CFS symptoms.
Yes indeed, I have Chronic Fatigue AND Epstein Barr. IS there any way to definitively get rid of the virus?
Until this company finalizes its drug to reverse the overload of Sodium and Calcium in the cells, undoing EBV seems to be the only path. CAN it be done?
Every Pharmacology Department in the major Med Schools has graduate students placing micro-fine electrodes into the calcium channels of mouse Pheochromocytoma cells to research the efficacy of new calcium channel blocking chemicals. Calcium channel blockers are very effective at treating severe hypertension. The technology to measure calcium levels in cells has existed for decades. We should be able to measure mitochondrial levels of calcium by now. Hundreds, if not thousands, of compounds have already been studied in terms of their ability to manipulate cellular channels. The solution may be already sitting in some grad student’s lab notes.
Please keep us updated about this promising german drug, Cort! Are there any news yet?
Great article. I have long been an admirer of Carmen’s work.
I do believe ME/CFS is Epstein Barr virus related. Hopeful for treatments in the future and continued funding for scientific studies.
Would this mean that it could potentially help to take a B2AdR blocker?
If I remember correctly, the problem with those receptors is that they’re not functioning properly and they need to function properly to stimulate the sodium-potassium pump so my guess is that a B2AdR blocker would not be helpful.
These researchers are speculating that there are antibodies to receptors. If that is, indeed, the case, Rituximab should throw many ME/CFS patients into a remission. Here we go again. More debris thrown from the never ending ME/CFS tornado!
I’ve read five abstracts now reporting antibodies to receptors in ME/CFS. Is the NIH trying to peer review, and replicate this data? If no, why not? ME/CFS does not afflict %0.3 of the US population. It’s much closer to %1.0, or 3 million Americans.
Did you try, Ingrid? Did it work? LG
Have you tried it, Ingrid? Did it help? LG
No, sorry, I haven’t tried it. Still looking for things that can help!
Aren’t there any already medicine to help this sodium calcium thing?
Discovering a new medicine may take years and will also take many years for trials
Are we doomed ?
As I am lying here 100% bedbound in dark.
Thank you medical science.
My understanding – hopefully, it’s correct – is that Wirth has identified a compound or drug that may helpful.
Hi Cort, could you please elaborate more on why you think he has identified existing drugs? I dont see why he would need to create a new company with seed investors in that case, he could just run a trial with the drug. Im afraid he wants to develop a brand new drug that completes phase 3 in approximately 500 years. Thanks
Coming away from communicating with him my recollection is that he’s found something. I don’t know if it’s an existing drug or a compound of some sort. Trials don’t seem like they’d be that expensive but they are actually EXTREMELY expensive to run, though.
Look at Cortene – it took them years to get enough funding to run their small trial and even though it was a success they’re still looking for funding. Getting funding is not easy. I sure hope he’s able to do that.
How would dietary or supplementary intake of calcium affect this?
Great question. Court, do you have any information about this?
I assume that it would be the same thing as with sodium: it’s the ineffectiveness of the sodium-potassium pump inside the cell that is causing the cell to get flooded with calcium – not the external environment. If I remember correctly, the amount of calcium in the cell is many, many times lower than outside the cell.
The reason I ask is that when I take supplementary calcium, my muscles become weak, wobbly with more fasciculation than usual. However my calcium levels are nowhere near optimal (in hair tissue mineral analysis). On a toxic metals test I found the mysterious sentence written with the results: ‘calcium in ion channels’ and I’ve never been able to find out what it means or why it was worthy of comment. Presumably it was where it wasn’t supposed to be and I’m now wondering if it has anything to do with this research.
Very interesting – something is clearly going on there. I believe that calcium is important for muscle contraction. Could you be leaking calcium into areas it’s not supposed to go?
Have you tried Low Dose Naltrexone?
Australian researchers at Griffith University have studies showing improvements in calcium function in the TRPM3 ion channel.
https://www.healthrising.org/blog/2021/09/07/ldn-low-dose-naltrexone-chronic-fatigue-syndrome-natural-killer-cell/
https://news.griffith.edu.au/2022/08/11/world-first-chronic-fatigue-syndrome-findings-could-fast-track-response-to-long-covid/
Yes thank you I’m already taking it and have been for some time.
