


Patterson has become a juggernaut in the long-COVID treatment realm.
Working out of a small laboratory called IncellDX, which Pitchbook states has just 23 employees, Bruce Patterson MD has managed to have an outsize effect on the long-COVID field. More patients (many more) are reportedly using his protocol via an array of doctors he works with – more than have been enrolled in the NIH’s $.1.5 billion RECOVER thus far.
Patterson’s managed to do this by making himself available again and again to the press and social media over the past two years. I counted over 2 dozen YouTube presentations featuring Patterson over the past two years. Recently, Patterson appeared on the Solve ME/CFS Initiative/BIO webinar and in a follow-up talk with Dr. Drew. He also recently published a case series of treatment reports.
Two plus years since Patterson reported he thought that he’d uncovered the cause of long COVID, it’s a good time to catch up using a couple of presentations Patterson has recently made with Dr. Drew and in the recent Solve M.E./BIO webinar.
Patterson probably wasn’t expecting Dr. Drew to start off his recent interview 3 months ago the way he did. Seemingly without having a clue about the fix he was putting his guest in, Drew blithely noted that the drug Leronimab, which had shown so much promise early in the pandemic, just didn’t work out. “What about that?”, he asked Patterson.
Leronimab, it turns out, was Patterson’s first choice several years ago. In fact, Patterson got slapped down by the FDA after stating in a TED talk that the drug was saving the lives of severely ill COVID-19 patients. Patterson also attempted, but failed, to take over the company that produces Leronimab.
Patterson, after understandably stumbling a bit, asserted that the CCR5 pathway – which Leronimab blocks – plays a key role in long COVID, chronic fatigue syndrome (ME/CFS), fibromyalgia, and post-treatment Lyme disease. He also suggested there may have been some issues with the “offering”.
With Patterson’s audio cutting out frequently, it was a bit of a rocky start. Dr. Ram Yogendra – a board-certified anesthesiologist researcher on several of Patterson’s papers – cut in.
Evolving Understanding
Yogendra provided a moderating counterpoint to Patterson’s sometimes more exuberant statements. Yogendra emphasized that their understanding of long COVID and diseases like ME/CFS, FM, and post-treatment Lyme disease syndrome (PTLDS) is still evolving. He said they get patients who expect that the 2-drug combo Maraviroc and Pravastatin is going to be it, and are disappointed to find their case more complicated and much more testing than they expected needed. He encouraged patients not to expect one magic drug or drug combination to do the trick.
Noting that they’re still trying to define what pure long COVID is, Yogendra said they were still very confident that the S1 protein from the spike protein of the coronavirus was driving long COVID but that “not everyone has that profile”.
Indeed, their main hypothesis remains the same as it was 2 1/2 years ago. They believe (and have found) that a SARS-CoV-2 virus protein called S1 is persisting in non-classical monocytes. They believe those monocytes – whose sole job is to bind to the blood vessels via the fractalkine receptor pathway – are making their way to the blood vessels and causing damage and clotting. Patterson also believes they’re making their way through the blood-brain barrier into the brain.
These monocytes usually live only a week or so, but the protein short circuits the apoptosis (suicide) pathway – causing them to live much longer than usual. These same monocytes can carry viruses into the brain.

Patterson called monocytes “a garbage can” for pathogens. Could they be harboring different pathogen proteins in ME/CFS?
Calling them “a garbage can for viruses and bacteria and proteins”, Patterson clearly believes similar monocytes may be playing a major role in diseases like “long-Lyme disease” and ME/CFS. One wonders if Patterson has begun a search to determine if a Lyme or Epstein-Barr virus protein is persisting in ME/CFS patients’ monocytes.
Reactivation of the herpesviruses or the Lyme virus, and other factors means, Yogendra said, that the etiology and pathophysiology is “all over the place” – indicating once again, that a cookie-cutter solution does not work. Yogendra also reported that over the past 6-12 months, they’ve been doing more and more testing – another sign that the 2-drug combo is not the be-all and end-all. In that vein, he reported kids – who haven’t been exposed to as many pathogens as adults – tend to respond more quickly; i.e. they’re not as complicated.
Yogendra stated that 20 to 30% of patients with ME/CFS and PTLDS are not having the response they wanted to see and they are exploring them further. (Patterson is apparently using the same immune factor set that popped up in long COVID to assess his ME/CFS and other patients, and one wonders if that is sufficient.) Patterson stated that viral reactivation – which honestly no one to this point has really seemed to treat well – is a really important part of ME/CFS and Lyme.
It was good to hear Yogendra moderate expectations. If Patterson has, at times, hedged his bets in the past (has he?), those hedges were largely lost in the excitement over his claims that he’s identified the cause of long COVID. As time has gone on, though, long COVID has clearly gotten more complicated – not less – with studies finding over 200 symptoms and suggesting that multiple kinds of long COVID exist.
Patterson believes there is one common factor, though, that’s present in all these diseases (long COVID, ME/CFS, FM, Post-treatment Lyme) – and that’s vascular inflammation. (Certainly, studies have found evidence of blood vessel or endothelial cell problems in all these diseases. Note, though, that thus far, clotting problems seem to be more prevalent in long COVID than ME/CFS).
Patterson said that CD40L – the first protein producing in the clotting pathway is increased in his long-COVID patients – indicating that an inflamed endothelium (blood vessels) is present. VEGF – which promotes blood vessel growth – is also commonly increased, and is associated with neuropathic pain and other symptoms.