From the very start of my illness, there have been only three CONSISTENT abnormalities showing up in my lab work; everything else shifts in and out of “normal range.”
The three? Reactivating EBV and HHV6, and high calcium. My PCP was, thankfully, willing to chase potential issues like parathyroid disease, malignancies, and such but of course found nothing. He chalked it up to the ME/CFS, although he admitted he couldn’t fathom the connection.
Is it possible that calcium is leaching into blood serum?
Good to know. Calcium supplementation, which is supposedly necessary for osteoporosis, seems to make me feel worse. Sounds like it doesn’t affect this mechanism however.
No mention of the new company name. I’ve just looked at one of the doctors profiles and found Kosa Pharma. The website is down. These guys need seed money. This sounds like a very promising avenue.
Good try, Sam! The only thing I know is that it is not KOSA Pharma 🙂
Its name is Mitodicure. See here:
https://mitodicure.com/
Thank you for a great article and being relentless. I will donate today.
Thanks!
Thank you for this very interesting article! I would love to know the name of the start-up!
Its name is Mitodicure. See here:
https://mitodicure.com/
OTC Guaifenesin apparently pushes out excess phosphate and related calcium while helping to improve ATP and mitochondrial function. For some, EBV, ME and pain symptoms are exacerbated then dissipate while guaifenesin does its work. Connection?
Thank you for bringing it up. I took Guaifenesin from mid-2008 to end of 2010. Hope someone knowledgeable will comment on it.
Did it benifitted you during 2008-10
I knew about the paper but not the start-up. Thanks for bringing the news from Germany to me in Germany, Cort 🙂
I don’t think I have classical POTS, but I do have a sort of orthostatic intolerance that feels as if caused by a tiring of the skeletal muscles, i.e. the muscles tiring of holding the body upright while sitting or standing. (I’ve been wondering if that might cause further ripple effects of compression on spine and nerves). On the other hand, on days of recovery I sometimes suddenly had a feeling of my spine rising up in itself. So I’m quite sure there is a muscle-related problem. Also on bad days like currently there is an acute feeling of a lack of energy in my body cells – like hunger or thirst, but for energy. There’s also a feeling as if cells were poisoned somehow. So I’m quite sure it’s something relating to muscles and metabolism.
I’m a literature teacher and so the language we use to try and describe our symptoms intrigues me, as I realize language is contributing to the failure to communicate the severity of our illness to our docs/NIH/congress representatives/family/friends/etc.
Your post includes language that I have been using since my triggering event and that I rarely see elsewhere–you say “it feels as if [your] cells have been ‘poisoned’.” Yes, that word (‘poisoned’) hits the mark.
(The other analogy I use to describe PEM is, “It feels like each of my muscles has its own stomach, and they are all nauseous.”)
I have wondered what a list of the most effective descriptors of PEM/fatigue/exercise intolerance would look like.
Dear Brian, I have always been amazed at how ME/CFS patients, independently from each other, have come up with the same descriptors and metaphors around the globe! For example, I came up with the word “crash” and the “battery & charger” analogy for myself before I even identified with ME/CFS. Then later when I joined the ME/CFS online community, I was electrified to see other patients (and as far as I remember even the then CDC website) use the same analogies. Yes, I’ve also been thinking it would be fun to have a list of creative metaphors.
I am also using “it’s like overdrawing your energy bank account, and having to pay a hefty penalty for it”, and many more. Yay for language for making things more palpable for healthy people, when they cannot relate to own lived experience.
Brian, yes I call my pain a “nauseating poisonous ache”
I was diagnosed with CFS many years ago (triggering event 1985) and finally in about 2017 I, too, came up with the word “poisoned.” “I feel like I am being poisoned.” Not long after that I tested positive for Mast Cell Activation Syndrome. Perhaps mast cells releasing 200 chemicals inappropriately would feel like poison! MCAS treatment has helped me, but it is not the whole enchilada. I’ve had lots of diagnoses and have a good idea of where many of my symptoms come from (mold remediation of our house following a super high ERMI score, Lyme disease (positive with IGENEX testing), hEDS (9/9 on Beighton), POTS (positive Tilt Table Test), etc. BUT, I still think there is something going on with my mitochondria or inside my cells. I was 32 when symptoms began in earnest, now I am 70. Not sure how much longer I can wait on studies, people seeking money from my pain and fatigue, etc. 🙁 “Doctor Google” has helped me find almost ALL of my eventual confirmed diagnoses!
Im wondering if he is working on an analog of 2.4-DNP low dose which is also trialed for MS and ALS by by Mitochon
https://www.mitochonpharma.com/pipeline/multiple-sclerosis/
“The ability of DNP to lower mitochondrial free radical generation and prevent mitochondrial Ca2+ accumulation could provide a therapeutic benefit in disorders involving cellular calcium overload. ”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5337177/
https://pubmed.ncbi.nlm.nih.gov/30909602/#&gid=article-figures&pid=figure-3-uid-2
“if the threshold for mitochondrial Ca2+ storage is exceeded, the mitochondrial permeability transition pore (mPTP) is formed and the mitochondria are destroyed setting up a cascade for neighboring mitochondrial destruction [16]. (B) Calcium overload of the mitochondria may be reduced, even in the presence of a sudden rise of cytosol Ca2+ from the ER, in TBI and other conditions of metabolic stress, in the presence of DNP by closing the calcium uniporter channel. Reduced intra-mitochondrial calcium removes the driving force toward apoptosis, thereby saving the neuron, myotube and other at-risk cell types.”
That is fascinating stuff! How about that…
At the very least it shows the detrimental effects too much intracellular calcium can have on the mitochondria.
This, again, shows how we need to keep our eyes on other diseases.
Thanks!
Thanks! What is the name of this substance in Europe? Is it available as a drug for MS patients?
What a great article you have written again Cort. Thanks. I feel that Scheibenbogen has landed on the right path. I don’t understand something. Can the calcium levels not be measured in the blood before and after exercise? And sodium increases blood volume?
I wonder if the adrenaline receptor is stimulated or blocked. Because the production of noradrenaline should promote blood flow to the muscles during exercise (especially when we are in the fight-flight mode). The blood is then removed from the intestines and less goes to the brain.
I think the problem is measuring it in the intracellular spaces. I asked him about this and he wrote:
“This is not possible in the patients. Calcium in the cell is about 5000 times lower in the cell than in the extracellular space. It cannot be measured in vivo with current techniques.”
“Plus, people with ME/CFS appear to have low levels of several other sodium-potassium pump stimulators (cortisol, aldosterone, and T3/triiodothyronine).”
These three hormones, cortisol, aldosterone and T3 are very helpful, I have found. I am 28 years into ME/CFS.
Can you elaborate how aldosterone Cortisol and T3 helped you?
These are all clinically low in me. Also testably low with the right kind of tests. The cortisol and T3 get my system up to a balanced/normal level of functioning—that is, energy. And the aldosterone keeps enough sodium onboard to maintain a more normal blood volume and effective hydration.
Are u prescribed fludocortisone and hydrocortisone for low cortisol?
I too have low morning cortisol( 5 normal range 6.5-20)
Yes
Thanks for this piece, very interesting. What’s the name of the company they founded?
I don’t know.
Thanks for the great article Cort. Does “have derived pharmacological mechanisms” mean they have a drug combination in mind and are establishing a company to do clinical trials? Some people (e.g. Raelan Agle), have had success recovering from ME/CFS through extremely slow anaerobic strength training, avoiding aerobic exercise for a year or more with heart rate monitors and other techniques. I wonder how slowly building muscle and presumably mitochondria would shift all these negative processes the researchers discovered? The recoverees also work really hard at avoiding stimulating their nervous system, using meditation, diet, and cognitive approaches to minimize anxiety, fight-or-flight states. Graded exercise therapy has harmed ME/CFS patients in general, but there was no differentiating between aerobic exercise and very slow strength training. I am going to try this specific approach over a year and see if it helps. It sounds more promising. I used to lift weights, but injured my muscles right away after I got ME. But this requires that you stay within your pacing limits and start with no weights and do tiny increments over time. So I’m feeling hopeful about it. It looks like after a year of slow muscle building, you may slowly be able to start aerobic exercise, a minute at a time. I imagine I’ll have plenty of time to wait anyhow for the various drug trials to finish. So it’s something to try.