An ME/CFS and Long-COVID Connection
If Patterson’s findings describe both long COVID and ME/CFS, they should have popped up in the ME/CFS research literature, and at times they have. CD40L and VEGF both popped up in a Stanford ME/CFS study that assessed levels of 51 immune factors before and 18 hours after exercise. The study found, though, reduced – not increased – levels of CD40L post-exercise.
Interestingly, three of the five most discriminatory cytokines post-exercise in ME/CFS (CD40L, platelet activator inhibitor, CXCL1) affect the blood vessels – the big battleground in Patterson’s scenario. Plus, two blood vessel factors – CXCL10, vascular endothelial growth factor (VEGF) (as well as IL-15) were reportedly “highly connected with other cytokines in the ME/CFS network.”
Both White (2010) and Hornig (2015) also found reduced levels of CD40L – in Hornig’s case, in the short-duration illness group. (She found comparatively higher levels in the longer duration group.) Hornig also found reduced levels of platelet-derived growth factor in short-duration ME/CFS patients when compared to controls.
On the other hand, CD40L was increased in patients with PI-CFS (post-infection CFS) and other persons with fatigue after Giardia infection, and correlated well with fatigue levels. Another study found a significant increase in CD40 receptors on mast cells in severe CFS/ME patients.
The CD40L findings, like so many immune findings, are all over the place in ME/CFS, but the main takeaway may be that one way or another, they keep popping up. Something appears to be going on with these cells that play a big role in Patterson’s long-COVID hypothesis. An EBV study also found increased CCR5/RANTES activation in ME/CFS.
Patterson’s core findings should be showing up in other long-COVID studies, and some of them have. Other studies have found evidence of T-cell exhaustion and Epstein-Barr virus reactivation.
The really big surprise, though, has been monocytes and ME/CFS. Patterson’s big goal is to stop coronavirus protein-carrying monocytes from migrating to the blood vessels in long COVID.
Monocytes, on the other hand, have hardly been mentioned in ME/CFS for decades. (They were a big deal in the RNase L hypothesis, which eventually fell apart.) They were basically a no-show in ME/CFS, until recently, when they suddenly showed up big time.
The more effective gene expression methods Grimson used in an NIH-funded ME/CFS research center study revealed a big surprise: monocytes might actually be at the heart of what’s going on in ME/CFS.
Grimson found the strongest center of immune dysregulation occurred in monocytes – particularly classical monocytes. (Note that Patterson is focused on non-classical monocytes). Grimson found that the percentage of diseased classical monocytes was highly, highly correlated with fatigue (P<.00057 (!)). He also found increased levels of the chemokine CCL4, which specifically attaches to CCR5 receptors – a key player in Patterson’s hypothesis. Grimson wrote that the highly activated and aggressive monocytes he found:
“could contribute to many of the symptoms of ME/CFS. This work sets up lots of questions that motivate our work now – where are the monocytes going in ME/CFS individuals, what is causing them to be dysregulated, and ultimately, can we reverse this dysregulation?”
Grimson’s further statement – “ME/CFS patients experience continual improper recruitment of monocytes to one or more tissues” – seems quite in line with what Patterson seems to be suggesting – similar, albeit with nonclassical monocytes – they are moving into and harming the blood vessels.
Nonclassical monocytes are generally thought to be anti-inflammatory and are confined to the blood vessels but can be associated with the disease. Classical monocytes, on the other hand, are considered pro-inflammatory and could be attacking many tissues.
Other monocyte findings have popped up in COVID-19 and long COVID. Avindra Nath found extremely aggressive monocytes that were associated with the blood vessels in COVID-19. A Swedish study found activated and unusually long-lived classical monocytes in long-COVID patients six months after infection. A German study found a “profound dysregulation” in almost all soluble factors associated with monocyte/macrophage biology and a study linked CD9+ monocytes to the development of long COVID.
While monocytes (and many other immune cells) have shown up in long COVID no one yet has tried to validate Patterson’s core finding – that nonclassical 16+ monocytes a) are carrying the S1 spike protein in long COVID and b) that they’re migrating to the blood vessels and causing harm. Two years later, the CD16+ monocytes Patterson is so focused on have received little study in long COVID.
Treatment
Patterson’s first goal is to treat the chronic inflammation. Maraviroc’s ability able to affect inflammatory cells across the body, but not suppress the immune system, makes it an ideal drug for him. A CCR5 antagonist, Maraviroc, was the first in a new class of retroviral drugs developed to treat HIV. It apparently blocks the receptor that allows immune cells to respond to the chemokines that direct immune cells to the tissues.
Statins – developed to prevent atherosclerosis – then prevent cells from binding to blood vessels. That turns off the anti-apoptotic (anti-suicide) process causing the uber-long-lived monocytes to finally die. That’s why the effect is purportedly permanent. Patterson stated that their testing shows the numbers of these cells dropping over time.
Precision Medicine Approach
Doctors are more and more using a precision medicine approach
At least three precision medicine approaches are being done in these diseases. Patterson’s, Hyman’s CIRS group, and an Australian group are using gene expression, or other results, to track their patients and modify their treatment regimens.
If Patterson sees high levels of cytokines, for instance, he may try to block them using a treatment. Patterson reported that his results indicate that increased levels of IL-2 and TNF-a, for instance, are strongly correlated with fatigue. CD40L and VEGF, on the other hand, are correlated with neural symptoms and dysautonomia. Their latest paper – which is not out yet – correlated cytokines/inflammatory markers with symptoms.
Validating that paper’s findings could open the door to targeting symptoms using cytokine blockers and other agents. It was recently argued in the Solve M.E./BIO webinar that associating symptoms with biological factors is a much better and more efficient way to approach chronic diseases. Instead of diagnosing a patient with x disease and trying to hit that very variable target with a drug, you simply show, probably through gene expression results, that x-factors are associated with say, fatigue, and then you target that factor.
The differences Patterson reports are intriguing given the really close fit we’ve seen between the results of ME/CFS and long-COVID studies. With his precision medicine approach, and his ability to compare disease cohort to disease cohort, Patterson is taking a more fine-tuned look at these diseases than most. The Bateman Horne Center/Jax Laboratory and Lenny Jason (and surely others) are also comparing ME/CFS and long-COVID cohorts. It’ll be fascinating to see how closely or not closely connected they end up being as they’re put more and more under the microscope.
The Case Series Report
Patterson, Yogendra et al. recently published an 18-person case series report: “Case series: Maraviroc and pravastatin as a therapeutic option to treat long COVID/Post-acute sequelae of COVID (PASC)“.
Sometimes patient results are presented in chronological order; that is. every patient seen between x and y date is reported on. This case series, though, plucked out patients who had seen improvement on the protocol.
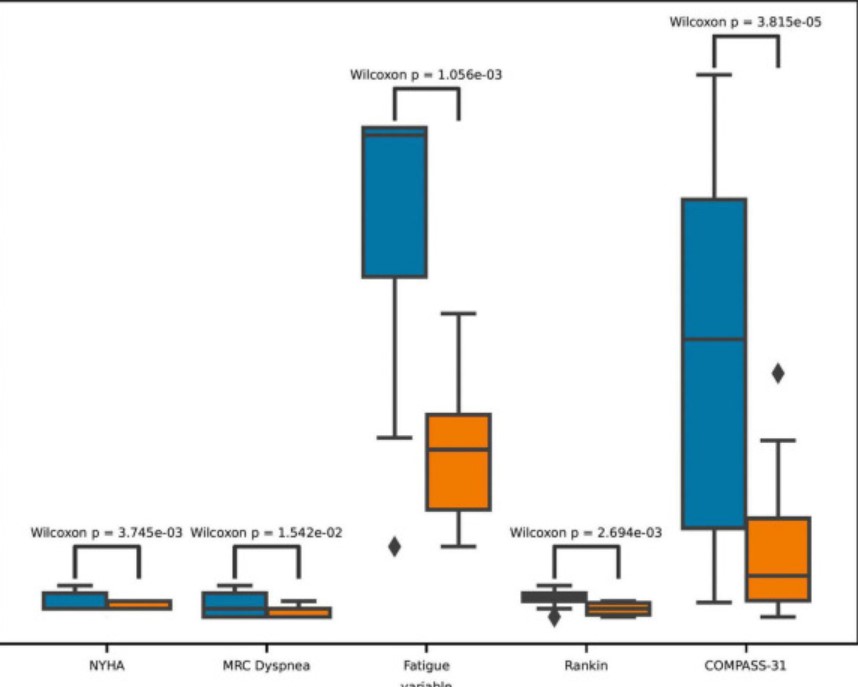
The study found that the treatment did change the levels of the cytokines Patterson believes are driving these diseases and that the levels of the cytokines were positively associated with some symptom scores; i.e. using the two-drug combo to drop the levels of the cytokines improved symptoms.
The results were not overwhelming, however. Changes in the Rankin disability scale, for instance, demonstrated a “low positive” correlation with changes in VEGF and sCD40L levels. Similarly, changes in autonomic nervous system scores (COMPASS) had low positive correlations with TNF-alpha and GM CSF levels and moderate positive correlations with VEGF and sCD40L. Changes in functional test scores had a low positive correlation with IL-8, and GM-CSF. Finally, changes in fatigue scores showed moderate positive correlations with IL-2, sCD40L, TNF alpha, and VEGF, and low positive correlations with IL-8, IL-10, and GM CSF.
With regard to the immune factors that Patterson believes are driving long COVID, all the authors could say was:
“Our results suggest that there are a number of biomarkers that appear to be positively associated in varying degrees with the various subjective scores.”
A similarly underwhelming result showed up when they assessed whether the treatments were sufficient to move a patient from one disease severity state (0 = Normal, 1 = Mild-Moderate, 2 = Severe, and 3 = PASC) to another; i.e. from mild/moderate to normal or severe to mild/moderate, etc. Although the statistical analysis showed that patients were better off after the treatment than before (see below), a machine learning model did not, if I’m reading this right, find their disease classifications, in general, had changed.
Simply assessing symptom scores (fatigue, autonomic, functional, etc.) before and after treatment, however, suggested that significant improvements – particularly in fatigue and autonomic nervous system symptoms – had occurred.
As this was a self-picked case series, it doesn’t tell us anything about the efficacy of Patterson’s protocol in general. That will have to wait for a placebo-controlled, randomized trial that Patterson stated is going to start later this year.
The Gist
- With 30,000 long COVID, ME/CFS, FM, and Lyme patients taking his tests and trying his protocol, and with tests available in the U.S., the UK, Europe, and Brazil, Bruce Patterson has become a juggernaut in the long COVID treatment realm.