Very interesting approach, Chris…What a creative group we have! Yes, I believe a compound or drug has been identified. I have no idea what it is.
I suspect it will be a drug combination because they are dealing with a couple complex feedback mechanisms. I wonder if it will end up us a drug cocktail like they developed in AIDS.
I have been practicing Raelan Agle’s approach about two months now, though not so much exercising yet (except for slow walks with my dog) and it seems to be having a small positive effect so far.
Strictly limiting my physical and mental exertions to few things each day and I’m able to minimize symptoms and seem to recover more after reach night’s sleep. But slow going….
I’ve heard good things about her approach, John – just talked to someone who is using it and other things and it’s really helping :). Good luck!
She also has a youtube channel with a lot of helpful videos
What’s that approach??
She describes her story and approach in her book, Finding Freedom. It’s a holistic approach involving plant-based diet, fasting, learning to think more positively, careful anaerobic exercise and other things.
I’d already found my own way to several aspects, but after reading her book I opted to go for the plant-based diet and hot Epsom baths, as these were new for me.
I have calcium overload and this all makes sense. I flare up when I consume vitamin D, phosphate and magnesium. Sodium and potassium are the only thing I can tolerate. Donating blood also makes my fatigue and burning pain subside for a while. I look forward to where this leads.
Google search: “The more salty you eat, the more calcium you lose. “Salt is known to cause excessive calcium excretion through the kidneys,” says Felicia Cosman, MD“
Are you donating blood? And you have ME/CFS? If you are sick it is not allowed to give blood.
Yes, I have hereditary hemochromatosis. A standard of care for this condition.
How interesting, may I ask you what the connection is between VitD and calcium?
I have low VitD but do not like taking it. I had started with a really high dose (as that helps some fatigued people) and it made me all wired-up.
I do not know why it negatively affects me. It could be related to a genetic mutation of the vdr gene. Either way, I am never deficient and often in the higher/toxic range.
Since you summoned Einstein, I, a former physics major, will have to chime in :-). A hypothesis is validated by testing its predictions apart from the observations that the hypothesis is derived from. In this case, however, it’s more like reaffirming the observations that was the basis, not the prediction, of the hypothesis. PEM, as it is defined, requires 24-48 hours delay. But the test measured sodium level only after minutes. This sodium overload in muscles is what prompted the MECFS hypothesis at the first place as far as I understand.
An additional problem with the study is that they compared the subjects with healthy controls. We don’t know if deconditioned or chronically ill subjects, in addition to MECFS patients, would have shown similar anomaly of sodium level.
Even if sodium overload is detected after 24 hours, it still doesn’t prove that it is the cause of MECFS. For that, they’ll need to preclude the possibility that b2adr anomaly is a consequence of MECFS. The sodium overload after 24 hours doesn’t do that.
I don’t really understand this – “A hypothesis is validated by testing its predictions apart from the observations that the hypothesis is derived from” but this is clearly just a step and I don’t know that we’re going to see the other steps. Wirth believes the best test for the hypothesis is in the end going to be a clinical trial that alters intracellular sodium levels in the cells and improves symptoms (or not) . I imagine we will see that first.
There is always the deconditioning factor to figure into this. Not everybody who has ME/CFS is deconditioned, of course. I’m not – I’m not conditioned but I’m also certainly not deconditioned either – I go for regular walks. At one point I looked for how many steps a day one needs to not be deconditioned and I couldn’t find it.
This deconditioning argument has been a thorn in our side for many years because deconditioning can result in orthostatic intolerance and can effect some exercise study results. Studies, though, have proven that while deconditioning may be present it’s is not causing either orthostatic intolerance or the exercise results in ME/CFS.
https://www.healthrising.org/blog/2018/12/16/deconditioning-denied-chronic-fatigue-syndrome-deconditioning-myth/
I don’t know if deconditioning affects sodium levels in the cell or the sodium potassium pump. Deconditioning doesn’t affect everything but I have no idea.