- Patterson asserted he’s found the cause of long COVID (and possibly ME/CFS/FM and Lyme) in dozens of Youtube presentations and interviews over the past two years.
- During a recent presentation featuring him and a fellow researcher, Dr. Yogendra, Yogendra presented something of a moderating viewpoint. While they believe their thesis – that unusually longlived immune cells called monocytes are honing in on the blood vessels in long COVID – still stands Yogendra said their understanding of long COVID is still evolving. Long COVID is turning out to be quite complicated and different kinds of it exist.
- That means that the two drug Maraviroc and Prevestatin combo is not always the be-all and end-all treatment. At times further testing and other treatments may come into play.
- ME/CFS, not surprisingly, is more complicated with 20-30% of patients not progressing like they hoped they would.
- One common factor, though, they believe that’s present in all these diseases (long COVID, ME/CFS, FM, Post-treatment Lyme) is vascular or blood vessel inflammation.
- Study results in ME/CFS present some similarities and dissimilarities. A key factor in Patterson’s model of long COVID has shown up in ME/CFS research but in reduced not increased levels.
- Interestingly, though, Patterson’s major focus in the immune system – monocytes – recently showed up big time in a major ME/CFS research study. That study suggested that monocytes (albeit a different kind of monocyte) are ground zero for the immune dysfunction found in ME/CFS. That was remarkable given monocytes have received almost in ME/CFS until now.
- Some of Patterson’s early findings – T-cell exhaustion, herpesvirus reactivation, Lyme reactivation and vaccine-induced long COVID – have been showing up elsewhere.
- Patterson’s recent report on a case series of long COVID patients who had improved on his treatment protocol had mixed results.
- Reductions in the key cytokines that he believes are driving long COVID (and ME/CFS/FM) symptoms were, at best, only moderately associated with improvements in symptoms. More importantly, though, the study did appear to find significant reductions in fatigue, dysautonomia, and other symptoms.
- Patterson has been taken to task at times for moving too quickly to promote his approach. During a recent talk, Patterson noted that being in the corporate world has allowed him to disseminate his findings and ideas to the long COVID patient population much more quickly allowing him to diagnose and treat many more long COVID and other patients than he would have otherwise.
- That’s undoubtedly true. Dr. Shungu’s fourteen-year NIH-funded assessment of the effectiveness of just one substance (NAC) in ME/CFS amply demonstrates how terribly long and drawn out and ultimately not feasible for patients the academic approach can be.
- Still, without a clinical trial – which Patterson says is coming this year – thousands of long COVID, ME/CFS/FM, and Lyme patients have been spending thousands of dollars in hopes that Patterson is correct. In light of that, despite the obvious shortcomings of any online poll one that attempts to give some idea of his protocol’s efficacy is provided.
Booming Business
It’s still hard to know what to make of Patterson. He came up with a novel hypothesis and approach to long COVID very early on. He was one of the first to acknowledge that herpesvirus reactivation sometimes occurs, was the first that I know to talk about Lyme reactivation – including in patients who didn’t know they’d ever been exposed to Lyme, and one of the first to find the vaccine-induced “long COVID” that sometimes occurs.
Whether Patterson is right or not, he’s another example of the creativity that’s been unleashed by long COVID and the novel approaches to it, and diseases like ME/CFS that have shown up. No one was talking about monocytes, antiretroviral drugs or statins in ME/CFS before Patterson showed up.
An academic with a long record of achievement, he’s now out on the skinny branches, though, asking sick people to trust him that his diagnostic and treatment protocol works. That’s engendered some criticism, but the patients have been responding to his message of hope.
His company, IncellDx is booming. It just launched in Australia and is already live in the European Union (EU), the United Kingdom (UK), and Brazil. It’s started a collaboration with Igenex in the US – which Patterson called a global leader in Lyme testing – to help them ferret out post-treatment Lyme patients.
This is in part because Patterson has made trying out his protocol very easy. At COVIDlonghaulers.com you can order $910 worth of tests (in the US), schedule a blood draw, get your results from the lab and talk to one of Patterson’s collaborating doctors. The doctor’s notes and recommendations will then be sent to your primary care provider, who will be responsible for prescribing the medications. It’s a wonderful system.
In a recent talk, Patterson, a former academic, pointed out an interesting advantage of being in the corporate world – the ability to scale up quickly and disseminate his findings. It’s that ability, he said, that is allowing him to reach more patients.
He certainly has a point. Take Dikoma Shungu’s NAC study in ME/CFS. It took 9 years for Shungu to definitively show that low brain glutathione levels are present in ME/CFS. In 2012, Shungu received an R21 (small exploratory) grant to investigate whether NAC – a glutathione enhancer – might be helpful.
Eight years later, in 2021, Shungu got his BIG grant – an RO1 grant – to thoroughly investigate the effects of NAC in ME/CFS. That study is projected to last through 2025, with the results hopefully ready sometime in 2026. It will have taken 14 years – a fifth or so of a normal person’s lifespan – to study the effects one substance in ME/CFS using the standard academic approach.
So yes, Patterson is bashing through some norms, but at least it won’t take 15 years to know if he’s right or not.
(Health Rising is not associated with Bruce Patterson or IncellDx in any way).
The Patterson Poll
It’s not really fair to assess treatment efficacy using an online poll. We don’t know who is taking the poll. It’s possible the poll could undercount people who had positive results and went on with their lives. We don’t know how well the participants adhered to the treatment protocol, or how well the doctors treating the patients did, or what additions they made to it, what other health issues the patients brought to the table, etc.