Here is an example of what I meant: A observes the stock market from 1990-2000 and concludes that growth stocks will always outperform value stocks; then B validates the hypothesis by testing it against 1990-2000 data. The paper is obviously not that extreme, but it tested sodium level minutes after rather than the next day as done in 2-day CPET test. And the hypothesis already observed altered sodium level from a2adr anomaly in MECFS patients, so it didn’t uncover anything new as far as I can tell. If b2adr anomaly predicts PEM, then it should have tested the sodium level 24 hours later, not minutes after.
And, yeah, I understand the aversion to deconditioning argument. But the hypothesis that MECFS is deconditioning is already debunked by PEM and 2-day CPET test. We just need to make sure that any new anomaly we see is unique to MECFS, not deconditioning or anything else,
I don’t get it. The hypothesis proposed that high sodium levels in the muscles were present – and the study was done to test that. I did a search of sodium and ME/CFS and don’t see any studies finding high intracellular sodium levels in ME/CFS before this.
Yes, in a perfect world, we would have similarly conditioned HC’s and a longer testing period and the PEM aspect has certainly not been proven. Bringing Einstein in again (sorry Klaus) I read it took a hundred years to prove some parts of his hypotheses. So yes there’s certainly more to do.
Looks like I misunderstood, Cort. I focused on “The Study” portion of the blog and thought that the study was about proving b2adr/sodium anomaly resulting in PEM. So I assumed the hypothesis already established sodium overload and the study only reestablished the sodium overload without proving PEM. I read again from the beginning: the hypothesis only observed high level of b2adr antibodies and surmised the resulting vasoconstriction and sodium overload would explain the MECFS symptoms. And then the study was about proving the elevated sodium level and its relation to fatigue, not PEM. So, mea culpa; you were right and I was wrong.
Appreciate that TK 🙂
Yes like the arteries. My CT Calcium score was 1842. My ONLY risk factor appears to be viral induced GBS leading to CFS/ME.
I’ve also had Epstein Barr in my teens. and for decades I had severe HSV outbreaks treated almost daily with acyclovir. Post GBS I have NOT had a single hsv outbreak in 10 years. GBS a cure for herpes simplex?
If done LDN with negligible results.
I should have had a zero calcium score.
I am sick for +10 yrs now and I think my muscles still look strong (not as if I lost a lot of muscle mass), and in particular under adrenaline I have a lot of grip strength; while sometimes I cannot lift a cup without exceeding the systemic energy limit of the body. I remember being deconditioned very well back from when I was a student and starting to go for runs for the first time ever; that felt totally different. So I think deconditioning is if at all only a plausible add-on layer, never causative.
I have even wondered if the fact of constant nervous hyperarousal (which also results in constant muscular tension) as well as the fact that we so often operate on the aerobic limit with small daily tasks are somehow keeping muscles in a training-like state and preserving muscle mass somehow?
“I have even wondered if the fact of constant nervous hyperarousal (which also results in constant muscular tension) as well as the fact that we so often operate on the aerobic limit with small daily tasks are somehow keeping muscles in a training-like state and preserving muscle mass somehow?”
Interesting idea. I like the connection between constant nervous system hyperarousal and constant muscular tension. That intuitively seems very true to me – but I’ve never heard it put that way…Thanks!
Interesting, I also have retained reasonable muscle,especially in the arms, even after so long not doing weights.
Thank you Cort. It’s amazing to me that they were able to zero in on an ionic channel/ receptor with MRI technology. It’s a very interesting explanation for OI as well as muscle dysfunction.I hope they get sufficient funding to trial a drug remedy !
Oh….one more thing…All these ionic pumps require energy of course …. Which brings us right back to impaired mitochondrial
energy production……..oh well !!!
Dear Cort, thank you for this encouraging article. Lost a bit in translation, do you have the link for the startup?? Can you ask the doctors if we can contribute in any way?
Sorry, I don’t have a link for the startup – it doesn’t have a website at this point.
thanks. When you talk to them in the next time, can you please ask them if they have a website. Also, maybe OMF would be interested in joint forces with them??
Will do.
Cort, I admire and appreciate your clear prose, good analogies and probing questions. Thanks for explaining this complex proposed mechanism.
Thanks!
No website yet – in fact, the company has not even been named yet.
Cort, thank you for asking about high dietary sodium intake, especially as I sit here guzzling my electrolyte drink ;). I’m relieved that’s not a factor.
An anti- Na/K ATPase antibody was used to increase its activity in a mouse model of Parkinson’s & was successful in clearing alpha synuclein & improving symptoms:
https://doi.org/10.1126/sciadv.abc5062
Whoa! Nice find. Good to see some potential options opening up.
Hi thanks for this thought provoking article. I’m writing because my 18 year old son who has had ME/CFS for 2 years has had abnormally high ionized calcium for at least 6 months. Interestingly his regular blood calcium has been normal except for once, otherwise we wouldn’t have caught it. (Parathyroid issues have been ruled out.). He just had a scan which showed low bone density.
He is not hearing of other ME/CFS patients with hypercalcemia, but I wonder if this is because they haven’t had ionized calcium measured as that isn’t standard.
Do you think his hypercalcemia is related to the theory of this article? Thanks!
I don’t anything about ionized calcium but Wirth reported that intracellular calcium levels are impossible to measure right now and that they are 5000 times lower than outside the cells. My guess is that they’re not related but I don’t know.
hypercalcemia is related to cancer…. generally
My calcium was trending high. It normalized, as I got better too. I worked on what I eat, my nutrition. Salt, vitamin D, vitamin K, and lots of calcium (which used to be almost nil before). The D I did supplement, because from the sun I was making none. My cholesterol was low too – may have something to do with it.
I never bothered with the bone scans – I did fracture bones very easily.
I did many more things. Read Ray Peat, you’ll get an idea of what things to try.
I would have rather more confidence in this article if it were properly referenced.
I’m also rather suspicious of the credibility of the researchers behind the hypothesis. Why? I’m seeing use of odd language here that I never read in papers in legitimate journals.
This is a blog – not a scientific journal. With regard to the references – check out the link in the blog to the article. You can find more blogs on the Wirth Scheibenbogen hypothesis papers below. Each blog contains a link to the paper which is, of course, loaded with references.
No Neuroinflammation Needed? An Epic ME/CFS Hypothesis Series Winds Up
Hypothesis Predicts Major Failure Point in Chronic Fatigue Syndrome (ME/CFS)
The Blood Vessel Crunch – A Unifying Hypothesis for ME/CFS
Thank you, Cort.
Carmen Scheibenbogen is the most well-known ME/CFS researcher in Germany and works at the Charité University Hospital in Berlin: https://immunologie.charite.de/en/metas/person_detail/person/address_detail/prof_dr_med_carmen_scheibenbogen/
I was wondering. How do we integrate the theory from Wirth and Lipkin?
I can’t remember what Lipkin’s theory is but I think the Wirth/Scheibenbogen theory ties in with Ron Davis’ Nanoneedle paper, I think Wirth/Scheibenbogen quoted it in their publication.
Lipkin thinks that the cause is in the gut (bacteria).
I was wondering similarly – is the NCNED calcium chanelopathy findings basically the same as Wirths intracellular Ca findings, perhaps in different body parts?
Surely the next obvious step would be for a far larger study to see if their findings do pan out. Isn’t this the only way that mainstream scientists take notice to the extent that others are then able to either confirm or disagree with the hypothesis?
I realise this takes large sums of money but if only these scientists would group together as Ron Davis’ group has done we might move forward much quicker and actually get a treatment that is accepted by the mainstream doctors and media, As a very long standing patient I find the situation we find ourselves in very frustrating and definitely not moving forward faster enough.
I did think that treatments of Long Covid patients would move quality research/treatments for ME forward but I don’t see much evidence this is actually going to happen.
An SGLT2 inhibitor could decrease intracellular sodium. Any reports of those drugs being helpful in real life?
https://pubmed.ncbi.nlm.nih.gov/32037659/
Calcium and sodium go into the cell during the stress response.
Also, in hypothyroidism.
Also, a hypothyroid state will trigger stress hormones.