Dr. Patterson, though, has promoted his diagnostic and treatment plan again and again over social media and the internet, and in the absence of a clinical trial, the only thing we can do is do a poll. So, take the results as you will – here’s the poll.






Great piece, Cort. Just one thing about the poll – I got significantly better with Patterson’s treatment, then crashed from adrenaline/over activity, then could not achieve any gains with his testing or treatment after that. So I answered the poll “moderate improvement” and that I would “probably not” do the program again, but my gains didn’t last. And I know I’m not the only one this happened to. Maybe an added question about whether the gains lasted?
Very good point, Amy! Just saw that recently in a LongCovid reddit forum, where someone advised to please report “recovery” only after 3 months or so, stating that the forum had so many “I am 80% recovered”-stories where patients crashed a couple of days or weeks later. D’ you think this could be covered by the question “If you improved at some point did the improvements stick?”
In general, with some supplements I initially got a rush of energy for a couple of days, then crashed from overactivity/adrenaline.
JR, 80% is exactly where I got to during the four or so months the treatment worked. At the time they said they had “put out the forest fire; the rest of recovery (the other 20%) is rebuilding the forest.” It sucked because I was able to with f/t fairly comfortably again and I even took a hiking trip to Sedona. But I had a horribly stressful week at work, followed by what would have surely been a near fatal car accident that I narrowly avoided (BIG adrenaline dump), followed by a planned hiking weekend trip (that I should have canceled but felt bad for my husband missing out so I didn’t). So a few weeks after I predictably crashed the following week, I sent IncellDx an email saying I had “relapsed” and nothing was working anymore. Patterson himself wrote back lightly scolding me for using the word “relapse,” but saying they would help me. They retested my spike proteins at that point and I had cleared them from the non-classical monocytes but still had them in my classical monocytes, so they adjusted all my medication to address the “rebuilding the forest” as they called it. Months and months on the new meds, and nothing changed. I got worse and had to leave my job. I even recently tried maraviroc again for 10 weeks; nothing.
I stopped giving them my money after I realized that scolding me for using the word relapse meant that it was more important to Patterson to stick with his theories and the program he built around them ($$$), even if they weren’t correct, than to switch gears at that point. A whole lot of folks are given that initial blast of hope by feeling better only to get let down when they crash, and then they’re stuck in IncellDx’s expensive mouse wheel. Very few have recovered 90-100% and stayed there, or else it’s all we’d be hearing about.
Sorry, long-winded way of saying yes, we need a poll question about whether the improvements lasted 🙂
Best wishes to you Amy!!
Thank you!! And to you as well 🙂
Thank you so much Cort. This is furiously interesting.
My theory is that it’s not infected monocytes that are creating problems, but simply monocytes crossing the blood brain barrier and hanging out in the CNS.
There are several potential ways that could potentially address this that I am interested in.
Cort, you may recall me mentioning this non- ME/CFS research a few times, quite a few years ago, and postulating that this might be causing ME/CFS:
https://medicalxpress.com/news/2009-02-inflammatory-disease-fatigue.html
That’s fascinating Matthias – I had forgotten all about that. It fits so well. Younger is assessing immune cell infiltration into the brain in ME/CFS now.
“New animal research in the February 18 issue of The Journal of Neuroscience may indicate how certain diseases make people feel so tired and listless. Although the brain is usually isolated from the immune system, the study suggests that certain behavioral changes suffered by those with chronic inflammatory diseases are caused by the infiltration of immune cells into the brain. ”
It fits so well. I see if I can find out what’s happened since then. 🙂
I contacted the researchers many years ago. Unfortunately ME/CFS didn’t seem to hold much interest for them, which was a bit disappointing.
I agree since ME/CFS is basically the epitome of sickness behavior….
“Sickness behavior significantly impacts quality of life. Our findings further our understanding and may generate potential new avenues for treatment of these often crippling symptoms,” said Swain.
Matthias, I agree, and this could be why MCP-1 is one of the most associative biomarkers for Long Covid (MCP-1 is involved in leucocyte trafficing across the BBB)
Matthias, to clarify for those who follow the link in your comment: MCP-1 = CCL2
By the way: EBV causes monocytes to secrete MCP-1:
https://journals.asm.org/doi/10.1128/JVI.00403-07
This could facilitate their migration into the CNS
+15yrs Monocytosis “(unknown cause”)
MCP-1 too high
Serum/csf albumin ratio: too high
(Only a few of the many lab findings.)
Dx: ME/CFS 21 yrs
started with mainly lymphopenia (nk’s) and went on to chronic monocytosis + increased cd19 Bcells.
Last few years eosinophilia was added (No parasites or allergy).
extreme (blood) (ebv, cmv, hsv) viral reactivations continue.
& Hhv6DNA was found in a stomach biopsy.
“no clue whatsoever.
Doesn’t fit any known disease.”
Again and again and again …
Bruce Patterson´s first goal is to make money. This is why he contends with “science” that serious scientists do not accept:
Cort, your description of Patterson´s case series says it all:
“Sometimes patient results are presented in chronological order; that is. every patient seen between x and y date is reported on. This case series, though, plucked out patients who had seen improvement on the protocol.”
Publishing this way is advertising, not disseminating data to learn from.