Addressing both seems like a much more effective way of doing things.
Also, will people be fear-mongered into not cosuming calcium and/or sodium because of this?
One of the many ways which contributed to me becoming severe with ME/CFS was limiting my salt intake and the absence of calcium in my diet.
One of the many changes I made from which I improved was to up their intake. I did calcium first, and then salt no longer had the effect of raising my blood pressure and heart rate.
Is it worth trying to drink a little baking soda w/water to help correct this? I’ve been ill a really long time, and haven’t really tried drinking baking soda until yesterday and today a little. Remission Biome retweeted this blog & opened a discussion about baking soda. I’m way too brain fogged to try to read scholarly articles about baking soda/inflammation. Maybe Remission Biome is focusing on the topical PR Lotion though? Thank you for any easy to understand insight. Thanks so much, Cort!
OK, we’re back to discussing antibodies. If there are antibodies to B2AdR receptors, Rituximab should help many people. If, indeed, B2AdR receptors are “the most potent activators of the Na/K/ATPase enzyme”. The woman who ran the 10K in the Norwegian film was not fueling her body with the “placebo effect”. That drug cured her.
On the idea of whether a high salt diet might exacerbate high intracellular sodium levels, here is a study that looked at that question: https://pubmed.ncbi.nlm.nih.gov/2842105/
The study defined a higher salt diet as 6-8.9 grams per day. The study found that intracellular sodium levels did not change BUT the activity of the sodium-potassium pump was suppressed fairly significantly on the higher salt diet. That is something that might indeed have an impact in an ME/CFS patient. I have seen other studies suggesting high salt diet negatively impacts mitochondria.
Might be one to put to Wirth sometime Cort. Perhaps this is a problem more from salt in dietary sources versus from ORS where I think it is absorbed more safely.
Thanks for the great write-up and I think this pair are majorly onto what is going on in ME/CFS.
What is the name of the start up doing the research? Id like to invest. As someone with ME/CFS and low blood pressure, this is very interesting. My doctor has always told me to increase salt in my diet to combat light headedness from low blood pressure. I really hope more research comes soon because I’m more confused than ever.
Hi M,
The company has actually not been named yet. I will post it when it is. I would follow your doctors recommendations to increase the salt in your diet as it could be helpful and will not affect the sodium situation in your muscles.
Why is the company not named?
I just emailed Klaus and the company has not been named yet. I will post it when it is.
The company is now Named and there were some Information in Wirths talk today at ME/CFS conference in Berlin.
Name is Mitodicure GmbH.
Human proof of concept in 3 years (Phase 2)
Critical step financing around 10 Mio. €
Novel first in class aproach, no drug repurposing
Thanks for the update! Good to hear 🙂
would oxygen therapy be a good treatment for CFS …ps: i do feel my muscle are weaker than i want…making the bed is a chore.
Some small studies indicate that hyperbaric oxygen therapy may be helpful in ME/CFS/FM – do a search on Health Rising to find some blogs on that 🙂
the one i talking about is the IV….
How would all of this fit into a possible diagnosis of AFib Atrial Fibrillation?
My Doctor found my pulse is very abnormal by using her fingers, she then sent me for a long ECG which shows I have a number of ectopic heartbeats which can be from AFib.
She sent her findings & my ECG to Cardiology. They use beta blockers, blood thinners, calcium channel blockers, pacemakers, Maze Surgery, ablation surgeries even resetting the heart in Emergency or Cardiology by shocking it back in to normal heartbeat rhythms.
I was told back in June I had POTS I told another Doctor I disagree with this & told them I was told I had Syncope but their meds never helped me he even offered another tilt table test I refused it right out.
That Doctor in June told me to increase Salt same as Johns Hopkins/Dr. Peter Rowe said years ago, it makes me worse.
I have not yet got this diagnosis yet but she feels this is why I am sick. It is not an easy illness but at least it is taken more seriously. There are articles on Facebook key in AFib.
Has anyone on here been diagnosed with AFib? It also can run in Families one is permanent or progressive some it goes away. Some can have Tachy Brady Syndrome instead.
What was the medicine the German Team used? Are these AFib or similar meds?