This slide from one of his early presentation says it all:
https://twitter.com/IamBreastCancer/status/1445452703564369930
He wants to reap as much money as possible from desperate patients (“market size”)
Why is his work not replicated? Because his collegues don´t take his work serious (they should, nevertheless, just to set the record straight).
Two years later, the CD16+ monocytes Patterson is so focused on have received little study in long COVID. << Why is this? Wouldn't this be easy to prove by another institute?
I often wonder why findings are not followed up on. Patterson’s finding of long-lived monocytes was novel – as was his finding that they were carrying the S1 protein. To me, a layman – that seemed like quite a finding but I don’t have the ability to assess the finding and the testing done, etc. – it’s too technical for me – all I can do is report that it happened. I hope somebody is following up on it.
Temporary remissions like those of many Patterson patients could be attributed to what the medications are altering in the microbiome. For those that did see improvement or short-lived remission, I’d suggest doing recurring microbiome testing (every 3 months or so) and running your results through MicrobiomePrescription.com
Dr. John Scott (Canada)” has spent three decades studying ticks and Lyme disease and has been recognized nationally and internationally for his multiple studies on the topic. He is responsible for identifying 37 tick species and publishing 35 peer reviewed scientific articles on the subject.” He states, “ ILADS-trained MDs and NPs in the US are testing long COVID patients for tick-borne diseases, and they are testing positive. The human immune system cannot fight COVID and also keep asymptomatic tick-borne diseases in check. It its well known that tick-borne diseases can lay dormant for years. When the immune system is under attack fighting COVID, these dormant pathogens become active. Everyone with long COVID should be tested for tick-borne diseases at a reputable lab like IGeneX or Armin.”
Lyme is a bacterial, not a viral infection as stated in the article. Most people carrying Lyme also have other tick borne diseases such as Babesia and Bartonella which can cause ME/CFS symptoms.
Dear Kat, this is very interesting! Would you have more information on the source of the quote, and by whom these labs are regarded most reputable? I just looked up Armin labs in Germany and it seems to me that it caters to the alternative health scene including Heilpraktiker.
JR, Igenex is the best lab in the US for testing for tick borne diseases See TreatLyme.net for DR Ross review of labs. https://www.treatlyme.net/guide/best-lyme-tests.
Thank you for this!
And that is what Dr Bruce Patterson does. His Ai and algorithms assessment of the 14 cytokine blood panel signposts where bacteria and tick borne need to be tested for. His latest discussion 5 weeks ago on drbeen YouTube channel gives an over view of what he is finding bacteria and virus wise.
Dr. Scott (Canadian) “has spent three decades studying ticks and Lyme disease and has been recognized nationally and internationally for his multiple studies on the topic. He is responsible for identifying 37 tick species and publishing 35 peer reviewed scientific articles on the subject.”
He states, “ ILADS-trained MDs and NPs in the US are testing long COVID patients for tick-borne diseases, and they are testing positive. The human immune system cannot fight COVID and also keep asymptomatic tick-borne diseases in check. It its well known that tick-borne diseases can lay dormant for years. When the immune system is under attack fighting COVID, these dormant pathogens become active. Everyone with long COVID should be tested for tick-borne diseases at a reputable lab like IGeneX or Armin. ”
Lyme (Borellia) is a bacteria, not a virus. Ticks seldom carry just one pathogen. Coinfections such as Bartonella and Babesia need to be tested for also as they can cause ME/CFS symptoms. These infections can also reactivate viruses such as EBV, herpes, etc.
Definitely worth testing for along with mold mycotoxins.
I have been to two prominently known ME/CFS doctors in the US who both quickly disregard my positive Bartonella results from iGenex (despite my multiple tick bites 40 years ago as a child). Perhaps it’s not worth spending the money on iGenex testing since results do not impact treatment at this time.
How very frustrating! Have you visited a LLMD (Lyme literate MD)? They would treat your Bartonella, as they regard tick borne illness a factor in chronic fatigue and PEM.
You need to find a LLMD (Lyme DR). I highly recommend visiting TreatLyme.net by Dr Marty Ross MD and searching for Bartonella. You’ll find a lot of info on treating Bart with both herbs and traditional meds plus he does a live online webinar to answer questions. Igenex is the premier lab for tick borne diseases in the US. If they show you have Bart, you have Bart and you won’t get better until you treat it. Good luck!
I marked myself as game changer because I truly believe it saved my life and the difference between now vs before I went on the protocol is astounding. I still have some issues but overall, the protocol has helped me get my life back. After I got the vaccine and Covid for a second time, I began to get worse but once I got back on the protocol, I got my life back again. I’m better now than I have been in years despite still having some smaller issues and finally have hope again.
Good to hear, Kate! Thanks for sharing your experience 🙂
Is “the protocol” you’re referring to Maraviroc and Prevestatin?
Something is very wrong with the math in the results of the poll. The percentages do not match the numbers.
If 1 represents 8%, how can 55 then be 42%? These mismatches can be found in most of the results.
Knowing what you now know, would you do it again?
Definitely yes 42% 55
Probably 19% 25
Not sure 21% 27
Probably not 10% 13
Definitely not 8% 1
According to my count they are off – not very off – but still off – which is very strange.
55/121 – 45%
25/121 – 21%
27/121 – 22%
13/121 – 11%
It’s the 1 that’s really off – it should be .8%
Kate, if you have a moment, can you clarify exactly what Dr. Patterson prescribed for you, please?
Is it case by case, depending on the symptoms, or is the protocol the same for everyone?
Thanks in advance
I was first introduced to corona viruses when our sweet two year old Ragdoll kitten died from Feline Intestinal Peritonitis. This horrible and usually 100% fatal disease is caused by a feline corona virus. We tried desperately to save him, even contacting a research team at the University of Tennessee.
Then in 2003, a Chinese-American businessman was the first diagnosed case of SARS (Severe Acute Respiratory Syndrome) which had a 9.5 % fatality rate. He died as did the doctor who first diagnosed him. SARS is caused by a corona virus.
This was followed by MERS (Middle Eastern Respiratory Syndrome) diagnosed in Saudi Arabia in 2012 by an Egyptian virologist. MERS (also a corona virus) had a fatality rate of 34.4 %.
Both SARS and MERS could have long term health effects.
https://www.rcpjournals.org/content/clinmedicine/21/1/e68
Rather than study these viruses in the same family, researchers are making (I believe) tenuous links to ME/CFS which is not caused by a corona virus.
ME?CFS is also known as Post Viral Syndrome and that probably isn’t actually correct either because I ended up with it after a VA Dr failed to treat me for Anaplasmosis for 6 months. Anaplasma is a bacteria. ME/CFS is better known as a post severe illness syndrome. It may have a link to other common viruses like EBV, HPV6, Parvo, etc because of the prevalence of those viruses in the general population. My theory is the immune system can only fight off “bugs” (including mold) for so just long and at so point is overwhelmed and goes haywire. Genetics likely plays a role as well.
Grateful for Dr. Patterson and his protocol. He gave me my life back. I was able to exercise again. One thing about the does it last question is it does until you get reinfected. I have repeated the protocol after reinfection and it worked again. Worked for my child and husband as well.
I saw moderate improvement with Maraviroc and a statin. (Not so much with Fluvoxamine and others.)
Most importantly, Dr. Patterson helped me realize that I have a complex case — what he calls “PASC Plus.” Once I learned that, I was able to pursue specialists that helped me find more clues, including ME/CFS, Small Fiber Neuropathy, SIBO, Candida, OCHOS Syndrome, pelvic floor dyssynergia and possibly Sjogren’s Disease.
I’m not cured, but Dr. Patterson’s group helped give me a boost along my health journey.
Good to hear you got such a thorough workout. This is the kind of thing that I expect – and which Yogendra alluded to – that many people have very complex cases – and that was missed in the earlier Patterson presentations that I saw – which can result in unrealistic expectations. I’m glad to see that aspect of this coming out. Thanks for sharing that and good luck.
It’ll be interesting to see how the clinical trial goes. The poll, such as it is, suggests that Patterson is pretty good at diagnosing long COVID but poor at diagnosing other diseases – which is actually what – despite his statements that he can do that – is what my reading of his paper showed.
With regards to treatment, I think the results are pretty good – 40% said they would definitely do it again and 27% said they probably would – not bad for these diseases at all. There weren’t that many home runs – which honestly is not a surprise for anyone with a longstanding case of ME/CFS – but there were some and there were a nice swatch of moderate and significant improvements.
On the other hand, a significant subset of the poll participants said the effects didn’t last.
It would be interesting to match these results up against those of an experienced ME/CFS physician.
They suggests that to me that Patterson has not solved this thing but he may have made a nice dent. We’ll learn much more when the clinical trial gets going (fingers crossed) this summer/fall. That will weed out the placebo effects from the treatment effects and placebo effects can be quite significant. Plus it will also not take into account other treatments these patients may be taking. Time will tell!
My opinion is he doesn’t need to “diagnose” long covid. People are going to Patterson saying they have it. And then he’ll say, “looks like your labs support that.” But any lab results can support it because there is no definitive biomarker, and there isn’t one for ME/CFS or fibro either. So if there’s any inflammation or anything that could have a general connection to LC symptoms, then that backs up the “diagnosis”. Ultimately, every doctor in the world is throwing stuff at the wall and seeing what sticks, including Patterson. He’s just found a mighty good way to brand and monetize it…
I totally agree! I have lyme and was told to try his protocol. I felt horrible while on it and it didn’t help at all. He is not a lyme doctor and I think he is just jumping on another bandwagon for the money,
The protocol helped with brain fog symptoms; I improved significantly. Everything else didn’t change: fatigue, shortness of breath, joint pain, and other less severe symptoms. Reducing brain fog helped me maintain my employment; I couldn’t have done that w/o Dr. Patterson’s protocol. Also, the diagnosis of Long Covid, and crazy high CCR5/Rantes, was a BIG DEAL for my mental health (when all other doctors were telling me I had mental health issues). Very grateful for Dr. Patterson’s work.
I would like to add that the 12 week therapy You point to in the poll is not what I was told to do.
“minimum 21 days max 30 days Sezentry and Pravastatin.
I assume because my blood test was not as severe as others.
I did go on the therapy a second time after my second blood test showed high VEGF and SDL40 but my long covid index was OK.
There was a delay up to two months getting my prescription filled because most pharmacies will not fill a prescription for long covid.
You need to investigate this major problem. A delay in care.
I included the 12-week because I think it was Yogendra who said it took up to 12 weeks to see results. Sorry to cut you out though!
I have dealt with long haul for two years, it’s so clear to me that the culprit is the spike proteins. Take for example Vaccine Long COVID, all that’s being injected into you the script for spike proteins, nothing else.