Dr. Peter Rowe’s regimen dramatically improved my symptoms the moment I started. Twenty years later, my body switched gears and I no longer take Fludrocortisone nor added salt.
However, I will forever be grateful for those years that Dr. Rowe’s regimen helped me. I’m glad I traveled from Massachusetts to Rhode Island when he was there to thank him personally.
(I’ve always thought that if those who might benefit from fludrocortisone would increase their dose instead of reducing it, or stopping it, when adverse symptoms arose, they would find it beneficial.)
Hi Court, thank you for keeping us updated. Does the calcium overload hypothesis contradict the previous findings about impared calcium transport in NK cells (NCNED study, 2017)? I remember that they hypothesized that a calcium deficit might be causing problems not only with NK cells but in all tissues.
Good question – It seems like it would but honestly, I don’t know.
I’m bedridden in a dark room with severe m.e.
If I eat sundried sea salt, I get agonisingly painful muscle spasms.
Apparently this is caused by an imbalance of electrolytes (which could be sodium, chloride, calcium, potassium or magnesium).
This presumably doesn’t happen to normal people or it wouldn’t be sold. It’s actually recommended for muscle cramps and to balance electrolytes.
So I’ve been Googling to see why this happens to me? Why is it causing an imbalance? Is it to do with a sodium imbalance in m.e.?
This doesn’t happen with pink Himalayan rock salt, processed table salt, or black sulphur salt. Only whole supposedly unprocessed, dried sea salt.
I looked it up and apparently it has less sodium relative to chloride than Himalayan rocksalt.
Very interesting and promising. I’m officially MCAS but also have numerous ME/CFS symptoms. I exercise lightly every other day to maintain muscle and tone, where if I don’t and lose it I cant exert and build back up. Was having bad days for seemingly months and was experiencing severe muscle weakness, pain, and soreness for past 4 days. Today I feel great, ALL things equal the only thing I did different was take Diphenhydramine/Benadryl instead of Cetirizine last night (both are options for me from MCAS). Today all muscle issues gone, clear minded, alert, woke early. From looking into Diphenhydramine appears to be a Sodium Channel Blocker as well which led me to this paper. Also interesting is other items tried (to some relief but lesser degree) and prescribed in past also happen to be sodium channel blockers.
With these findings of depleted oxygen levels, has anyone tried hyperbaric oxygen chamber therapy two boost The oxygen levels? There is a place nearby me that offers it, but it’s not supervised by a doctor. Have there been any studies showing improvement in CFS/FM symptoms with this therapy?
We’ve done several blogs and a poll on HBOT. Some studies have been done.
https://www.healthrising.org/blog/2022/12/27/hbot-hyperbaric-oxygen-chronic-fatigue-fibromyalgia-long-covid/
Prof. Wirth has developed a drug for #MECFS and #PCS together with
@C_Scheibenbogen
Here he presents the basis of #PEM and its treatment.
https://youtube.com/watch?v=8iRMTnG2Rms&ab_channel=Fatigatioe.V.
There is not enough money for the necessary approval.
Unfortunately in German.
Ok, yes a hypothesis, in fact too much sodium stops calcium from being used. The calcium ion channel is indicated with Kalemic Periodic paralysis as well, but it’s main problem is potassium.
I have met online 2 people that have gotten cfs from using heart meds that block calcium ion channels, proving again this is a major problem.
Also a very interesting case ,a woman in the USA,with cfs used a bone healing enhancing machine, which increase the calcium ion channel function by ultrasound for a broken arm, and when on the treatment, had a complete remission of cfs, returning after not using the bone stimulator.
I’m trying to find out if pwME with high muscle sodium have low renin with normal aldosterone plus low serum sodium levels. I have the chronic low serum sodium, newly discovered low renin and normal aldosterone which led to several hypertensive crises recently. I ended with hypertension and on a calcium channel blocker.
I also am looking for references to share with my healthcare providers.
Also which blood pressure medications do pwME tolerate the best? It seems like calcium channel blockers might not be the best option.
This would be an ideal problem to put to an advanced AI model. Take every single ME/CFS study and have AI figure out all the puzzle pieces.