Some peoples bodies cannot break down the spike proteins.
So then the real solution seems to be what breaks down spike proteins?
Here’s a question. Since thus far the ME-CFS subset of long COVID looks almost exactly like ME/CFS pathophysiologically (similar brainstem, HPA axis, dysautonomia, clotting/blood vessel problems, gut flora alterations and exercise challenge results) and the coronavirus wasn’t around when people with ME/CFS got sick – what’s the common denominator? It’s clearly not the spike protein…
The body respond to the spike proteins ( from the vaccine or the virus) by producing T-cells. T-cells irregularity seems to be the problem.
You did not include vaccine injury resulting in long covid like symptoms. Many of vaccine injured, including my loved one, got improvement once starting Dr. Patterson’s program.
Your article and survey suggest a bias against Dr. Patterson’s program working. You are not going to get good survey results. I am happy to see that the survey showed that many people got positive results from Dr. Patterson’s program.
Glad your loved one got good results and the survey showed that some people got good results and others didn’t – which is actually pretty typical. I don’t understand this, though, you stated the survey was not going to get good results – yet it did?
Regarding the crashing after 80% improvement. I myself experienced this from a different protocol that Dr. Patterson’s. That’s why I hate that each Long COVID theorist looks at theirs as the only one valid. Each holds a piece to this puzzle. I saw Dr. Patterson deny that MCAS or microclots were much of an issue since “treating them didn’t seem to help much”. I’m not sure if he meant from what he’d done for treatment or from what research has shown. But the research is lagging the very active and organized community. MCAS treatment gave me a 20% bump that is sustainable as long as I am in treatment. Using enzymes like nattokinase, serrapeptase, the Vedicinals 9 antioxidant mix, and aspirin 81mg for 42 days gave me 80% resolution that once again crashed. No one is addressing the repurfusion injury. Endothelial damage/vasculitis leads to microclots. And yes, treating them is not a quick fix. Because the issue is so wide spread in the body that breaking down these clots causes a systemic repurfusion injury. Mitochondria die, cells die, releasing toxic ROS that further inflame tissues, organs, the nervous system. Everyone wants to say their treatment is the end all be all, but they are putting people in harms way until they learn that treatment must go low and slow, giving patients the chance to rebuild these mitochondria as we go. Otherwise we end up with a deficit in the energy needed to rebuild. If we destroy all the energy factories in hopes for rapid resolution, we won’t have energy to make more factories. Enter severe MECFS and multiple organ failure. Why is no one seeing this? Its driving me nuts!
For everyone here:
Try neurophysics (Australia) for dysautonomia/ME/CFS/long covid. Especially if the nervous system is reacting strongly (which is in most long haulers). You might just need a reset of your brain and nervous system (but also pay attention to molds, EBV, Lyme, toxins). It’s a long road to recovery…
Could someone advise me the Australian group mentioned in this article that is doing specialised along the lines of the Patterson group .thankyou.
My lyme doctor suggested Patterson’s protocol after numerous lyme treatments did not get rid of my symptoms. I started on the Maraviroc and Pravastatin in January. Several days later, I started feeling extremely ill. I did a zoom call with Dr. Patterson and told him that I felt like I was being poisoned. He was adamant that it was not from his protocol. He told me there were “a lot of things going around” and then said maybe I had covid again. His zoom call lasted about 7 mins and cost me $249. I agreed to stay on the plan for 12 weeks hoping things would change. I made it to the 12 weeks and he recommended redoing the cytokine 14 panel test. Much to my disappointment, all my abnormal markers went through the the roof. My VEGF, Gamma, CDL40 and Rantes all were significantly higher. I sent him lab results and told him I was disappointed and financially, another follow would be a hardship but was willing to speak with him to discuss the fact that the protocol worsened my symptoms. He responded that I should get someone to treat the lyme. I responded by saying I wasn’t interested in discussing lyme, I specifically wanted to talk about his protocol and find out why all my numbers were higher. He never responded. If he really cared about people and was truly interested in the lyme/covid connection, I think a follow up would have been helpful. I’m sure he didn’t want to talk to me because he couldn’t justify the fact that now my inflammation is much worse and it is due to the meds. Took my money but didn’t follow through. Extremely disappointing.
Sorry to hear but thanks for relaying your experience. Not what you would expect at all!
Please give the dosage and how many times a day you are taking Maraviroc and pravastatin. Please Peace
Sorry, but I am not taking. I’m just reporting on it!
Was asking the community. Thanks for everything. I have Ben sick a long time as well. But thinking I bet it was those vaccines around the time I got sick.
My son is in the UK and has chronic long covid brought on by 2nd vaccine – no improvement in over 2 years, despite trying different treatments. Can I please ask how we get in touch with Dr Patterson for a consultation.
I read a press release from Dr Patterson’s company that the FDA approved their clinical trial but they are not listed on clinicaltrials.gov?
For those who took maraviroc + statin, how hard was it to take? Did it make you miserable before it made you better (if you got better) and how long did the side effects last, if you had them? Thanks.
For those of you who improved on his protocol, how long had you been ill before starting? I’m wondering whether his protocol works for people who have been sick for years or only for those who have been sick for shorter amounts of time (and might just be getting better spontaneously).
Bruce has a large placebo-controlled clinical trial going on – great to see that he’s putting his money where his mouth is 🙂
I 100% agree. Any idea when results are expected?